Abstract
Salinity stress is the major abiotic stress that affects crop production and productivity as it has a multifarious negative effect on the growth and development of the plant. Salinity stress stimulates the accumulation of reactive oxygen species (ROS) which is toxic to cells at higher concentrations. At lower concentrations, these molecules help in the mitigation of salinity stress through a series of signal transduction mechanisms. The respiratory burst by NADPH oxidase leads to an increase in ROS generation. It is a key signalling node in the plant gene network and helps to integrate the signal transduction with ROS signalling. Reactive nitrogen species (RNS) are free radical and non-radical reactive molecules that are also produced under salinity stress and lead to nitrosative stress by regulating SOS, MAPK dependent, Ca2+ dependent and G-protein dependent pathways. The reactive sulphur species (RSS) is a strong oxidizing agent that preferably attacks the thiol functional group. Activation of the different signalling components like ROS, RNS, RSS, SOS, Calcium, MAPK signalling and cross-talk between different signalling pathways and phytohormones have been considered as the main mechanism for ion homeostasis and Na+ exclusion at the cellular level. These reactive species and their interaction upregulate the gene expression and phosphorylation level of different membrane transporters viz., PM H+-ATPase and Na+/ H+ antiporter which might endure salinity tolerance in plants. This review aims to describe the interplay/crosstalk amongst reactive species and phytohormones under salinity stress. Moreover, mechanistic insight of reactive species-mediated stress regulation and the response has also been discussed which will be helpful for the development of stress-tolerant cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity stress is one of the major abiotic stresses that drastically affects plant growth, development, crop production and productivity that further leads to the deterioration of the quality of the field and horticultural crops (Sanower and Sultan 2019; Lal et al. 2020; Chourasia et al. 2021). Soil salinity is identified as the main cause of land degradation thereby making the land inappropriate for the cultivation of crops (Basu et al. 2021). Generally, soil salinity is described by the presence of excess mineral ions viz., Na+, Cl−, SO4−2 above the threshold level predominantly Na+ ions in the soil (Chourasia et al. 2021). Salinity stress affects more than 1 billion hectares of land in about 100 countries around the world (Ivushkin et al. 2019). Salinity accounts for nearly 20% of the world's arable land and more than 50% of irrigated farmland (Basu et al. 2021). However, this number is expected to increase in the future which might be the consequence of unsuitable land management and artificial irrigation in the crop fields.
Salinity stress hinders the growth and development of plants by interfering with major physiological processes such as sodium ion toxicity, inducing early leaf senescence, nutritional disorders, membrane disorganization, necrosis and ultimately cell death (Kolomeichuk et al. 2020; Chourasia et al. 2021). Due to impaired root membrane selectivity, excessive Na+ and Cl− influx and K+ efflux may cause ion imbalance that leads to K+ deficiency because excess Na+ ions compete with K+ ions for the major binding site (Hussain et al. 2021). Salt stress induces the accumulation of reactive oxygen species (ROS) which includes 1O2, O−2 and H2O2, which is detrimental to cells at higher concentrations as they trigger oxidative damage to nucleic acids, lipids and proteins (Devireddy et al. 2021). However, the ROS at lower concentrations might act as the signalling molecules which help in mitigating the salt stress by their signalling pathway. The RNS synthesized in the plant in the form of nitric oxide (NO) is responsible for conferring nitrosative stress in plants (Fatima et al. 2021; Tomar et al. 2021). Reactive sulphur species (RSS) has been proposed to denote a group of sulphur (S) related molecules (GSH) that plays an important role in preventing oxidative damage caused by salt stress (Astolfi and Zuchi 2013). The RSS itself is a strong oxidizing agent that preferably attacks the thiol functionality (Giles et al. 2002). So reactive oxygen, nitrogen and sulphur species (RONSS) plays a crucial role as signalling molecules in response to salt stress in plants (Antoniou et al. 2016). To cope with salinity stress, resulting from either osmotic stress or ionic toxicity, not only the RONSS but phytohormones have been reported to affect salt tolerance by regulating several physiological, biochemical and molecular mechanisms (Tiwari et al. 2020a, 2020b, 2021a; Kim et al. 2018; Wei et al. 2019). The crosstalk of these signalling molecules (ROS, RNS and RSS) with each other and phytohormones have been reported to be crucial in the adaptation of the plant under salinity stress.
The primary mechanism developed by the plant for salinity tolerance involves ionic homeostasis and regulation of the scavenging mechanism of toxic compounds in plant tissue. Plants use complex multi-component signalling pathways to rebuild cell homeostasis and promote survival in response to salt stress (Hussain et al. 2021). Activation of the different signalling components viz., like ROS, RNS, SOS, calcium, MAPK signalling and cross-talk between different phytohormones acts as a principal mechanism for ion homeostasis control and Na+ exclusion at the cellular level. ABA is mainly induced in vascular tissues under salinity stress which is further exported from the biosynthesis site to the site where it is utilized (Martínez-Andújar et al. 2021). The uptake and distribution of ABA are stimulated to other cells by the specific ATP-dependent carriers thereby distribution and redistribution in the neighbouring tissues. The signalling mechanism of ROS molecules controls a wide array of biochemical processes, from the regulation of development and growth to responses to salinity stress (Suzuki et al. 2011). Besides, Ca2+ also plays an important role in the signalling of salt-mediated response which integrates many physiological and biochemical processes that regulate plant growth and development (reviewed by Manishankar et al. 2018). The salt overly sensitive (SOS) pathway which appears to be conserved in the almost all plant species have been studied in many plant species under salinity stress condition (Ji et al. 2013). MAPK signalling cascade is activated in stress conditions in plants including salinity stress (Xing et al. 2015). The genes involved in salinity stress tolerance can be divided into the following functional categories: (i) ion transport (SOS, AtNHX and H+-ATPase genes) (ii) ROS scavenging (SOD, APX, CAT, GR) (iii) molecular chaperones (HSPs) and (iv) dehydration related transcription factors (Acosta-Motos et al. 2017; Kearl et al. 2019; Demirelbose et al. 2020).
A plethora of studies highlights the crucial role of ROS (Bose et al. 2014; Tanveer and Ahmed 2020), RNS (Saddhe et al. 2019), RSS (Nawaz et al. 2019) and phytohormone (Fahad et al. 2015) for salinity stress tolerance in the plants. However, to our knowledge, there are very limited reviews that have elucidated the cross-talk of all three reactive species with phytohormone. In this review, the interaction amongst reactive species (RONSS) along with their crosstalk with phytohormones has been discussed under salinity stress. Per contra, the mechanism of these three reactive species (RONSS) and their signalling component which acts upstream or downstream in salinity stress regulation and response have also been elucidated.
Phytohormones Interaction in Plants Under Salinity Stress
Phytohormones are important molecules for plants by which they regulate different stress responses (Zhang and Shi 2013). Previous reports revealed that the synthesis of endogenous plant hormones under osmotic/salinity stress conditions was correlated with tolerance (Fahad et al. 2015; Ryu and Cho 2015). During the plant life cycle from germination to maturity, auxin (IAA) modulates various growth and development processes of plants. These mechanisms rely heavily on the auxin concentration, gradient and spatiotemporal expression of receptors and signalling genes (Wiggins 2015). Under salt stress, there is a quick reduction in endogenous auxin availability due to strong regulation of auxin synthesis and these changes in endogenous levels provide convincing evidence about its role in response to these environmental changes (Sharma et al. 2015). Jung and Park 2011 found that under high salt levels in Arabidopsis auxin signals are incorporated into the NTM2 (membrane-bound transcription factor) which modulate seed germination via IAA30 gene. Reports on expression micro RNA (miRNA) suggest that the salinity-mediated expression of miR393 negatively regulates TIR1 and AFB2 mRNA which is shown to have stabilization of Aux/IAA repressor, thereby leading to inhibition of auxin signalling (Iglesias et al. 2014). On the other hand, root architecture was also reported to be affected by auxin-mediated salinity stress (Pandey et al. 2019). Similarly, cytokinin was also reported to affect plant growth and developmental processes under abiotic stress (Reviewed by Ryu and Cho 2015; Pavlů et al. 2018). The positive and beneficial effect of exogenous application of kinetin under salinity stress might play a crucial role as a free radical scavenger and antioxidative mechanism involved in the protection of purine breakdown. Cytokinin might alleviate salinity stress by various mechanisms such as preservation of the photosynthetic mechanism, enhancement of antioxidant enzyme activity, improvement in water balance regulation, decreasing ABA concentration in wheat (Iqbal et al. 2006; Lal et al. 2021a), increasing proline content in brinjal (Wu et al. 2014) and modulation of plant growth and differentiation. Maggio et al. 2010 also reported in tomato plants that under salinity stress, exogenous application of gibberellic acid (GA) showed to have decreased stomatal resistance via enhancement of Cl− ion in leaves. Ahmad et al. 2021 suggested that seed priming with exogenous GA application in pea under salinity stress might alleviate the expression of Na+/H+ antiporter (NHX1 and SOS1) proteins that enhance Na+ sequestration. Magome et al. (2008) reported that under salinity stress the endogenous GA level was reduced due to lowering of GA2-oxidase genes in Arabidopsis thaliana. However, the upregulation of the GA2-oxidase gene, expression of DDF1 (Dwarf and Delayed Flowering 1) gene is responsible for salinity stress. Another classical hormone, ethylene has also emerged as one of the crucial mediator for salinity tolerance in plants such as Arabidopsis thaliana (Yang et al. 2013), rice (Yang et al. 2015), cucumber (Shakar et al. 2016) and tomato. In Arabidopsis, EIN3 and EIL1 regulate ethylene responses and confer salt tolerance but in rice Yang et al. 2015 observed that MHZ6/OsEIL1, a master transcription factor in the ethylene signalling pathway and analogous to EIN 3 as well as OsEIL2 (analogous to EIL1) negatively regulate salt tolerance. Yang et al. 2013 suggested that ethylene mediates salinity tolerance in Arabidopsis thaliana by retaining K+ in shoots and roots rather than lowering tissue Na+ content in plant tissues. Zhao et al. 2017 in their study on soybean found that exogenous ABA applications increased the expression of all RAV genes significantly under salinity stress. Out of all RAV genes in soybean, they characterized the GmRAV-03 gene which might be involved in salinity tolerance. It was reported that RAV transcription factors play a role major in the regulation of the plant response to different abiotic stresses (Wang et al. 2020). The exogenous application of ABA in rice might upregulate the expression of OzCan1-1 (salt-stress-responsive calmodulin) gene which plays a crucial role in signalling and enhance proline accumulation (Sripinyowanich et al. 2013).
