Abstract
Laser-supported laparoscopic partial nephrectomy is a promising new technique, but remains under experiment. We presented our single institutional experience of laparoscopic partial nephrectomy using the 980/1470-nm dual-diode laser system to investigate its feasibility, and oncological and functional outcomes. The study retrospectively evaluated 25 patients with small exophytic renal tumors, who underwent laparoscopic partial nephrectomy using a 980/1470-nm dual-diode laser. The demographics, surgical data, complications, pathological variables, oncological, and functional outcomes were reviewed. The changes in hemoglobin and estimated glomerular filtration rate (eGFR) before and after surgery were statistically analyzed. The investigators operated on a total of 25 patients. The off-clamping technique was performed for 23 cases, while the other two cases required renal artery clamping due to unsatisfactory hemostasis. The tumor diameter was 24.6± 6.2 mm, and the mean operative time was 104.4± 23.4 min. The median estimated intraoperative blood loss (EBL) was 100 ml (range 50–600 ml). No major complications (Clavien-Dindo >II) occurred perioperatively. The mean change in hemoglobin before and after the operation was 9 g/l, with a P value of <0.001. The mean decrease in eGFR from before the surgery to the 6-month follow-up was 1.4 ml/min, with a P value of 0.463. The postoperative histopathology evaluation did not demonstrate a positive surgical margin. No recurrence or metastasis was found during the follow-up (mean 24 months). Laparoscopic partial nephrectomy using a 980/1470 nm dual-diode laser appears to be a feasible and oncological satisfactory technique for the treatment of small renal mass (SRM), with the advantages of reducing warm ischemia time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of small renal masses (SRMs), which are defined as masses of ≤4.0 cm in maximum diameter, has consistently increased during the past decades due to the wide use of imaging techniques [1], and this accounts for up to 66% of all newly diagnosed renal tumors [2].
Laparoscopic partial nephrectomy (LPN) has become the prevailing treatment for SRM, since this offers the equivalent oncological treatment outcome and maximum renal function preservation on the involved kidney, when compared to radical nephrectomy [3, 4]. At present, the temporary clamping of renal hilar vessels during LPN remains as the most commonly used hemostasis technique to provide a bloodless surgical field, allowing for a precise tumor removal and parenchyma reconstruction. However, the blockage of blood perfusion leads to varied degrees of warm ischemic damage to the kidney, depending on the warm ischemic time (WIT). In clinic, WIT serves as an indicator to predict the postoperative renal function, and the accepted WIT for successful LPN has been reported to range within 20–30 min [5]. In such a limited time, it would be challenging for an experienced urologist to finish the LPN, especially in some complicated cases. Furthermore, WIT was also identified as a risk factor to deteriorate the renal function in patients with existing chronic renal insufficiency [6, 7].
Several surgical techniques have emerged to minimize the WIT, such as targeted renal blood flow interruption, selective renal artery clamping, selective renal parenchymal clamping, radio frequency-assisted minimal invasive partial nephrectomy, and sequential preplaced suture renorrhaphy. However, most of these techniques are not widely accepted due to the inherent and practical defects [8].
In recent years, laser-supported LPN (LLPN) has been experimentally investigated [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Due to its properties of precise cutting and excellent coagulation, laser-supported LPN can potentially exempt the need for renal hilar clamping [9, 13,14,15, 18, 21, 23, 24]. Among the various laser techniques, the 980/1470-nm dual-diode laser system (Biolitec AG, Germany) represents as a new and promising technique. This is absorbable in both water and hemoglobin, thereby possessing better performance in incision and hemostasis. Several studies have reported successful results in the treatment of benign prostate hyperplasia (BPH) using the 980/1470-nm dual-diode laser [25]. However, its role for partial nephrectomy remains under investigation.
The present study aims to evaluate the efficacy of dual-diode laser-supported laparoscopic partial nephrectomy (DDL-LPN) without renal hilar clamping in a relatively populous study population. To our knowledge, the present study has the largest series to date on this issue.
