Abstract
Purpose
To assess the effects of warm ischaemia time (WIT) on renal function after laparoscopic partial nephrectomy (LPN) for renal masses in patients with a normal contralateral kidney.
Methods
From October 2006 to December 2008, 53 patients treated with LPN were enrolled in this prospective study. Effective renal plasma flow (ERPF) was estimated with 99mTc-mercaptoacetyltriglycine renal scintigraphy before the intervention and after 3 and 12 months. Multiple linear regression analysis was used to assess the effects of demographic and operative variables on postoperative renal function. Logistic regression analysis was used to evaluate the associations between the same variables and a ≥20% reduction in postoperative ERPF compared with baseline (defined as significant loss of renal function–LRF). ROC curve analysis was used to identify potential ischaemia time cut-off points.
Results
Fifty-one patients were eligible. The mean lesion size was 30 mm, and the mean WIT was 21.9 min. Longer WIT was associated with lower postoperative ERPF values (P < 0.001). A logistic regression model confirmed that longer WITs were significantly associated with ERPF decreases ≥20% (OR 1.454 and 1.741, for each 1-min increase, respectively). ROC analysis identified 25 min as a ‘safe’ cut-off for WIT (AUC 0.874, P < 0.001). Postoperative ERPF differences between the two groups (WIT ≤25 and >25 min) were significant.
Conclusions
Longer WIT was associated with LRF, as estimated with renal scintigraphy. LRF occurred within 3 months and remains stable until the 12th month after LPN. Every effort should be made to minimise warm ischaemic intervals during LPN, and the limit of 25 min should be not exceeded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic partial nephrectomy (LPN) has become the first choice for treating small RMs in centres with high laparoscopic expertise [1], and these centres are expanding to the treatment for larger lesions as well [2].
Renal artery clamping is vital to decrease blood loss during LPN and to allow improved visualisation, which is paramount to achieving negative oncological margins and performing precise kidney reconstruction. Prolonged ischaemia time can lead to some degree of renal ischaemic damage, but there has been no consensus on the maximum duration of renal warm ischaemia time (WIT) that would avoid a loss of renal function (LRF) and chronic kidney disease (CKD) [3–5]. On the other hand, in patients with a solitary kidney, serum creatinine level variations and the glomerular filtration rate (GFR) are adequate markers of kidney damage [6, 7]; however, there is still uncertainty about how to evaluate LRF in patients with a normal contralateral kidney. In this scenario, the 99mTc-mercaptoacetyltriglycine (99mTc-MAG-3) clearance is widely used to measure renal function and is considered the best method for precisely determining LRF after tissue resection and ischaemic injury [5, 8].
To contribute to this discussion, we planned the present prospective study to assess the impact of WIT during LPN on the function of the treated kidney in patients with a normal contralateral kidney.
Methods
From October 2006 to December 2008, patients with a single, organ-confined, contrast-enhanced RM for whom LPN was recommended were invited to participate in this prospective study, which was approved by our ethic committee.
Inclusion criteria
Baseline estimated glomerular filtration rate (eGFR) (Cockroft–Gault equation) was >60 ml/min, and baseline split renal function (SRF) assessed by 99mTc-MAG-3 renal scintigraphy was ranging from 45 to 55% in the kidney with the tumour.
Exclusion criteria
Patients presenting any anatomical abnormality. Patients were excluded from the analysis if they experienced intra- or postoperative complications, such as significant bleeding (causing severe hypotension), urinary fistula or other conditions that prevented them from undergoing postoperative renal scintigraphy.
Surgical technique
LPN was performed according to the previously described technique by the same surgeon (FP) [9]. All patients received proper hydration and mannitol infusion (0.25 g/kg) 20 min before clamping, and 20 ml of lidocaine (2%) was injected above the renal artery immediately prior to clamping in order to prevent vascular spasm [10]. The renal artery was then clamped with a bulldog clamp. After tumour resection and parenchymal reconstruction, the renal artery was unclamped, and 20 mg of furosemide was injected intravenously.
For each case, demographic, perioperative, and pathological data were recorded. Growth pattern of lesions was classified as cortical, cortico-medullar or central, according to a previously published experience [9]. Location of lesion was classified according to R.E.N.A.L. nephrometry score [11]. Comorbidities were classified according to Charlson’s index [12], whereas postoperative complications were classified according to the Clavien system [13].
Evaluation of renal function
Serum creatinine (SCr), eGFR, and renal scintigraphy effective renal plasma flow (ERPF), and SRF of the treated kidney were assessed before the procedure and at different time points after surgery. For the purposes of this study, the results at baseline and after 3 and 12 months were considered, and ERPF was used to assess the renal function of the damaged kidney. An ERPF decrease ≥20% respect to baseline was considered a significant LRF.