Salicylic acid also involved in the regulation of responses under different biotic and abiotic stresses. Recent studies revealed that SA plays a role in response to salinity stress also in many crops such as rice (Kim et al. 2018), strawberry (Samadi et al. 2019), cucumber (Miao et al. 2020), Limonium bicolor (Liu et al. 2019), safflower (Shaki et al. 2018) and barley (Pirasteh-Anosheh et al. 2017). Application of salicylic acid in all these crops under artificial salinity conditions different types of morphological and biochemical changes were observed which is mentioned in Table 1. Brassinosteroids are a group of naturally occurring plant steroidal hormones (Fahad et al. 2015) that are involved in different physiological, biochemical and molecular responses in plants. It is well known that they can also improve the detrimental effects of salinity on plant growth performance by external application in different crops like rice, Arabidopsis and cucumber. Application of brassinosteroid under salinity stress reported increased stomatal conductance, chlorophyll concentration, intercellular CO2 concentration, net photosynthetic rate, antioxidant activity and osmolyte accumulation in plants (Fahad et al. 2015). Melatonin a low molecular weight compound having multiple functions protects the plants from various environmental conditions (Tiwari et al. 2021b; Lal et al., 2021b; Altaf et al., 2021). Elsayed et al. 2020 and Kamiab 2020 reported that exogenous melatonin application mitigates excess ROS production in peanut and pistachio seedlings under salinity stress.
Reactive Oxygen Species (ROS) Signalling Under Salinity Stress
Role of Reactive Oxygen Species (ROS)
The synthesis of ROS is an inevitable mechanism of aerobic life that is essential for the regulation of all biological processes in nature (Castro et al. 2021). Salt stress enhances the production and signalling of ROS in the cell which ultimately affects the phenotype of the plant. Under salinity stress, the stomatal conductance of the plant is affected, that reduces the supply of CO2 in the plant, thereby increases the photorespiration rate in C3 plants. The increased photorespiration rate is also responsible for the production of ROS (mainly H2O2). The hyperaccumulation of Na+ and Cl− ions occurs in the cytosol that affects the electron transport rate in the photosystem (PS) thereby inhibiting the electron acceptors in the vicinity of PS I and PS II (Baxter et al. 2014; Bose et al. 2014). Molecular oxygen receives these electrons and responsible for the reduction of O2 or the formation of superoxide and other different reactive oxygen species (ROS) in plants (Shabala 2019; Chourasia et al. 2021). ROS are versatile reactive molecules that include both oxygen radicals (unpaired electrons) and other non-radicals mentioned in Table S1.
The major site for ROS synthesis is the subcellular compartment viz., chloroplasts (Suo et al. 2017), mitochondria (Che-Othman et al. 2017) and peroxisomes (Del Río and López-Huertas 2016) under abiotic stress conditions. In chloroplast, salinity stress can enhance ROS synthesis in the PSI and PSII are the crucial sites for production and signalling of ROS under illuminated conditions. Complex I and III of mitochondrial electron transport chain was reported as the major source for salinity stress-induced ROS production. The excess reduction of the ubiquinone pool under salinity stress might allow electrons to leak into molecular oxygen from complex I and III that results in the synthesis of superoxide (O2.−) radical (Miller et al. 2010). The oxidative stress caused by higher salinity conditions might lead to the generation of the higher amount of H2O2 during photorespiration glycolate oxidase reaction, fatty acid β-oxidation and disproportionation of O2.− in the plant cell (Betti et al. 2016; Acosta-Motos et al. 2017). The cell wall-associated peroxidase that is located in the apoplastic region of the cell also generate O2– by oxidizing NADPH and transferring the electron to molecular oxygen (Qi et al. 2017).
The ROS molecules are produced in different metabolic pathways as a normal by-product (del Río 2015). The synthesis of ROS in the specific compartments are produced in a controlled manner, however, the synthesis of these molecules reported to be increased manifold under salinity stress condition. Thereby, uncontrolled salinity-mediated oxidative stress might lead to cellular damage and eventually cell death. Therefore, ROS production and ROS scavenging occur simultaneously in the cell thereby balancing production and the scavenging rate that maintains ROS level (Mittler et al. 2011). The response of ROS to salinity stress is expected to be different in the tissue and cell compartment. So to prevent damage under different salinity stress conditions, plants employ a mechanism that can maintain net photosynthesis with limited CO2 supply, use alternative electron sinks or the antioxidant defences must keep active oxygen under control (Bose et al. 2014).
Mechanism of ROS-Mediated Response Under Salinity Stress
Salinity stress leads to oxidative burst in the cell which has a detrimental effect on the lipid membrane system (Naeem et al. 2020). The respiratory burst by NADPH oxidase (respiratory burst oxidase homologues, RBOHs) leads to an increase in ROS generation. It is a key signalling node in the plant gene network and helps to integrate the signal transduction with ROS signalling (Suzuki et al. 2011). Under salinity stress conditions, Ben Rejeb et al. 2015 reported higher activity of cell membrane-bound RBOSH in Arabidopsis. In contrast, Rodríguez et al. 2009 reported lower activity of cell membrane-bound RBOHS in maize plants. The intricate network of signalling in the plant against salinity is highly complicated that include metabolic and genetic changes. The plants also have evolved adaptive and survival strategies to counterbalance the changing environmental conditions such as salinity in which ROS play valuable roles as signal molecules. It includes systemic and retrograde signalling between chloroplast and nucleus in tobacco (Seo and Park 2021), programmed cell death in rice (Kim et al. 2014) and tobacco (Monetti et al. 2014) and maintenance of ionic balance and Na+/K+ content in rice (Shen et al. 2015).
The detrimental effects of ROS at higher concentrations in cells might lead to lipid peroxidation in cellular membranes (Su et al. 2019), DNA damage (Qiu et al. 2019), protein denaturation (Khan and Shahwar 2020), carbohydrate oxidation (Davaritouchaee et al. 2019), pigment breakdown (Nemat Alla and Hassan 2020) and impairment of enzymatic activity (Bharti and Barnawal 2019). The production of different types of ROS under salinity stress in maize (Mansour et al. 2005) revealed that ROS attack polyunsaturated fatty acids of membrane system (both cellular and organelle) lipids and induced peroxidation which formed small hydrocarbon molecules like malondialdehyde (MDA) and ketones (Gill and Tuteja 2010). Essential fatty acids such as linolenic and linoleic acid are more prone to the damage caused by hydroxyl and singlet oxygen radicals. Higher lipid peroxidation along with the increased MDA level was reported to be observed in rice (Khan and Panda 2008; Khare et al. 2015), Cicer arietinum (Kukreja et al. 2005), Glycyrrhiza (Pan et al. 2006) and maize (Carrasco-Ríos and Pinto 2014; AbdElgawad et al. 2016). The ROS molecule attacks protein molecules by the mechanism of covalent modification known as protein oxidation. Methionine and cysteine are more easily destroyed by singlet oxygen and hydroxyl radicals under abiotic stress conditions (Gill and Tuteja 2010). Under salinity stress conditions in canola susceptible genotypes (Sarigol), Bandehagh et al. 2011 observed a reduction in the abundance of Cu/Zn SOD, due to the production of high levels of hydroxyl radicals (OH·) in this genotype. The ROS molecules can remove the hydrogen atom in cysteine which results in the thiyl radical attached to other thiyl radicals forming disulphide bridges (Gill and Tuteja 2010).
Reports by Saha et al. 2015 in mungbean suggested that salinity stress can damage DNA due to the accumulation of ROS. Similarly, Zvanarou et al. 2020 also suggested the ROS-induced DSBs (double-strand breaks) and SSBs (single-strand breaks) in DNA under NaCl treated Physcomitrella patens protonema. The mechanism behind the degradation of DNA might be due to the attack of ROS on purines, pyrimidines and the DNA backbone of DNA structure (Halliwell and Gutteridge 2015). Salt induced DNA damage has also been discovered in other plants, such as Arabidopsis (Roy et al. 2013) and mungbean (Ghosh et al. 2015). Therefore, the cell must need tight control of ROS content and its homeostasis in the cell to prevent the cell and nucleic acid from ROS-mediated oxidative damage. Different types of enzymatic and non-enzymatic antioxidants are responsible for the detoxification of ROS (Table S2 and S3).