Patients and methods
Patients enrollment
The present retrospective study was approved by the Institutional Review Board and Ethics Committee of our hospital. Between December 2016 and June 2019, the medical records of our hospital were reviewed to identify adult patients, who were diagnosed as SRM and underwent zero ischemic LLPN using the 980/1470-nm dual-diode laser system. The exclusion criteria included pediatric patients, centrally located tumors, unilateral multiple renal tumors, solitary kidney, and combined with coagulation disorders.
The indications for the procedure for zero ischemic DDL-LPN were strictly controlled before the operation. The SRM diagnosis and LLPN decision-making were based on the computed tomography (CT) and/or magnetic resonance imaging (MRI), and merely tumors with a diameter of ≤4 cm were considered. Other cases, such as recurrent tumors, tumors with a minimum distance of <5 mm from the urinary collecting system, previous ipsilateral upper abdominal surgery, and congenital renal malformation, were not indicated for the procedure.
All DDL-LPN procedures were performed by the same experienced surgical team. Before the operation, the benefits and risks of the laser approach were introduced to the patients, and conventional partial nephrectomy with renal hilum vessel clamping was chosen as the backup plan. The surgeons ensured that the patients fully understood the proposed surgical procedure, and fully addressed their concerns and questions.
Data collection
The preoperative R.E.N.A.L [26] and PADUA [27] scores were calculated for each patient to evaluate the operation complexity. The pre- and post-operative eGFR (CKD-EPI) was calculated based on the serum creatinine for renal function evaluation. The modified Clavien-Dindo scores were calculated based on the medical and surgical complications within 3 months after the surgery, in order to evaluate the procedure safety [28]. The demographics, surgical data, histopathological results, and oncological outcome were also collected.
Instruments
The synchronous 980/1470-nm dual-diode laser system (Biolitec AG, Germany) was utilized for these cases. The laser system can simultaneously emit two different wavelength lasers (980 and 1470 nm). The 600-um laser fiber was utilized in the operation, and placed into the operation area through the fiber guidance instrument. A continuous wave was used for all cases, with a power setting of 60 w (980/1470 ratio 2:1).
Surgical techniques
The patient was placed in the flank lateral position, and underwent general anesthesia with endotracheal intubation. The procedure was performed through the retroperitoneal approach, and the retroperitoneal cavity was formed by blunt dissection and balloon dilation from a small incision located at 2 cm above the iliac crest of midaxillary line.
After the establishment of the retroperitoneal space, four trocars were inserted. One 10-mm trocar was placed at the beginning incision for the endoscope. The other three trocars were inserted for the laparoscopic instruments, including one 12-mm trocar below the costal margin of the posterior axillary line, one 5-mm trocars below the 12th costal margin of the anterior axillary line, and another 5-mm trocar between the second and third trocars.
The renal tumor was resected based on the following steps:
-
(1)
Tumor exposure and hilar preparation: Depending on the location of the tumor, the kidney was dissected until tumor and surrounding normal renal tissue were fully exposed (Fig. 1a). The principle of kidney dissection was to facilitate the tumor resection and parenchyma suture. The renal arteries were separated from the surrounding tissue for all cases, in case renal vascular occlusions are required during the operation.
-
(2)
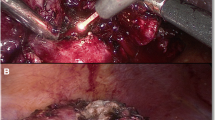
Laser excision: The laser fiber was introduced via a guidance instrument through a trocar. With a laser output power of 60 W, the laser procedure started from the incision of the renal parenchyma at 5 mm beyond the visible tumor margin (Fig. 1b). During the tumor excision, a low flow of saline was routinely flushed through the channel of the guidance instrument to the fiber tip, in order to reduce the smoke production. During excision, the laser fiber was placed in nearly direct contact with the parenchymal tissue (1–2 mm from the tip of the fiber to the tissue) (Fig. 1c). In the hemostasis, the investigators adjusted the distance from the fiber to the tissue, according to the condition of the bleeding. For arterial bleeding, the investigators adjusted the fiber tip to aim at the root of the bleeding artery and stop bleeding. For venous bleeding, the fiber was moved a little further (3–5 mm) from the tissue in a non-contact fashion for coagulation. The adjustment of the distance may increase the hemostatic area and improve the hemostatic effect. At the same time, the investigators reduced the velocity of the fiber to achieve a better hemostasis effect. One trocar was opened to evacuate the smoke, and suction was used when necessary. Suturing was not regularly performed unless the hemostasis was not satisfactory, or the calyx was opened (Fig. 1d). If necessary, additional hemostatic gel was used. After the tumor was removed through an endosac, a drainage catheter was placed through the 10-mm port site.