Pathology assessment
A single uropathologist reviewed all pathological analyses and classified the surgical margins as positive or negative [14]. The distance between the inked margins and the tumour was measured along with the minimum, maximum and average thickness of the healthy peritumoural tissue.
Statistical evaluation
Means and standard deviation were used to summarise continuous variables, while frequencies and proportions were used to summarise categorical variables. Differences between means of continuous variables were tested using student’s t test after verifying that variables analysed were approximately normally distributed. Multiple linear regression analysis was used to assess the effect of various independent variables, such as patient age, BMI, comorbidity index, lesion size, R.E.N.A.L. nephrometry score, baseline ERPF, average thickness of healthy peritumoural tissue and WIT on ERPF at 3 and 12 months after LPN. The same variables’ association with an ERPF decrease ≥20% after 3 and 12 months compared with baseline was evaluated after univariate analysis was performed, using logistic regression models and summarised with odds ratios and 95% confidence intervals (C. I.).
ROC curve analysis was used to identify the WIT cut-off with the best combination of sensitivity and specificity to predict a significant LRF after 12 months. All tests were two-sided, and P values <0.05 were considered significant.
Scatterplotting LOWESS fitted was used to graphically represent the trend of ERPF after 3 and 12 months after LPN with respect to WIT.
Results
Of the 53 patients enrolled in the study, 51 met the inclusion criteria and were considered. Two patients were excluded: the first for acute bleeding that caused transitory severe hypotension during the immediate postoperative period and the second for urinary fistula.
Patients
Baseline characteristics and perioperative results are shown in Table 1. The mean WIT was 21.9 min. Grade I–II complications were recorded in 4 cases (7.8%) and included postoperative bleeding requiring transfusion (2 cases), pneumonia (1 case) and pleural effusion (1 case). No complications were greater than those of Grade II were recorded.
Pathology assessment
Complete pathological results are reported in Table 1.
Evaluation of renal function
Figure 1a shows the ERPF values for the damaged kidney at different time points.
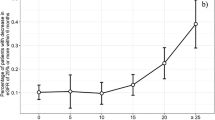
a ERPF variations for the operated kidney, calculated by 99mTc-MAG-3 renal scintigraphy at baseline and after 3 and 12 months. Data are presented as mean (±CI 95%). ERPF differences at 3 months versus baseline and 12 months versus baseline were significant (P = 0.003 and P = 0.001, respectively), whereas no difference was found between ERPF at 3 months versus 12 months (P = 0.128). b ERPF variations for the operated kidney, calculated by 99mTc-MAG-3 renal scintigraphy at baseline and after 3 and 12 months in the two groups of patients identified using a 25-minute cut-off. Data are presented as mean (±95% CI). In the group of patients with WIT ≤25 min, ERPF differences at 3 months versus baseline and at 12 months versus baseline were significant (P = 0.045 and P < 0.001, respectively); however, no difference was observed between ERPF at 3 months and 12 months after LPN. The same trend was noted in group of patients with WIT >25 min (3 months vs. baseline and 12 months vs. baseline: P < 0.001). c Scatterplot LOWESS fitted representing the trend of ERPF for operated kidney, calculated by 99mTc-MAG-3 renal scintigraphy at 3 (r = −0.5433, P = 0.00004) and 12 (r = −0.4489, P = 0.0010) months in our case study. Blue circles and red squares represent ERPF’s trends at 3 and 12 months after LPN, respectively
The regression analysis showed that longer WITs were associated with lower ERPF values at both 3 and 12 months postoperatively (R 2 = 0.67, F = 10.85, P < 0.001 and R 2 = 0.45, F = 4.96, P < 0.001), respectively. Postoperative renal function was significantly related to patients’ baseline ERPF 3 and 12 months after surgery (P < 0.001).
Overall, 16 (31%) patients developed a significant LRF as previously defined after 3 or 12 months.
Univariate regression analysis did not show significant correlation between R.E.N.A.L. nephrometry and significant LRF (P = 0.552 and 0.630 after 3 and 12 months, respectively).
Logistic regression model confirmed that longer WITs were associated with ERPF decreases ≥20% after both 3 and 12 months compared with baseline (OR 1.454 [CI 1.116 ÷ 1.896], P = 0.005 and OR 1.741 [CI 1.153÷2.630], P = 0.008 for each 1-min increase, respectively) (Table 2).
A ROC curve analysis based on 12-month data showed that the best combination of sensitivity and specificity to predict a significant LRF was observed at 25 min (area under curve 0.874, P < 0.001) of warm ischaemia. Using this cut-off, the sensitivity and specificity for significant LRF prediction were 75 and 88.5%, respectively (Table 3).