Crosstalk of ROS with Phytohormone
Understanding ROS-hormonal crosstalk is essential to elucidate how plants control salinity-mediated redox metabolism under salinity stress (Mishra et al. 2017). ROS may interact with different signalling molecules or biosynthetic pathways of phytohormones (Fig. 1). ROS molecules are the effective regulators for hormonal interplay and associated developmental changes in the plant under salinity stress (Devireddy et al. 2021). Fu et al. 2019 in Arabidopsis characterized the crosstalk of ROS and IAA which enhanced the formation of primary root growth via IAA-CONJUGATE-RESISTANT 4 (IAR4). They reported this cross-talk might regulate primary root growth under salinity stress through ROS-mediated auxin distribution. The distribution of auxin in different tissue-level might be beneficial to provide tolerance to plant stress conditions. Iglesias et al. 2010 reported in Arabidopsis that auxin signalling interacts with redox metabolism in case of adaptative response against oxidative stress and salinity. Similarly, Wang et al. 2015 in their study with Arabidopsis thaliana suggested that endogenous cytokinin overproduction resulting from inducible overexpression of AtIPT8 negatively affects plant salt tolerance by regulating stress-sensitive gene expression, chlorophyll homeostasis and ROS production. Moreover, salinity stress tolerance is also reported to be conferred by GA content and signalling. GA biosynthetic pathway was suppressed (by down-regulation of the GA biosynthetic genes) by elevated ROS level which served as a stress signal that activated the multiple stimulus responses in cotton after 3 h of treatment with NaCl (Shi et al. 2015). Saline soil generates excess Na+ ions and these ions stimulate ethylene-induced soil-salinity tolerance by its signalling mechanism. Ethylene might promote salinity tolerance by enhancing Na+/K+ homeostasis. In Arabidopsis, the ratio of Na+:K+ ratio is regulated by RBOHF-dependent as well as independent regulation of Na and K accumulation (Jiang et al. 2013). Peng et al. 2014 also found that ethylene pre-treatment might prevent the excess ROS accumulation in the cell which showed increased tolerance to salt stress in Arabidopsis. Whole-genome transcriptome analysis revealed Salt-Induced and EIN3/EIL1-Dependent (SIED) genes may contribute to salinity stress responses and amongst these genes, several genes encoding ROS scavengers were also involved. In Arabidopsis, the synthesis of ethylene was reported to improve salinity tolerance by modulating ROS redox, sucrose and antioxidant system (Yang et al. 2013; Tiwari et al., 2021c). Lin et al. 2012 suggested that in response to salinity stress ethylene insensitive and ethylene sensitive mutant of Arabidopsis showed promoted germination of seed which might be due to regulation of endogenous H2O2 concentration in germinated seeds. AtrbohD and AtrbohF are two genes expressing NADPH oxidase enzyme which involved in the impairment of ABA signalling under salinity stress (Kwak et al. 2003). AtrbohD and AtrbohF gene-mediated synthesis of ROS regulate Na+/K+ homeostasis in the cell which might help ABA-mediated stomatal regulation, increased ABA-induced cytosolic Ca2+ and ABA-mediated activation of plasma membrane Ca2+-permeable channels in guard cells (Ma et al. 2012). Luo et al. 2021 suggested that an elevated level of RbohD gene which is responsible for more ROS accumulation is dependent on ABI4 (Abscisic Acid-Insensitive 4). ABI4-RbohD/VTC2 (Vitamin C Defective 2) regulatory module integrates both cell membrane integrity and ROS metabolism, ultimately repressing seed germination under salinity stress. Rady 2011 in their study with bean observed that 24-Epibrassinolide showed enhanced level of the antioxidant systems (SOD, CAT, POX, GR and proline) under salinity stress.
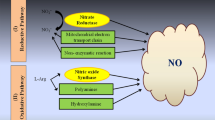
A schematic model illustrating ROS, RSS, RNS–phytohormone crosstalk in relation to other hormone signals under salinity stress conditions. Blackline indicates activation, whereas the red line indicates the repression mechanism. ABA abscisic acid, GA gibberellins, AUX auxins, MAPK mitogen-activated protein kinase, ROS reactive oxygen species, SA salicylic acid, ET ethylene, CT cytokinin, IPT isopentyl transferase, JA Jasmonic acid, NHX1 Na+/H+ exchanger 1, RSS reactive sulphur species (Color figure online)
Reports also suggested that novel phytohormones such as strigolactones (SL) are also involved in the regulation of stress adaptation (Ha et al. 2014). In Arabidopsis under salinity stress, it was reported that SsMAX2 (a key component in SL signalling) plays a pivotal role in the regulation of salinity-mediated oxidative stress via the regulation of antioxidative enzymes. Liu et al. 2020 in their study with rice root tip under salt stress observed increased expression of DEGs which encodes respiratory burst NADPH oxidases (OsRBOHA and OsRBOHF), calcium-dependent protein kinase (OsCDPK) and calcineurin B-like/calcineurin B-like-interacting protein kinase (OsCBL/OsCIPK). The role of different phytohormones and their effects on plants under salinity stress condition has been mentioned in Table 1. Further, researches on these versatile signalling switches are required which will provide the future path for the plant to adapt under dynamic environment.
Reactive Nitrogen Species (RNS)-Mediated Response to Salinity Stress
Role of Reactive Nitrogen Species (RNS)
Similar to ROS, reactive nitrogen species (RNS) are another set of free radical and non-radical reactive molecules that are synthesized under salinity stress, thereby leading to nitrosative stress (analogous to oxidative stress) (Tan et al. 2007; Kapoor et al. 2019). The RNS is synthesized from nitric oxide (·NO) which plays a vital role in plant growth, development, physiological processes and key signalling mechanism in different processes that are involved in various stress tolerance mechanisms (Molassiotis et al. 2010; Mur et al. 2013). The RNS synthesis is initiated in the plant with a quick reaction of nitric oxide (·NO) with superoxide (O2●−) to form peroxynitrite (ONOO–) which is a powerful oxidative agent. Peroxynitrite molecules further react with new molecules to form other different types of RNS (Ferrer-Sueta et al. 2018). The RNS molecules that are produced in the plant in response to abiotic stress are.NO, nitrogen dioxide (.NO2), S-nitrosoglutathione (GSNO), nitrate (NO3+), non-radicals S-nitrosothiols, peroxynitrite (ONOO−), dinitrogen tetroxide (N2O4), nitrosonium cation (NO+), dinitrogen trioxide (N2O3), nitroxyl anion (NO −), nitryl chloride (NO2Cl) and nitrous acid (HNO2) (Laxa et al. 2019; Kapoor et al. 2019).
RNS and its derivatives are generated in the apoplastic regions (Farvardin et al. 2020), cytoplasm, plasma membrane and different subcellular organelles such as the chloroplast, mitochondria (Gupta et al. 2018) and peroxisomes (Corpas et al. 2020) during salinity stress. Despite intensive work revealing its diverse functions, reactivity, signalling molecule and nitrosative stress, NO production pathway in plants is still poorly understood and remains one of the most puzzling topics in the field (Astier et al. 2018). NO can originate from different paths in the cell, however, two modes of RNS production viz., oxidative pathway (using nitric oxide synthases (NOSs) enzyme) and the reductive pathway (reduction of nitrites to NO using nitrate reductase (NR) enzyme) were mainly proposed. In Arabidopsis, Zhao et al. 2007 used NOS inhibitor (L-NNA) and observed a very low endogenous level of NO under salinity stress, which favoured the oxidative pathway of NO production. A reductive pathway under salinity environments was observed by Kataria et al. 2020 in soybean by using nitrate reductase inhibitor (sodium tungstate), resulting in low NO endogenous level.
The RNS synthesized in the plant in the form of NO is responsible for conferring nitrosative stress in plants. RNS signalling can mitigate the adverse effects of high salinity by regulating SOS pathway in rapeseed (Zhao et al. 2018), MAPK dependent pathway in maize (Bai et al. 2011), Ca+2 dependent pathway in the roots of Hylotelephium erythrostictum (Chen et al. 2019) and G-protein dependent pathway in Arabidopsis (Li et al. 2009). Recent reports on the role of RNS revealed positive effects in response to salinity stress in different plant species such as rice (Cai et al. 2015), maize (Kaya and Ashraf 2020), wheat (Sehar et al. 2019), tomato (Manai et al. 2014), citrus (Tanou et al. 2012), cucumber (Campos et al. 2019) and halophytes (Chen et al. 2013). Several studies demonstrate the beneficial role of the application of exogenous NO under salinity stress in the plant has been highlighted in Table 2.
Mechanism of RNS on Plant Cell
Salinity stress conditions in the soil might trigger NO metabolism in plants. NO metabolism leads to an increase in NO synthesis in rapeseed which showed an increase in the amount of S-nitrosylation (Zhao et al. 2018) and nitration in sunflower proteins (David et al. 2015) under salt stress. Several studies report that NO appears to modulate and reduce electrolyte leakage in strawberry (Kaya et al. 2019), lower synthesis of MDA and H2O2 content in wheat (Ali et al. 2017), induced H2S biosynthesis for mitigation of oxidative stress in tomato (da-Silva et al. 2018) and increased the protein expression of plasma membrane H+-ATPase and vacuolar Na+/H+ antiporter in Kandelia obovata (Chen et al. 2013) under salinity stress. Nitric oxide and NO-derived molecules can alter target proteins by the phenomenon of post-translational modifications (PTMs) which plays a pivotal role in the modification of RNS signalling pathways (Chaki et al. 2020).