Key steps for the DDL-LPN without renal hilar vessel blockage. The procedure started from the tumor exposure (tumor size 2.8 × 2.2 cm) (a), followed with the laser incision of the renal parenchyma at 5 mm beyond the tumor margin (b) and tumor removal (c). The post-resection tumor bed presented a clear margin, and there was no bleeding (d). DDL-LPN, dual diode laser supported laparoscopic partial nephrectomy
Statistical analysis
The statistical analyses were performed using SPSS software version 20.0 (SPSS, IBM Corp., Armonk, NY, USA). Continuous variables with normal distribution were presented as mean and standard deviation (SD), and categorical data were recorded as proportion, respectively. Non-normal distribution data were expressed as medians with interquartile ranges (25th and 75th percentiles). Paired t tests were performed to compare the pre- and postoperative Hb and eGFR changes. P<0.05 was considered statistically significant.
Results
Patients and baseline data
Between December 2016 and June 2019, a total of 25 patients with SRM, who were initially proposed to receive the zero ischemic DDL-LPN procedure, were enrolled from our institute. Among these patients, 23 patients were successfully completed as planned, while two patients failed due to insufficient hemostasis during the resection, and were transferred to conventional LPN with renal artery clamping. The demographics and tumor characteristics are outlined in Table 1. The mean tumor diameter was 24.7± 6.2 mm (range 10–40 mm). The maximum intraparenchymal depth of the tumors was 5–17 mm (median 9 mm), according to the preoperative CT or MRI. Furthermore, 13 tumors were located on the left side, while 12 tumors were located on the right side. Among these tumors, seven tumors were in the upper pole, 10 tumors were in the mid-parenchymal area, and eight tumors were in the lower pole. The R.E.N.A.L score was low (4–6) in 20 patients and moderate (7–9) in five patients. The Padua score was low (6–7) in 21 patients and moderate (8–9) in four patients.
For patients who failed the laser procedure, the tumors were in the left and right kidney, with a maximum diameter of 32 mm and 40 mm, respectively. Both cases had moderate complicated tumors, with a R.E.N.A.L score of 7 and 9, respectively, and a Padua score of 8 and 9, respectively.
Peri- and post-operational data
The operative time was 104.4 ± 23.5 min (range 70–160 min), with a median estimated intraoperative blood loss (EBL) of 100 ml (range 50–600 ml). WIT was 0 min for all cases, except for the two failed cases. Parenchymal sutures were performed for five cases due to insufficient coagulation or opened calyx system, which included two failed cases. No blood perioperatively transfusions were needed. The hemoglobin (Hb) changed from 144 g/l before surgery to 135 g/l after surgery, P<0.001. The eGFR decreased from 94.0±15.5 ml/min before surgery to 92.6±15.5 ml/min at the 6-month follow-up (t=0.746, P=0.463). No significant change in eGFR was measured. The postoperative hospital stay was 6.2±1.8 days (4–12 days).
In the two failed cases, the laser resection at the initial stage was smoothly performed. Bleeding occurred when the base of the tumor was cut. A higher laser power (up to 100 w) was used for better coagulation; this could not achieve a satisfactory hemostasis. Therefore, the investigators suspended the laser procedure, and transferred to conventional LPN with the renal hilar clamped. The subsequent resection and suture were uneventfully completed. The operative time was 130 and 160 min, respectively, with a WIT of 12 and 19 min, respectively, for the conventional LPN. The EBL for these two cases was 400 ml and 600 ml, respectively. No blood transfusions were perioperatively needed.