Figure 1b shows the ERPF values for the damaged kidney of the two groups created by the fixed cut-off (≤25 and >25 min) at different time points. Differences between the two groups were not significant at baseline (P = 0.575) but were statistically significant 3 months (P = 0.003) and 12 months (P = 0.005) postoperation. Figure 1c shows the results of LOWESS analysis.
Discussion
Vascular clamping is often fundamental for safe LPN, although new techniques such as zero ischaemia, early unclamping and ‘on demand’ clamping have been proposed to limit it [5, 15, 16]. When the renal artery is clamped, ischaemic damage is certain to occur; however, an upper limit of WIT that minimises this damage and its related renal function deterioration remains controversial. Some authors have published their experience with LPN and reported a WIT safety cut-off ranging from 20 to 40 min [5, 6, 17–23]. In a recent review, Becker suggested that the tumour should be removed within 20 min of warm ischaemia to limit LRF [5]. The majority of authors in urological literature are insisting on WIT reduction during LPN, while other authors are studying how to pharmacologically improve renal tolerance to ischaemia (e.g. mannitol in association with furosemide; angiotensin converting enzyme inhibitors and others [24, 25]). Moreover, when a long ischaemia time is prospected, renal cooling has to be considered in order to increase kidney’s tolerance to ischaemia [4].
Recently, Thompson et al. [6] studied 362 patients with a solitary kidney undergoing partial nephrectomy, using eGFR as a renal function marker. The authors concluded that WIT should be limited to 25 min and that each minute of WIT is associated with a 5% increased chance of developing acute renal failure and a 6% increased risk of new-onset Stage IV CKD during follow-up [6]. Although this study greatly clarified the relationship between WIT and LRF, it should be noted that the solitary kidney seems more resistant to ischaemic damage than paired kidneys [7]. Moreover, although SCr and eGFR are adequate markers of renal function for solitary kidneys, their usefulness is less clear in cases with a normal contralateral kidney, which is more common.
We, therefore, planned this study to evaluate the effects of WIT on renal function in patients with a normal contralateral kidney. Based on our clinical experience and literature results, we chose 99mTc-MAG-3 renal scintigraphy, which is widely used to separately estimate ERPF and split renal function, to assess the function of the damaged kidney [5, 8, 19, 26, 27, 29, 30].
Overall, the ERPF decrease occurred within 3 months and remained stable until the twelfth month after LPN (Fig. 1a).
Our results demonstrated that longer WIT during LPN is associated with lower ERPF values at both 3 and 12 months postsurgery. Baseline renal function is a positive predictor of postoperative renal function, which could support the findings that unmodifiable factors are also basic determinants of total renal function [5, 28].
A logistic analysis confirmed that WIT is related to significant LRF. For example, each additional minute of warm ischaemia was associated with a 45% increased chance of developing an ERPF decrease ≥20% after 3 months and a 74% increased chance after 12 months compared with baseline. These observations confirmed the results of Thompson et al. [6] and suggest that every minute is important when the renal artery is clamped during LPN.
Surprisingly, baseline ERPF was not related to significant LRF. This suggests that while baseline renal function is positively associated with postoperative renal function, it is not crucial in determining LRF as defined in our study.
In an attempt to identify a potential WIT cut-off point, we used ROC curve analysis and found that 25 min of WIT had the best combination of sensitivity and specificity to predict a significant LRF after 12 months. In other words, when WIT is longer than 25 min, the chance of having a significant LRF is 6.5 times higher that when WIT is less than 25 min. Interestingly, significant LRF occurred even in a case with a short WIT (see Table 3), suggesting that no cut-off time can guarantee that the kidney will be free of harm. This finding could have influenced LOWESS plotting, which showed lots of dispersion both at third and twelfth months.
Finally, the decrease in renal function occurred within 3 months in both groups and did not change significantly after 12 months.
This study has some limitations, including the number of patients involved and the duration of follow-up. Nevertheless, it should be noted that this study has one of the largest data series and longest follow-up periods currently available for prospectively studied patients with renal scintigraphy. Finally, we arbitrarily fixed the ERPF decrease (20%) to define significant LRF. We acknowledge the limitations of this choice; however, this value reflects previously reported experiences and is close to the mean renal function decrease reported in many papers comparing pre- and postoperative renal function after LPN [8, 19, 28–30].
Conclusions
There is a strong correlation between WIT and ERPF estimated with renal scintigraphy in patients with a normal contralateral kidney who underwent LPN. LRF occurred within 3 months and remained stable until the twelfth month after LPN. Every minute of warm ischaemia can decrease postoperative renal function, and surgeons should minimise warm ischaemic intervals and make every effort to not exceed the 25-minute limit.
Other studies are needed to further evaluate LRF after a longer follow-up.