RNS modify protein residues and modulate a broad range of molecules like tyrosine nitration, sulphydration, metal nitrosylation, S-glutathionylation and S-nitrosylation in response to a stressful environment (Hasanuzzaman et al. 2018b). The binding of different RNS like NO to cysteine and peroxynitrite (ONOO−) to a tyrosine of target proteins caused S-nitrosylation (Zhang and Liao 2019) and tyrosine nitration (NO2-Tyr), respectively, and this phenomenon can alter the function of different proteins. Similarly, enhanced level of total S-nitrosylation following salt stress has also been reported in citrus (Tanou et al. 2012), Arabidopsis (Fares et al. 2011), pea (Camejo et al. 2013; Begara-Morales et al. 2015), rapeseed (Zhao et al. 2018) and sunflower seedlings (Jain et al. 2018). This tyrosine nitration may be a good biomarker or footprint for nitrosative stress in many crop plants (Arora and Bhatla 2017). Most studies focus on the tyrosine nitration in plants. However, in the protein constitute of amino acid such as cysteine, tyrosine, methionine and tryptophan are preferentially nitrated. Tyrosine nitration as a nitrosative stress has been reported in Arabidopsis (Corpas et al. 2009), sunflower (David et al. 2015) and citrus (Tanou et al. 2012). It is very important to understand here that this PTM’s is not always harmful to plants because in some cases this nitrosylation of cysteine residues inhibit the carbonylation phenomenon in plants so ultimately prevent oxidative damage to proteins (Tanou et al. 2012). Therefore, it is cleared that the above PTMs are an integral part of the salt stress signalling intermediates that are controlled by different RNS molecules. Exogenous NO is also beneficial for plants subjected to salinity, where it increases salinity tolerance via different mechanism mentioned in Table 3. Therefore, NO-mediated mechanisms have been identified in plants under salinity stress, most of which can improve plant growth and reproduction (Sharma et al. 2020).
RNS Crosstalk with Phytohormone
Previous reports suggested that NO interplays with signalling pathways of different phytohormones (Fig. 1) to control metabolism, growth and development in plants under different physiological conditions (Sanz et al. 2015; Nawaz et al. 2017; Asgher et al. 2017; Zhou et al. 2021). Several synergistic and antagonistic interactions have also been observed during NO and phytohormone crosstalk.
The elevated NO accumulation in Arabidopsis under to salt stress down-regulates the expression of PIN (PINFORMED) genes, leading to the reduction of auxin levels and stabilizes IAA17 (INDOLE-3-ACETIC ACID17)/AXR3 (AUXIN RESISTANT3), thereby repressing auxin signalling (Liu et al. 2015). Under salinity stress condition in cotton, Kong et al. 2016 observed that NO plays an important role in delaying leaf senescence thereby maintaining plant growth. The NO-mediated delay of senescence might be due to increased expression of NHX1, SOS1 genes, cytokinin biosynthesis genes, K+ content and decreased the ABA biosynthesis genes (NCED2, NCED9) and Na+ content in leaves. Poór and Tari 2011 also found an antagonistic relationship between NO and ethylene in tomato cell suspension cultures treated with NaCl (100 and 250 mM). However, ethylene and NO act synergistically in stimulating plasma membrane H+-ATPase activity to regulate ionic homeostasis under salinity stress in Arabidopsis. Therefore ethylene might be a part of the downstream signal mechanism in NO-mediated regulation of salinity stress (Wang et al. 2009). Santos et al. 2020 studied ABA-NO-Auxin integrated signalling network in tomato roots in response to salinity stress condition and noted that NO promotes auxin distribution as well as modulates plasma membrane H+-ATPase activity and NO is required for ABA-mediated root antioxidant enzyme induction. Reports on cucumber seedlings under salinity stress revealed that NO might enhance salinity tolerance by regulation of free polyamines (putrescine, spermidine and spermine) proportion (Fan et al. 2013). Arora and Bhatla (2017) studied the interaction of melatonin and NO under salinity stress in sunflower seedlings and observed differential modulation of SOD isoforms (Cu/Zn SOD and Mn-SOD). Stress tolerance response might occur due to transportation of metabolic signals that leads to melatonin-mediated mitigation of nitrosative and oxidative stress (Sun et al. 2021). Ahanger et al. 2020 in their study with salt-stressed Vigna angularis noticed that combined application of salicylic acid and NO proved much more beneficial in mitigating salt stress as compared to their individual application. This was correlated with the upregulation of the antioxidant system to counter the deleterious effect of salinity stress. Similar types of results were obtained by Ahmad et al. 2018 in Solanum lycoperscum for mitigating NaCl stress by supplementation of jasmonic acid and nitric oxide. The reports on the cross-talk of phytohormone and RNS under salinity stress are elusive which need more in-depth research on these aspects.
Reactive Sulphur Species (RSS)-Mediated Response to Salinity Stress
Role of Reactive Sulphur Species (RSS)
Reactive sulphur species (RSS) is a molecule that contains sulphur compounds (GSH and thiol group) that have strongly nucleophilic properties and are suitable for biological redox reactions which play a vital role in preventing oxidative damage caused by salinity stress (Nazar et al. 2011). The enzymes of the sulphur assimilation pathway are induced by the application of sulphur under salinity stress that helps to neutralize or eliminate ROS in mustard and other plant species (Fatma et al. 2013; Nazar 2014; Nazara et al. 2015). In these molecules, sulphur is present in non-oxidizing states but some naturally occurring molecules like sulphinic and sulphonic acids where sulphur is present in higher oxidation states (Giles et al. 2001) that gives an idea of the involvement of reactive sulphur species (RSS)-mediated signalling. The term RSS has been proposed for the first time in animal systems where sulphur related molecules are formed under in vivo oxidative stress. These molecules include thiyl radicals (RS·), disulphide (RSSR) disulphide-S-monoxides [RS(O)SR] disulphide-S-dioxides [RS(O)2SR] and sulphenic acids (RSOH) (Giles et al. 2001; Jacob et al. 2004).
Till now, amongst all the reactive species ROS and RNS have gained significant attention and works of literature are available on this aspect but the involvement of RSS is also an important area to understand salinity stress signalling conditions in the plant. Moreover, these molecules also mitigate the abiotic stress condition through the regulation of sulphur metabolism as well as the synthesis of various sulphur metabolites (Ijaz et al. 2019). Sulphur attenuated the toxic effects of salinity by better uptake of P and K and a lower Na+/K+ ratio in lettuce plants (Freitas et al. 2019). S-mediated alleviation of salinity stress was reported to be achieved in mustard through higher GSH synthesis and lower concentration of Na+ and Cl− ions (Fatma et al. 2014). Moreover, Salicylic acid application also alleviates the salt-induced decrease in photosynthesis mainly through inducing the activity of ATP-sulphurylase (ATPS) and nitrate reductase (NR) enzyme in mungbean (Nazar et al. 2011).
Mechanism of RSS on Plant Cell
The RSS itself has strong oxidizing agents that preferably attack the thiol functionality (Giles et al. 2002). They regulate and modulate the redox status of biological thiols and disulphides, thereby positively act towards conferring stress tolerance (Corpas and Barroso 2015; Nawaz et al. 2019). Recent reports on the pepper plant suggested that GSH-induced salinity stress tolerance can be achieved by increasing H2S due to the activation of the L-DES enzyme (primary producer of H2S) (Kaya et al. 2020) and therefore H2S may improve the activities of antioxidant enzymes under saline stress. Sulphur and its reactive compounds are actively involved in different biochemical reactions due to its wide range of oxidation states. Like nitric oxide and superoxide, thiols are key molecules with formal oxidation states of S-2 that are an important resource for RSS production. The synthesis of novel compounds and molecules in plant peroxisomes such as GSH, GR, GSNO and sulphite oxidase (SO) shows the participation of these species in the metabolism of sulphur (Ijaz et al. 2019). Different types of RSS and their major function are mentioned in Table 3.
There is also some research evidence that validates the signalling mechanism of reactive sulphur species such as GSH, H2S, methionine (Met), cysteine (Cys), phytochelatin (PC), ATP-sulphurylase (ATPS) and protein thiols. Capaldi et al. 2015 reviewed the crucial mechanisms involved in S containing compound-mediated oxidative stress in plants. In presence of S, the GSH content in barley and mustard (Astolfi et al. 2010; Nazar et al. 2014), the ratio of GSH/GSSG in Dactylis glomerata (Zagorchev et al. 2012), AsA content in capsicum (Kaya et al. 2020), the ratio of AsA/DHA in mungbean (Nahar et al. 2015), APX in mungbean and lettuce (Nahar et al. 2015; Freitas et al. 2019), GR in mustard (Fatma et al. 2014) and CAT in tomato (Zhou et al. 2017a, b) were reported to be improved in different crops under salinity stress condition. Exogenous application of S in the plant might mitigate the deleterious effect of salinity by lowering Na+/K+ ratio, enhanced P and K uptake and enhancing activities of antioxidant enzymes (CAT and APX) (Freitas et al. 2019). Similarly, exogenous application of GSH also showed to have increase tolerance against salinity-mediated oxidative stress by increased antioxidant enzyme system, regulation of GSH biosynthesis and maintenance of cellular redox homeostasis (Zhou et al. 2017a, b).
In salt-treated plants of barley Astolfi and Zuchi 2013 observed that sufficient application of sulphur allows adequate GSH synthesis (high-thiol concentration) thereby preventing the adverse effects of ROS on photosynthetic machinery. Moreover, external application of GSH and S to the mustard plant was reported to have increased endogenous GSH production with improved photosynthetic rate and growth parameters (Fatma et al. 2014). In the aforementioned study, Fatma et al. 2016 suggested that the combination of the application of NO (sodium nitroprusside) and sulphur showed increased activity of ATP-sulphurylase (ATPS), CAT, APX and GR. Therefore, S and NO-mediated increase in activities of antioxidant enzymes might improve the photosynthetic performance of the plant. S-methyl methionine was also reported to be involved in the salinity tolerance of Arabidopsis thaliana plants during germination and early growth stages (Ogawa and Mitsuya 2012). Zagorchev et al. 2012 also showed that total glutathione (GSH + GSSG) concentrations and upregulation of Cys synthesis are crucial for scavenging ROS under salinity stress. Overall, sulphur or sulphur-containing compounds are integral molecules in the alleviation of salinity stress in plants (Nazar et al. 2011; Khan et al. 2013). Moreover, glutathione molecules might act as a centre of ‘hub’ molecule in the cellular metabolism and redox signalling mechanism under salinity stress.