Complications
There were no major intraoperative complications. Postoperative complications occurred in three cases, which included one pneumonia case, one lymphatic leakage case, and one hematuria case. These were all cured by conservative treatment. No perirenal hematoma was observed after the 3-month follow-up period. Furthermore, no reoperation or interventional therapy was needed. The mean follow-up duration was 24.9 ±10.1 months (range 10–42 months), during which no recurrence or metastasis was detected in any of the cases.
Histopathology and surgical margin
The postoperative histopathology demonstrated 18 clear cell carcinomas, two chromocytomas, two papillary carcinomas, one multicystic renal tumor, and two angiomyolipomas. Except for the two cases of angiomyolipoma, the pathological stage of the remaining cases was T1a. The surgical margin was negative in 21 cases and indistinct in four cases, due to charring of the tumor base by the laser. The perioperative outcomes and histopathological characteristics are presented in Table 2.
Discussion
Study overview
Although laser has been widely used in various fields of medicine and urology, its application remains scarce for renal surgery. In the present series, 23 of 25 DDL-LPNs were successfully performed without clamping the renal artery, and these had reasonable operative time and EBL. Furthermore, neither blood transfusions nor major complications occurred peri- and postoperatively. Two cases were transferred to conventional LPN due to insufficient hemostasis during the operation, and successfully underwent the subsequent treatment.
Characteristics of the 980/1470-nm dual-diode laser
The 980/1470-nm dual-diode laser system can simultaneously produce two kinds of laser beams, and the proportion of these two sources of laser beams can be adjusted according to clinical needs. The 980-nm wavelength can be absorbed by hemoglobin and water, which offers a good coagulation effect, while the 1470-nm wavelength is more absorbed by water, which has a better cutting effect. The combination of these two wavelength lasers offers a high cutting rate and good hemostasis. This appears to strike a balance between cutting and coagulation. At the same time, the dual diode laser has a shallow penetration depth, which reduces the damage to deep structures.
The dual diode laser has achieved good results in the treatment of benign prostate hyperplasia (BPH) [25]. Nikola Knezevic et al. reported 16 cases of LLPN using the 980-nm diode laser and one case using the 980/1470-nm dual-diode laser, and suggested that the 980/1470-nm dual-diode laser has better coagulation properties, when compared to the 980-nm laser. Furthermore, the tumor resection was more effective and faster [15]. The experience reported by the investigators has the largest series of DDL-LLPNs.
Operative time in LLPN
Compared with a previous study [24] on the treatment of localized exophytic renal tumors with the 1318-nm diode laser, the investigators spent a significantly shorter operation time (104.4 min in the present study vs. 179.4 min in their study) during the excision of relatively larger tumors (2.3 cm in the present study vs. 1.9 cm in their study). In another study [15], LLPN using a 980-nm diode laser was performed for 17 patients with solitary exophytic small renal tumors. However, the renal arteries were clamped to avoid bleeding, and the WIT was 16 min (range 9–20 min) in that study, which is longer than that in the present data. In another literature, blue laser was reported to perform zero-ischemia laparoscopic partial nephrectomy in the porcine model. Based on the analysis of the study, the blue laser might produce fast cutting, but is inadequate to control the bleeding [29]. Therefore, the investigators consider that the 980/1470-nm dual-diode laser-supported LPN is efficient and effective.
WIT in LLPN
The primary advantage of LLPN is that this can reduce the WIT. Considering the ischemic renal injury, the WIT has been identified as a significant risk factor in partial nephrectomy. Lane et al. estimated a GFR decline of 2.2 ml/min/1.73 m2 per 5 min of WIT [30], and the proposed WIT should be limited to 20 min in a successful nephron-sparing surgery [5]. However, the reduction of WIT in PN remains challenging in minimal invasive procedures due to the increased complexity, when compared to conventional operations. In the present series, relying on the laser coagulation hemostasis, no kidney was involved in warm ischemia in all successful laser cases, and no significant decrease in eGFR was observed during the 6-month follow-up period. This is a great step in terms of renal function preservation, and indicates that LLPN may potentially and peculiarly benefit patients with renal function insufficiency. However, further investigations are warranted for this group of patients in the future.