References
Ljungberg B, Hanbury DC, Kuczyk MA et al (2007) Renal cell carcinoma guideline. Eur Urol 51:1502–1510
Simmons MN, Chung BJ, Gill IS (2009) Perioperative efficacy of laparoscopic partial nephrectomy for tumour larger than 4 cm. Eur Urol 55(1):199–207
Simmons MN, Schreiber MJ, Gill IS (2008) Surgical renal ischemia: a contemporary overview. J Urol 180:19–30
Wickham JE, Hanley HG, Joekes AM (1967) Regional renal hypothermia. Br J Urol 39:727–743
Becker F, Van Poppel H, Hakenberg OW et al (2009) Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 56:625–634
Thompson RH, Lane BR, Lohse CM et al (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58:340–345
Jablonski P, Howden B, Rae D et al (1985) The influence of the contralateral kidney upon recovery from unilateral warm renal ischemia. Pathology 17:623–627
Funahashi Y, Hattori R, Yamamoto T et al (2009) Ischemic renal damage after nephron-sparing surgery in patients with normal contralateral kidney. Eur Urol 55:209–215
Porpiglia F, Volpe A, Billia M et al (2006) Assessment of risk factors for complications of laparoscopic partial nephrectomy. Eur Urol 53:590–596
Klos I (1971) Lysis of mechanically produced spasms of small arteries through local drug administration. Z Exp Chir 4(5):262–266
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. Sep 182(3):844–853
Nuttalla M, van der Meulena J, Embertona M (2006) Charlson scores based on ICD-10 administrative data were valid in assessing comorbidity in patients undergoing urological cancer surgery. J Clin Epidemiol 59:265–273
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Minervini A, Di Cristofano C, Lapini A et al (2009) Histopathologic analysis of peritumoural pseudocapsule and surgical margin status after tumour enucleation for renal cell carcinoma. Eur Urol Jun 55(6):1410–1418
Gill IS, Eisenberg MS, Aron M et al (2010) “Zero ischemia” partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol Jan 59(1):128–134
Nguyen MM, Gill IS (2008) Halving ischemia time during laparoscopic partial nephrectomy. J Urol 179(2):627–632
Shekarriz B, Shah G, Upadhyay J (2004) Impact of temporary hilar clamping during laparoscopic partial nephrectomy on postoperative renal function: a prospective study. J Urol 172:54–57
Porpiglia F, Volpe A, Billia M, Scarpa RM (2008) Laparoscopic versus open partial nephrectomy: analysis of the current literature. Eur Urol 53(4):732–742
Porpiglia F, Renard J, Billia M et al (2008) Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? One-year results of a prospective study. Eur Urol 52:1170–1178
Laven BA, Orvieto MA, Chuang MS et al (2004) Renal tolerance to prolonged warm ischemia time in a laparoscopic versus open surgery porcine model. J Urol 172:2471–2474
Thompson RH, Blute ML (2007) At what point does warm ischemia cause permanent renal damage during partial nephrectomy? Eur Urol 52:961–963
Bhayani SB, Rha KH, Pinto PA et al (2004) Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol 172:1264–1266
Desai M, Gill IS, Ramani A et al (2005) The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int 95:377–383
Redfors B, Swärd K, Sellgren J, Ricksten SE (2009) Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption blood flow and glomerular filtration after cardiac surgery. Int Care Med 35:115–122
Humke U, Uder M (1999) Renovascular hypertension: the diagnosis and management of renal ischemia. BJU Int 84:555–569
Pouliot F, Pantuck A, Imbeault A et al (2011) Multivariate analysis of the factors involved in loss of renal differential function after laparoscopic partial nephrectomy: a role for warm ischemia time. Can Urol Assoc J 5(2):89–95
Choi JD, Park JW, Choi JY et al (2010) Renal damage caused by warm ischaemia during laparoscopic and robot-assisted partial nephrectomy: an assessment using Tc 99m-DTPA glomerular filtration rate. Eur Urol 58:900–905
Lane BR, Russo P, Uzzo RG et al (2011) Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol 185:421–427
Lane BR, Novick AC, Babineau D et al (2008) Comparison of laparoscopic and open partial nephrectomy for tumor in a solitary kidney. J Urol 179:847–851
Kobayashi Y, Usui Y, Shima M et al (2006) Evaluation of renal function after laparoscopic partial nephrectomy with renal scintigraphy using 99m technetium-mercaptoacetyltriglycine. Int J Urol 13:1371–1374
Acknowledgments
The authors would like to thank Luca Pozzi for his collaboration and efforts in the first draft of the paper.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porpiglia, F., Fiori, C., Bertolo, R. et al. The effects of warm ischaemia time on renal function after laparoscopic partial nephrectomy in patients with normal contralateral kidney. World J Urol 30, 257–263 (2012). https://doi.org/10.1007/s00345-011-0729-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-011-0729-5