Crosstalk of RSS with Phytohormone
Sulphur can interact with different phytohormones (Fatma et al. 2013; Hasanuzzaman et al. 2018a) such as AUX, GA, CK, ABA, ET, JA and SA under salinity stress conditions and thereby positively regulates plant defence by accumulating GSH (Fig. 1). Therefore, the need for phytohormones is essential to cope with salinity stress through antioxidant enzymes and for S-regulation. Koprivova et al. 2008 studied a complex signalling network in Arabidopsis roots under salinity stress and found that GA signalling is essential for the increase in APR (adenosine 5′-phosphosulphate reductase: a key enzyme of sulphate assimilation) mRNA level under salt treatment. The improvement of photosynthesis might be controlled by S-induced alleviation under salinity stress conditions (Nazar et al. 2014). Recent reports suggested that the mustard plant when treated with 100 mM NaCl stress along with the ethephon showed an increased level of the antioxidant system, ABA accumulation in guard cells, enhanced photosynthetic efficiency and maintenance of membrane stability (Fatma et al. 2021). Cao et al. 2014 found that synthesis of the phytohormone ABA in developing seedlings and germinating seeds of Arabidopsis, was dependent on sufficient Cys availability and as a consequence affected by external sulphate supply. The increase in leaf sulphur (S) content through the higher activity of serine acetyltransferase (SAT) and ATP-sulphurylase (ATPS) by external salicylic acid application was associated with the higher accumulation of glutathione (GSH) and lower levels of oxidative stress (Nazara et al. 2015). The application of salicylic acid showed an increased photosynthetic rate which might be due to the increased activity of nitrate reductase and antioxidant mechanism (Nazar et al. 2011).
Tripartite Interaction Mechanism of RONSS Under Salinity Stress
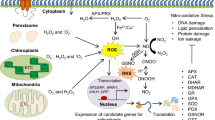
One reactive species affects the production, perception and further signalling of another reactive species. RNS and ROS produce peroxynitrite (ONOO −) which generates new signals when they react with H2S (Fig. 2). The ROS synthesis regulates the level of RNS (mainly NO) in the cell which regulates the signalling mechanism. The salinity-mediated synthesis of H2O2 by NADPH oxidase enhanced the production of NO which prevent the further accumulation of excess ROS in the cell. The ROS synthesis under salinity stress also enhances the production of RSS (H2S or GSH). NO react with GSH to form GSNO which is further reduced by GSNO reductase into oxidized glutathione (GSSG). Higher NO accumulation in the cell leads to inhibition of GSNOR and thereby preventing GSNO degradation in the cell.
Conceptual illustration of interaction/interplay between ROS, RNS and RSS in the plant cell under salinity stress condition. ROS (O2.−, H2O2) is synthesized mainly in the four pools which include apoplastic, chloroplastic, mitochondrial and peroxisomal pools under salinity conditions. The ROS synthesis also regulates the level of RNS (mainly NO) in the cell which regulates the signalling mechanism. The salinity-mediated synthesis of H2O2 by NADPH oxidase enhanced the production of NO which prevent the further accumulation of excess ROS in the cell. The ROS synthesis under salinity stress also enhances the production of RSS (H2S or GSH). NO react with GSH to form GSNO which is further reduced by GSNO reductase into oxidized glutathione (GSSG). Higher NO accumulation in the cell leads to inhibition of GSNOR and thereby preventing GSNO degradation in the cell. However, the GSNO interaction with ROS is not deciphered (dotted line). RSS interacts with H2O2 in the plant cell and counterbalances the level of RSS and ROS in the cell. CAT, catalase; GSNOR, nitrosoglutathione reductase; GSSG glutathione, oxidized form, H2O2 hydrogen peroxide, NO nitric oxide, O2.− oxygen radical, ONOO − peroxynitrite, RNS reactive nitrogen species, ROS reactive oxygen species, RSS reactive sulphur species, SO sulphite oxidase, SO42− sulphate, SOD superoxide dismutase (Color figure online)
Gohari et al. 2020 demonstrated a positive interplay between H2O2 and NO to counter salinity stress in basil (Ocimum basilicum L.) plant and reported enhanced guaiacol peroxidase (GP) and APX activity after combined treatment of H2O2 and SNP (NO donor). Hajihashemi et al. (2020) studied crosstalk between H2O2, NO and Ca2+ under the saline condition in quinoa and recorded enhanced amylase activity and a strong positive correlation between germination rate with germination index and relative seed germination which ultimately mitigated the adverse effect of salinity. Dinler et al. (2014) studied the interplay between nitric oxide (NO) and Glutathione-s-transferases (GSTs) in soybean plants in response to salinity stress and suggested that NO regulates the expression of GST genes and isoenzyme activities (GST1 and GST4) were significantly induced after SNP pretreatment. Tanou et al. (2012) in their study with citrus plants under salinity conditions demonstrated that pre-exposure to NO and H2O2 alleviated the negative effect of salinity and their interaction with each other either systematically or locally in citrus plants. In the same study, they noticed that on one side H2O2 pretreatment stimulated endogenous NO production in roots and leaves, and on another side SNP treatment also caused the buildup of H2O2 in roots and leaves. Chen et al. (2015) studied the interplay between H2S and NO under salinity-induced barley seedlings and observed enhanced endogenous NO production after external application of H2S. Both NO and H2S upregulated the expression of HvHA1, HvSOS1, HvVNHX2 and HvVHA-β genes which resulted in increased protein expression of PM H+-ATPase and vacuolar Na+/H+ antiporter in salt-induced barley seedlings which indicate that H2S enhanced salt tolerance in barley seedlings via NO regulated ion homeostasis. A similar type of result was obtained by Wang et al. (2011) where hydrogen sulphide enhanced salinity tolerance in alfalfa at the time of seed germination by diminishing oxidative damage which might be due to interaction with nitric oxide. In both the studies use of NO scavenger transposed the hydrogen sulphide effect on barley seedlings and alfalfa, which indicates the effect of H2S on endogenous NO levels. da-Silva et al. (2018) investigated whether H2S acts downstream or upstream of NO during mitigation of salinity stress in tomato. They found that H2S levels increased by 12–18.9% via NO donor whilst H2S donor enhanced 10% more NO in tomato roots. Along with this NO comes first in the signalling and involved in the induction of those genes that encodes enzymes responsible for H2S formation cascade, during attenuation of oxidative stress. This indicated that NO acts upstream of H2S in the mitigation of salinity stress in tomato. Li et al. (2014) in their study with Arabidopsis thaliana root in response to salt stress found that H2S function as upstream signal of H2O2 during alleviating the salinity stress. H2S enhanced the endogenous H2O2 level through regulating the activity of G6PDH (glucose-6-phosphate dehydrogenase) and (PM) NADPH oxidase. Apart from this H2S upregulated the genes expression and the phosphorylation level of PM H+-ATPase and Na+/H+ antiporter protein level. Mostofa et al. (2015) observed that in rice under salinity stress H2S pretreatment contributed to the reduction of H2O2, LOX activity, MDA content, the elevation of CAT and SOD activity, maintaining GSH level resulting in ROS homeostasis. Lai et al. (2014) found that H2S pretreatment enhanced the enzymatic activity of GR, DHAR and MDHAR and the abundance of Mn/Cu/Zn-SOD and CAT transcripts as well as regulate the AsA-GSH/hGSH pathway under salinity stress in Medicago sativa.
Conclusion and Future Thrusts
The generation of different reactive species (RONSS) and phytohormones in plants in response to salinity stress has been well established and studied. Plants use a combination of different signalling pathways to restore cell homeostasis and survival rate to mitigate salt stress. The ROS production and scavenging always occur simultaneously in the cell, tipping the balance between these, will cause rapid change in ROS levels, thereby generating signals. To prevent damage under different salt stress conditions, plants employ a mechanism that can maintain net photosynthesis with limited CO2 supply, use alternative electron sinks or the antioxidant defences must keep active oxygen under control. RNS signalling can mitigate the injurious effects of salinity by regulating the SOS pathway, MAPK dependent pathway, Ca2+ dependent pathway and G-protein dependent pathway in different crops. Nitric oxide and NO-derived molecules can alter target proteins by the phenomenon of post-translational modifications which plays a pivotal role in the modification of RNS signalling pathways. So far, ROS and RNS have received great attention in all reactive species. However, the participation of RSS is also an important area to understand the salinity stress signalling in plants because these molecules also mitigate the salinity stress through the regulation of sulphur metabolism as well as the synthesis of various sulphur metabolites. H2S was reported to act as a signalling molecule that interacts with NO and H2O2 which impart salinity tolerance. During the plant life cycle from germination to maturity, auxin, cytokinin, gibberellin, ethylene and abscisic acid modulates various growth and development processes of plants. The positive and beneficial effect of exogenous application of different phytohormones under salinity stress might play a crucial role as a free radical scavenger and antioxidative mechanism. Application of some novel phytohormones like salicylic acid, Brassinosteroids and strigolactones (SL) in crops under artificial salinity conditions different types of morphological and biochemical changes were observed.