The experiences of the investigators on LLPN
The major issue of the laser technique is excessive smoke production, which weakens the visibility in the laparoscopic surgical field. Several methods were used to deal with this issue. It was found that submersing the laser tip by slow saline irrigation can eliminate smoke, reduce carbonization, and thereby achieve a better surgical field visualization. Although some experts have concerns on possible tumor seeding due to irrigation [31], no recurrence was found in the present series after a long period of follow-up. In addition, continuous suction also helps to evacuate the smoke. However, since this may cause loss of pneumoperitoneum pressure, continuous suction can only be used as an auxiliary measure to control the smoke generation.
Another disadvantage of LLPN is the impaired visibility of the resection plane caused by tissue coagulation and burn, which may increase the possibility of positive surgical margin (PSM). In order to address this issue, the investigators suggest a prior demarcation of the resection line around the tumor to achieve tumor-free margins. In the present series, 21 cases had a negative surgical margin, four cases had an indistinct surgical margin, and no patient had PSM, which was comparable to results reported by previous literatures. Several literatures have reported no occurrences of PSM in their series [9, 15, 16], while other literatures also reported a low incidence of PSM (2–9%) [18, 24]. In the opinion of the investigators, careful patient selection, the surgeon’s adequate laser surgical technique, and careful preoperative planning can contribute to the reduction in frequency of PSM. In addition, it is noteworthy that the tissue necrosis zone after laser resection creates an additional safe margin, which may make an indistinct surgical margin that is not clinically important.
The lessons obtained from the failed-LLPN cases
To date, all studies on LLPN are limited to small renal tumors [23]. Based on the experience of the investigators, small exophytic renal tumors may be better candidates for LLPN. Both failed cases in the present study, in which hilar clamping were applied due to intraoperative hemorrhage, were complicated cases with a larger tumor size (3.2 and 4 cm), with relatively higher R.E.N.A.L and Padua scores. The laser procedures were suspended due to heavy hemorrhage caused by the large feeding artery at the base of tumor. It was noted that the laser technique may not be able to achieve satisfactory hemostasis on large feeding vessels. Specifically, the investigators strongly recommend the full pre-preparation of the renal hilum before starting the laser resection, in case significant feeding vessel hemorrhage occurs.
Study limitations
In the present preliminary study, some promising results were found on the efficacy and safety of the new surgical technique. However, some limitations should be mentioned. First, the present study included a relatively large number of patients, when compared to prior reports. However, further studies with more patients are needed to validate these present results before these can be widely generalized. Second, the investigators did not include a control group in the present study, and only compared the results with those of prior reports, in order to show the pros/cons of the laser technique. Due to the variations in study populations and laser techniques among studies, the comparison results may not be strong enough. Finally, the present study was conducted by a group of experienced laparoscopic urologists in a high-volume center, and these present results may not be repeatable in terms of the varied technical experiences of the surgeons.
Conclusion
These present single-center preliminary results demonstrate that the laparoscopic partial nephrectomy with dual 980/1470 diode laser technique is a safe and oncological satisfactory technique for the treatment of SRMs, and that this especially has the advantage of reducing WIT, thereby maximally preserving renal function. Further studies with more cases and longer follow-ups are needed to establish the oncological and functional efficacy of the procedure.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ferlay J et al (2018) Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–387
Conti A et al (2015) Small renal masses in the era of personalized medicine: tumor heterogeneity, growth kinetics, and risk of metastasis. Urol Oncol 33(7):303–309
Ljungberg B et al (2010) EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58(3):398–406
MacLennan S et al (2012) Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol 62(6):1097–1117