Despite intensive work revealing nitric oxide diverse functions, reactivity, a signalling molecule and nitrosative stress, the NO production pathway in plants is still poorly understood and remains one of the most puzzling topics in the field. Understanding RONSS-hormonal crosstalk at the gene level is essential to elucidate how plants control salinity-mediated redox metabolism under salinity stress. So far RONSS crosstalk with classical phytohormone is well established but their interaction with novel phytohormones is still in the infancy stage. Identification of different NO/H2S donors and exact dose of phytohormones for external application will help to mitigate salt stress and to understand their crosstalk with each other. Understanding the complex signalling pathway of different reactive species and using advanced biotechnological approaches like gene editing, RNAi might help researchers to develop varieties with enhanced salinity tolerance. Those genes that impart tolerance against salt stress like Na+ transporters, ROS scavengers, HKTs and NHXs and others could be combined which might have a positive impact on the plant to cope up with stress conditions. Identification of phenotypic traits with limited genetic complexity and strong correlation to salinity tolerance is crucial for the successful discovery of novel candidate genes and allelic variation therein.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the review.
Code availability
Not applicable.
References
AbdElgawad H, Zinta G, Hegab MM et al (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276. https://doi.org/10.3389/fpls.2016.00276
Abdel-hamid AME (2016) The effect of the exogenous gibberellic acid on two the effect of the exogenous gibberellic acid on two salt stressed barley cultivars. Eur Sci J 10:228–245
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A et al (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18
Adamu TA, Mun BG, Lee SU, Hussain A, Yun BW (2018) Exogenously applied nitric oxide enhances salt tolerance in rice (Oryza sativa L.) at seedling stage. Agronomy. https://doi.org/10.3390/agronomy8120276
Ahanger MA, Aziz U, Alsahli AA et al (2020) Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate- glutathione cycle in salt stressed Vigna angularis. Biomolecules 10:42. https://doi.org/10.3390/biom10010042
Ahmad P, Ahanger MA, Alyemeni MN et al (2018) Mitigation of sodium chloride toxicity in solanum lycopersicum l. By supplementation of jasmonic acid and nitric oxide. J Plant Int 13:64–72. https://doi.org/10.1080/17429145.2017.1420830
Ahmad F, Kamal A, Singh A et al (2021) Seed priming with gibberellic acid induces high salinity tolerance in Pisum sativum through antioxidants, secondary metabolites and up-regulation of antiporter genes. Plant Biol 23:113–121. https://doi.org/10.1111/plb.13187
Akram NA, Hafeez N, Farid-ul-Haq M, Ahmad A, Sadiq M, Ashraf M (2020) Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol Plant. https://doi.org/10.1007/s11738-020-03140-x
Ali Q, Daud MK, Haider MZ et al (2017) Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol Biochem 119:50–58. https://doi.org/10.1016/j.plaphy.2017.08.010
Altaf MA, Shahid R, Ren MX et al (2021) Protective mechanisms of melatonin against vanadium phytotoxicity in tomato seedlings: insights into nutritional status, photosynthesis, root architecture system, and antioxidant machinery. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10513-0
Antoniou C, Savvides A, Christou A, Fotopoulos V (2016) Unravelling chemical priming machinery in plants: the role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr Opin Plant Biol 33:101–107. https://doi.org/10.1016/j.pbi.2016.06.020
Ardebili NO, Iranbakhsh A, Ardebili ZO (2019) Efficiency of selenium and salicylic acid protection against salinity in soybean. Iran J Plant Physiol 9:2727–2738. https://doi.org/10.22034/ijpp.2019.664577
Arora D, Bhatla SC (2017) Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic Biol Med 106:315–328. https://doi.org/10.1016/j.freeradbiomed.2017.02.042
Asgher M, Per TS, Masood A et al (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res 24:2273–2285. https://doi.org/10.1007/s11356-016-7947-8
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69:3401–3411
Astolfi S, Zuchi S (2013) Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol Plant 35:175–181. https://doi.org/10.1007/s11738-012-1060-5
Astolfi S, Zuchi S, Hubberten HM et al (2010) Supply of sulphur to S-deficient young barley seedlings restores their capability to cope with iron shortage. J Exp Bot 61:799–806. https://doi.org/10.1093/jxb/erp346
Avalbaev A, Yuldashev R, Fedorova K, Somov K, Vysotskaya L, Allagulova C, Shakirova F (2016) Exogenous methyl jasmonate regulates cytokinin content by modulating cytokinin oxidase activity in wheat seedlings under salinity. J Plant Physiol 191:101–110
Bai X, Yang L, Yang Y et al (2011) Deciphering the protective role of nitric oxide against salt stress at the physiological and proteomic levels in maize. J Proteome Res 10:4349–4364. https://doi.org/10.1021/pr200333f
Bandehagh A, Salekdeh GH, Toorchi M et al (2011) Comparative proteomic analysis of canola leaves under salinity stress. Proteomics 11:1965–1975. https://doi.org/10.1002/pmic.201000564
Banerjee A, Roychoudhury A (2019) Melatonin application reduces fluoride uptake and toxicity in rice seedlings by altering abscisic acid, gibberellin, auxin and antioxidant homeostasis. Plant Physiol Biochem 145:164–173
Basu S, Kumar A, Benazir I, Kumar G (2021) Reassessing the role of ion homeostasis for improving salinity tolerance in crop plants. Physiol Plant 171:502–519. https://doi.org/10.1111/ppl.13112
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240. https://doi.org/10.1093/jxb/ert375
Begara-Morales JC, Sánchez-Calvo B, Chaki M et al (2015) Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J Exp Bot 66:5983–5996. https://doi.org/10.1093/jxb/erv306
Ben Rejeb K, Benzarti M, Debez A et al (2015) NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J Plant Physiol 174:5–15. https://doi.org/10.1016/j.jplph.2014.08.022
Betti M, Bauwe H, Busch FA et al (2016) Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. J Exp Bot 67:2977–2988. https://doi.org/10.1093/jxb/erw076
Bharti N, Barnawal D (2019) Amelioration of salinity stress by PGPR. PGPR amelioration in sustainable agriculture. Elsevier, Amsterdam, pp 85–106
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Cai W, Liu W, Wang WS et al (2015) Overexpression of rat neurons nitric oxide synthase in rice enhances drought and salt tolerance. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0131599
Camejo D, del Romero-Puertas Carmen M, Rodríguez-Serrano M et al (2013) Salinity-induced changes in S-nitrosylation of pea mitochondrial proteins. J Proteomics 79:87–99. https://doi.org/10.1016/j.jprot.2012.12.003
Campos FV, Oliveira JA, Pereira MG, Farnese FS (2019) Nitric oxide and phytohormone interactions in the response of Lactuca sativa to salinity stress. Planta 250:1475–1489. https://doi.org/10.1007/s00425-019-03236-w
Cao MJ, Wang Z, Zhao Q et al (2014) Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J 77:604–615. https://doi.org/10.1111/tpj.12407
Capaldi FR, Gratão PL, Reis AR et al (2015) Sulfur metabolism and stress defense responses in plants. Trop Plant Biol 8:60–73
Carrasco-Ríos L, Pinto M (2014) Effect of salt stress on antioxidant enzymes and lipid peroxidation in leaves in two contrasting corn, “Lluteño” and “Jubilee.” Chil J Agric Res 74:89–95. https://doi.org/10.4067/S0718-58392014000100014
Castro B, Citterico M, Kimura S et al (2021) Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat Plants 7:403–412
Chaki M, Begara-Morales JC, Valderrama R et al (2020) Role of nitric oxide–dependent posttranslational modifications of proteins under abiotic stress. Plant Life Under Chang Environ 2:793–809. https://doi.org/10.1016/b978-0-12-818204-8.00035-7
Chen J, Xiong D-Y, Wang W-H et al (2013) Nitric oxide mediates root K+/Na+ balance in a mangrove plant, Kandelia obovata, by enhancing the expression of AKT1-Type K+ channel and Na+/H+ antiporter under high salinity. PLoS ONE 8:e71543. https://doi.org/10.1371/journal.pone.0071543
Chen J, Wang WH, Wu FH et al (2015) Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 5:1–19. https://doi.org/10.1038/srep12516
Chen Z, Zhao X, Hu Z, Leng P (2019) Nitric oxide modulating ion balance in Hylotelephium erythrostictum roots subjected to NaCl stress based on the analysis of transcriptome, fluorescence, and ion fluxes. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-54611-2
Che-Othman MH, Millar AH, Taylor NL (2017) Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C3 plants. Plant, Cell Environ 40:2875–2905
Chourasia KN, Lal MK, Tiwari RK et al (2021) Salinity stress in potato: understanding physiological. Biochem Mol Responses Life 11:545. https://doi.org/10.3390/life11060545
Corpas FJ, Barroso JB (2015) Reactive sulfur species (RSS): possible new players in the oxidative metabolism of plant peroxisomes. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00116
Corpas FJ, Hayashi M, Mano S et al (2009) Peroxisomes are required for in vivo nitric oxide accumulation in the cytosol following salinity stress of Arabidopsis plants. Plant Physiol 151:2083–2094. https://doi.org/10.1104/pp.109.146100
Corpas FJ, González-Gordo S, Palma JM (2020) Plant Peroxisomes: a factory of reactive species. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00853
da-Silva CJ, Mollica DCF, Vicente MH et al (2018) NO, hydrogen sulfide does not come first during tomato response to high salinity. Nitric Oxide—Biol Chem 76:164–173. https://doi.org/10.1016/j.niox.2017.09.008
Davaritouchaee M, Hiscox WC, Martinez-Fernandez J et al (2019) Effect of reactive oxygen species on biomass structure in different oxidative processes. Ind Crops Prod 137:484–494. https://doi.org/10.1016/j.indcrop.2019.05.063
David A, Yadav S, Baluška F, Bhatla SC (2015) Nitric oxide accumulation and protein tyrosine nitration as a rapid and long distance signalling response to salt stress in sunflower seedlings. Nitric Oxide—Biol Chem 50:28–37. https://doi.org/10.1016/j.niox.2015.08.003
de Freitas WE, de Oliveira AB, Mesquita RO et al (2019) Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake, lower Na/K ratio and an efficient antioxidative defense system. Sci Hortic. https://doi.org/10.1016/j.scienta.2019.108764
del Río LA (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66:2827–2837. https://doi.org/10.1093/jxb/erv099
Del Río LA, López-Huertas E (2016) ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol 57:1364–1376
Demirelbose U, Morris WL, Ducreux LJM et al (2020) Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive potato genotypes. Front Plant Sci 11:169. https://doi.org/10.3389/fpls.2020.00169
Devireddy AR, Zandalinas SI, Fichman Y, Mittler R (2021) Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J 105:459–476. https://doi.org/10.1111/tpj.15010
Dinler BS, Antoniou C, Fotopoulos V (2014) Interplay between GST and nitric oxide in the early response of soybean (Glycine max L.) plants to salinity stress. J Plant Physiol 171:1740–1747. https://doi.org/10.1016/j.jplph.2014.07.026
Elsayed AI, Boulila M, Rafudeen MS et al (2020) Melatonin regulatory mechanisms and phylogenetic analyses of melatonin biosynthesis related genes extracted from peanut under salinity stress. Plants 9:1–21. https://doi.org/10.3390/plants9070854
Fahad S, Hussain S, Matloob A et al (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404
Fan HF, Du CX, Guo SR (2013) Nitric oxide enhances salt tolerance in cucumber seedlings by regulating free polyamine content. Environ Exp Bot 86:52–59. https://doi.org/10.1016/j.envexpbot.2010.09.007
Fares A, Rossignol M, Peltier JB (2011) Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem Biophys Res Commun 416:331–336. https://doi.org/10.1016/j.bbrc.2011.11.036
Farvardin A, González-hernández AI, Llorens E et al (2020) The apoplast: a key player in plant survival. Antioxidants 9:1–26
Fatima A, Husain T, Suhel M et al (2021) Implication of nitric oxide under salinity stress: the possible interaction with other signaling molecules. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10255-5
Fatma M, Khan MIR, Masood A, Khan NA (2013) Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: involvement of phytohormones. Ann Rev Res Biol 3:267–295
Fatma M, Asgher M, Masood A, Khan NA (2014) Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot 107:55–63. https://doi.org/10.1016/j.envexpbot.2014.05.008
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521. https://doi.org/10.3389/fpls.2016.00521
Fatma M, Iqbal N, Gautam H et al (2021) Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 10:1–22. https://doi.org/10.3390/plants10010180
Ferrer-Sueta G, Campolo N, Trujillo M et al (2018) Biochemistry of peroxynitrite and protein tyrosine nitration. Chem Rev 118:1338–1408. https://doi.org/10.1021/ACS.CHEMREV.7B00568
Fu Y, Yang Y, Chen S et al (2019) Arabidopsis IAR4 modulates primary root growth under salt stress through ros-mediated modulation of auxin distribution. Front Plant Sci 10:522. https://doi.org/10.3389/fpls.2019.00522
Gadelha CG, de MirandaAlencar RNLM, Costa JH, Prisco JT, Gomes-Filho E (2017) Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J Plant Physiol 212:69–79. https://doi.org/10.1016/j.jplph.2017.02.005
Ghosh N, Das SP, Mandal C, Gupta S, Das K, Dey N, Adak MK (2012) Variations of antioxidative responses in two rice cultivars with polyamine treatment under salinity stress. Physiol Mol Biol Plants 18:301–313. https://doi.org/10.1007/s12298-012-0124-8
Ghosh S, Mitra S, Paul A (2015) Physiochemical studies of sodium chloride on mungbean (Vigna radiata L. Wilczek) and its possible recovery with spermine and gibberellic acid. Sci World J. https://doi.org/10.1155/2015/858016
Giles GI, Tasker KM, Jacob C (2001) Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radical Biol Med 31:1279–1283. https://doi.org/10.1016/S0891-5849(01)00710-9
Giles GI, Tasker KM, Collins C et al (2002) Properties of disulphide S-oxides. Society 585:579–585
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gohari G, Alavi Z, Esfandiari E et al (2020) Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol Plant 168:361–373. https://doi.org/10.1111/ppl.13020
Gupta P, Srivastava S, Seth CS (2017) 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 411:483–498. https://doi.org/10.1007/s11104-016-3043-6
Gupta KJ, Kumari A, Florez-Sarasa I et al (2018) Interaction of nitric oxide with the components of the plant mitochondrial electron transport chain. J Exp Bot 69:3413–3424
Ha CV, Leyva-Gonzalez MA, Osakabe Y et al (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA 111:851–856. https://doi.org/10.1073/pnas.1322135111
Hajihashemi S, Skalicky M, Brestic M, Pavla V (2020) Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol Biochem 154:657–664. https://doi.org/10.1016/j.plaphy.2020.07.022
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Hasanuzzaman M, Bhuyan MHMB, Mahmud JA et al (2018) Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal Behav. https://doi.org/10.1080/15592324.2018.1477905
Hasanuzzaman M, Oku H, Nahar K et al (2018b) Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol Rep 12:77–92. https://doi.org/10.1007/s11816-018-0480-0
Hussain S, Hussain S, Ali B et al (2021) Recent progress in understanding salinity tolerance in plants: story of Na+/K+ balance and beyond. Plant Physiol Biochem 160:239–256. https://doi.org/10.1016/j.plaphy.2021.01.029
Iglesias MJ, Terrile MC, Bartoli CG et al (2010) Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol 74:215–222. https://doi.org/10.1007/s11103-010-9667-7
Iglesias MJ, Terrile MC, Windels D et al (2014) MiR393 regulation of auxin signaling and redox-related components during acclimation to salinity in Arabidopsis. PLoS ONE 9:e107678. https://doi.org/10.1371/journal.pone.0107678
Ijaz M, Ali Q, Fahad S et al (2019) Role of reactive sulfur species in the oxidative metabolism in plants. Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Taylor and Francis, England, pp 729–742
Iqbal M, Ashraf M (2013) Salt tolerance and regulation of gas exchange and hormonal homeostasis by auxin-priming in wheat. Pesqui Agropecu Bras 48:1210–1219. https://doi.org/10.1590/S0100-204X2013000900004
Iqbal M, Ashraf M, Jamil A (2006) Seed enhancement with cytokinins: changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul 50:29–39. https://doi.org/10.1007/s10725-006-9123-5
Ivushkin K, Bartholomeus H, Bregt AK et al (2019) Global mapping of soil salinity change. Remote Sens Environ 231:111260. https://doi.org/10.1016/j.rse.2019.111260
Jacob C, Lancaster JR, Giles GI (2004) Reactive sulphur species in oxidative signal transduction. Biochemical society transactions. Portland Press, London, pp 1015–1017
Jain P, von Toerne C, Lindermayr C, Bhatla SC (2018) S-nitrosylation/denitrosylation as a regulatory mechanism of salt stress sensing in sunflower seedlings. Physiol Plant 162:49–72. https://doi.org/10.1111/ppl.12641
Ji H, Pardo JM, Batelli G et al (2013) The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant 6:275–286
Jiang C, Belfield EJ, Cao Y et al (2013) An Arabidopsis soil-salinity-tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis. Plant Cell 25:3535–3552. https://doi.org/10.1105/tpc.113.115659
Jung JH, Park CM (2011) Auxin modulation of salt stress signaling in Arabidopsis seed germination. Plant Signal Behav 6:1198–1200. https://doi.org/10.4161/psb.6.8.15792
Kamiab F (2020) Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J Plant Nutr 43:1468–1484. https://doi.org/10.1080/01904167.2020.1730898
Kapoor D, Singh S, Kumar V et al (2019) Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 19:100182. https://doi.org/10.1016/j.plgene.2019.100182
Kataria S, Jain M, Tripathi DK, Singh VP (2020) Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol Plant 168:422–436. https://doi.org/10.1111/ppl.13031
Kaur G, Asthir B (2020) Impact of exogenously applied ABA on proline metabolism conferring drought and salinity stress tolerance in wheat genotypes. Cereal Res Commun 48:309–315. https://doi.org/10.1007/s42976-020-00041-0
Kaya C, Ashraf M (2020) Nitric oxide is required for aminolevulinic acid-induced salt tolerance by lowering oxidative stress in maize (Zea mays). J Plant Growth Regul 40:617–627. https://doi.org/10.1007/s00344-020-10126-z
Kaya C, Ashraf M, Dikilitas M, Tuna AL (2013) Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients—a field trial. Aust J Crop Sci 7:249–254
Kaya C, Ashraf M, Sönmez O, Tuna AL, Aydemir S (2015) Exogenously applied nitric oxide confers tolerance to salinity-induced oxidative stress in two maize (Zea mays L.) cultivars differing in salinity tolerance. Turk J Agric For 39:909–919. https://doi.org/10.3906/tar-1411-26
Kaya C, Akram NA, Ashraf M (2019) Influence of exogenously applied nitric oxide on strawberry (Fragaria × ananassa) plants grown under iron deficiency and/or saline stress. Physiol Plant 165:247–263. https://doi.org/10.1111/ppl.12818