Thompson RH et al (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58(3):340–345
Volpe A et al (2015) Renal ischemia and function after partial nephrectomy: a collaborative review of the literature. Eur Urol 68(1):61–74
Muramaki M et al (2013) Prognostic factors influencing postoperative development of chronic kidney disease in patients with small renal tumors who underwent partial nephrectomy. Curr Urol 6(3):129–135
Hou W, Ji Z (2015) Achieving zero ischemia in minimally invasive partial nephrectomy surgery. Int J Surg 18:48–54
Loertzer H, Strauß A, Ringert RH et al (2013) Laser-supported partial laparoscopic nephrectomy for renal cell carcinoma without ischaemia time. BMC Urol 13:31
Gofrit ON et al (2012) Laparoscopic partial nephrectomy using a flexible CO2 laser fiber. JSLS 16(4):588–591
Clayman RV (2005 Apr) Laparoscopic partial nephrectomy using holmium laser in a porcine model. J Urol 173(4):1200–1201
Rioja J et al (2017) Laparoscopic partial nephrectomy with potassium-titanyl-phosphate laser versus conventional laparoscopic partial nephrectomy: an animal randomized controlled trial. Urology 99:123–130
Khoder WY et al (2012) Outcome of laser-assisted laparoscopic partial nephrectomy without ischaemia for peripheral renal tumours. World J Urol 30(5):633–638
Wang Y et al (2019) Thulium laser-assisted versus conventional laparoscopic partial nephrectomy for the small renal mass. Lasers Surg Med
Knezevic N et al (2014) Laparoscopic partial nephrectomy with diode laser: a promising technique. Photomed Laser Surg 32(2):101–105
Thomas AZ et al (2013) Zero ischemia laparoscopic partial thulium laser nephrectomy. J Endourol 27(11):1366–1370
Anderson JK et al (2007) Large-volume laparoscopic partial nephrectomy using the potassium-titanyl-phosphate (KTP) laser in a survival porcine model. Eur Urol 51(3):749–754
Khoder WY et al (2011) The 1,318-nm diode laser supported partial nephrectomy in laparoscopic and open surgery: preliminary results of a prospective feasibility study. Lasers Med Sci 26(5):689–697
Hindley RG et al (2006) Laparoscopic partial nephrectomy using the potassium titanyl phosphate laser in a porcine model. Urology 67(5):1079–1083
Arkhipova V et al (2019) Ex vivo and animal study of the blue diode laser, Tm fiber laser, and their combination for laparoscopic partial nephrectomy. Lasers Surg Med. https://doi.org/10.1002/lsm.23158
Browne C et al (2017) A single centre experience of zero-ischaemia laparoscopic partial nephrectomy in Ireland. Ir J Med Sci 186(4):1023–1026
Lotan Y et al (2002) Clinical use of the holmium: YAG laser in laparoscopic partial nephrectomy. J Endourol 16(5):289–292
Kyriazis I et al (2015) Current evidence on lasers in laparoscopy: partial nephrectomy. World J Urol 33(4):589–594
Drerup M et al (2018) Non-ischemic laparoscopic partial nephrectomy using 1318-nm diode laser for small exophytic renal tumors. BMC Urol 18(1):99
Rosenthal BD, DiTrolio JV (2012) Photoselective vaporization of the prostate in office and outpatient settings. Can J Urol 19(2):6223–6226
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182(3):844–853
Ficarra V et al (2009) Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 56(5):786–793
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Arkhipova V et al (2020) Ex Vivo and animal study of the blue diode laser, Tm fiber laser, and their combination for laparoscopic partial nephrectomy. Lasers Surg Med 52(5):437–448
Lane BR et al (2008) Comparison of laparoscopic and open partial nephrectomy for tumor in a solitary kidney. J Urol 179(3):847–851 discussion 852
Bui MH et al (2007) Less smoke and minimal tissue carbonization using a thulium laser for laparoscopic partial nephrectomy without hilar clamping in a porcine model. J Endourol 21(9):1107–1111
Funding
This work was supported by institutional research funding provided by the Fundamental Research Funds for the Central Universities (grant no. 3332020004) (J.D.).
Author information
Authors and Affiliations
Contributions
JD, WFX, and GHL performed the research; YX and YQ collected clinical data; JD wrote the manuscript; WFX and ZGJ supervised the study and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in accordance with relevant guidelines and regulations and was approved by the PUMCH Ethics Committee.
Consent for publication
Informed consent was achieved from all patients for the utilization of their medical records.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, J., Xu, W., Liu, G. et al. Retroperitoneoscopic partial nephrectomy using a 980/1470-nm dual-diode laser for small exophytic renal tumors. Lasers Med Sci 37, 471–477 (2022). https://doi.org/10.1007/s10103-021-03284-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03284-3