Kaya C, Murillo-Amador B, Ashraf M (2020) Involvement of L-cysteine desulfhydrase and hydrogen sulfide in glutathione-induced tolerance to salinity by accelerating ascorbate-glutathione cycle and glyoxalase system in capsicum. Antioxidants 9:1–29. https://doi.org/10.3390/antiox9070603
Kearl J, McNary C, Lowman JS et al (2019) Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01849
Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89. https://doi.org/10.1007/s11738-007-0093-7
Khan Z, Shahwar D (2020) Role of heat shock proteins (HSPs) and heat stress tolerance in crop plants. Sustainable agriculture in the era of climate change. Springer International Publishing, Cham, pp 211–234
Khan MIR, Asgher M, Iqbal N, Khan NA (2013) Potentiality of sulphur-containing compounds in salt stress tolerance. Ecophysiology and responses of plants under salt stress. Springer, New York, pp 443–472
Khare T, Kumar V, Kishor PBK (2015) Na+ and Cl- ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 252:1149–1165. https://doi.org/10.1007/s00709-014-0749-2
Kim Y, Wang M, Bai Y et al (2014) Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca2+ level with elevated K+ efflux in NaCl-induced PCD in rice. Plant Physiol Biochem 80:168–175. https://doi.org/10.1016/j.plaphy.2014.04.002
Kim Y, Mun BG, Khan AL et al (2018) Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 13:e0192650. https://doi.org/10.1371/journal.pone.0192650
Klein A, Hüsselmann L, Keyster M, Ludidi N (2018) Exogenous nitric oxide limits salt-induced oxidative damage in maize by altering superoxide dismutase activity. South Afr J Bot 115:44–49. https://doi.org/10.1016/j.sajb.2017.12.010
Kolomeichuk LV, Efimova MV, Zlobin IE et al (2020) 24-Epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth Res 146:151–163. https://doi.org/10.1007/s11120-020-00708-z
Kong X, Wang T, Li W et al (2016) Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38:1–9. https://doi.org/10.1007/s11738-016-2079-9
Koprivova A, North KA, Kopriva S (2008) Complex signaling network in regulation of adenosine 5′- phosphosulfate reductase by salt stress in arabidopsis roots. Plant Physiol 146:1408–1420. https://doi.org/10.1104/pp.107.113175
Kukreja S, Nandwal AS, Kumar N et al (2005) Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant 49:305–308. https://doi.org/10.1007/s10535-005-5308-4
Kwak JM, Mori IC, Pei Z-M et al (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633. https://doi.org/10.1093/EMBOJ/CDG277
Lai D, Mao Y, Zhou H et al (2014) Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci 225:117–129. https://doi.org/10.1016/j.plantsci.2014.06.006
Lal MK, Kumar A, Raigond P et al (2020) Impact of starch storage condition on glycemic index and resistant starch of cooked potato (Solanum tuberosum) tubers. Starch—Stärke. https://doi.org/10.1002/star.201900281
Lal MK, Tiwari RK, Gahlaut V et al (2021a) Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep 1:1–18. https://doi.org/10.1007/s00299-021-02784-4
Lal MK, Tiwari RK, Kumar R et al (2021b) Effect of potato apical leaf curl disease on glycemic index and resistant starch of potato (Solanum tuberosum L.) tubers. Food Chem 359:1239. https://doi.org/10.1016/j.foodchem.2021.129939
Laxa M, Liebthal M, Telman W et al (2019) The role of the plant antioxidant system in drought tolerance. Antioxidants 8:94
Li JH, Liu YQ, Lü P et al (2009) A signaling pathway linking nitric oxide production to heterotrimeric g protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in arabidopsis. Plant Physiol 150:114–124. https://doi.org/10.1104/pp.109.137067
Li J, Jia H, Wang J et al (2014) Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251:899–912. https://doi.org/10.1007/s00709-013-0592-x
Lin Y, Wang J, Zu Y, Tang Z (2012) Ethylene antagonizes the inhibition of germination in Arabidopsis induced by salinity by modulating the concentration of hydrogen peroxide. Acta Physiol Plant 34:1895–1904. https://doi.org/10.1007/s11738-012-0989-8
Liu W, Li RJ, Han TT et al (2015) Salt stress reduces root meristem size by nitric oxidemediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168:343–356. https://doi.org/10.1104/pp.15.00030
Liu X, Wang X, Yin L, Deng X, Wang S (2018) Exogenous application of gibberellic acid participates in up-regulation of lipid biosynthesis under salt stress in rice. Theor Exp Plant Physiol 30:335–345. https://doi.org/10.1007/s40626-018-0129-y
Liu J, Li L, Yuan F, Chen M (2019) Exogenous salicylic acid improves the germination of limonium bicolor seeds under salt stress. Plant Signal Behav. https://doi.org/10.1080/15592324.2019.1644595
Liu J, Shabala S, Zhang J et al (2020) Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant, Cell Environ 43:2591–2605. https://doi.org/10.1111/pce.13759
Luo X, Dai Y, Zheng C et al (2021) The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol 229:950–962. https://doi.org/10.1111/nph.16921
Ma L, Zhang H, Sun L et al (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na +/K + homeostasis in Arabidopsis under salt stress. J Exp Bot 63:305–317. https://doi.org/10.1093/jxb/err280
Ma X, Zhang J, Huang B (2016) Cytokinin-mitigation of salt-induced leaf senescence in perennial ryegrass involving the activation of antioxidant systems and ionic balance. Environ Exp Bot 125:1–11
Maggio A, Barbieri G, Raimondi G, de Pascale S (2010) Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul 29:63–72. https://doi.org/10.1007/s00344-009-9114-7
Magome H, Yamaguchi S, Hanada A et al (2008) The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56:613–626. https://doi.org/10.1111/j.1365-313X.2008.03627.x
Manai J, Kalai T, Gouia H, Corpas FJ (2014) Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J Soil Sci Plant Nutr 14:433–446. https://doi.org/10.4067/S0718-95162014005000034
Manishankar P, Wang N, Köster P et al (2018) Calcium signaling during salt stress and in the regulation of ion homeostasis. J Exp Bot 69:4215–4226. https://doi.org/10.1093/jxb/ery201
Mansour M, Salama K, Ali F, Abou Hadid A (2005) Cell and plant responses to NaCl in Zea mays L. cultivars differing in salt tolerance. Gen Appl Plant Physiol 31:29–41
Martínez-Andújar C, Martínez-Pérez A, Albacete A et al (2021) Overproduction of ABA in rootstocks alleviates salinity stress in tomato shoots. Plant, Cell Environ 44:2966–2986. https://doi.org/10.1111/pce.14121
Miao Y, Luo X, Gao X et al (2020) Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci Hortic. https://doi.org/10.1016/j.scienta.2020.109577
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mishra S, Srivastava V, Mehrotra S, Quadri SN (2017) The regulation of plant development: cross-talk of reactive oxygen species and plant hormones. Reactive oxygen species in plants: boon or bane—revisiting the role of ROS. Wiley, Chichester, pp 243–260
Mittler R, Vanderauwera S, Suzuki N et al (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Molassiotis A, Tanou G, Diamantidis G (2010) No says more than “YES” to salt tolerance salt priming and systemic nitric oxide signaling in plants. Plant Signal Behav 5:209–212. https://doi.org/10.4161/psb.5.3.10738
Monetti E, Kadono T, Tran D et al (2014) Deciphering early events involved in hyperosmotic stress-induced programmed cell death in tobacco BY-2 cells. J Exp Bot 65:1361–1375. https://doi.org/10.1093/jxb/ert460
Mostofa MG, Saegusa D, Fujita M, Phan Tran LS (2015) Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front Plant Sci 6:1055. https://doi.org/10.3389/fpls.2015.01055
Mur LAJ, Mandon J, Persijn S et al (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB PLANTS. https://doi.org/10.1093/aobpla/pls052
Naeem M, Sadiq Y, Jahan A et al (2020) Salicylic acid restrains arsenic induced oxidative burst in two varieties of Artemisia annua L. by modulating antioxidant defence system and artemisinin production. Ecotoxicol Environ Saf 202:110851. https://doi.org/10.1016/J.ECOENV.2020.110851
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015) Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant 59:745–756. https://doi.org/10.1007/s10535-015-0542-x
Nawaz F, Shabbir RN, Shahbaz M et al (2017) Cross talk between nitric oxide and phytohormones regulate plant development during abiotic stresses. Phytohormones—signaling mechanisms and crosstalk in plant development and stress responses. InTech, Rijeka. https://doi.org/10.5772/intechopen.69812
Nawaz F, Majeed S, Ahmad KS et al (2019) Reactive sulfur species-key regulators of abiotic stress tolerance in plants. Reactive oxygen nitrogen and sulfur species in plants. . Wiley, New Jersey, pp 685–713
Nazar R (2014) Involvement of salicylic acid in sulfur induced salinity tolerance: a role of glutathione. Ann Res Rev Biol 4:3875–3893. https://doi.org/10.9734/arrb/2014/11773
Nazar R, Iqbal N, Masood A et al (2011) Understanding the significance of sulfur in improving salinity tolerance in plants. Environ Exp Bot 70:80–87
Nazar R, Khan MIR, Iqbal N et al (2014) Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol Plant 152:331–344. https://doi.org/10.1111/ppl.12173
Nazara R, Umara S, Khanb NA (2015) Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal Behav. https://doi.org/10.1080/15592324.2014.1003751
Nemat Alla MM, Hassan NM (2020) Nitrogen alleviates NaCl toxicity in maize seedlings by regulating photosynthetic activity and ROS homeostasis. Acta Physiol Plant 42:93. https://doi.org/10.1007/s11738-020-03080-6
Ogawa S, Mitsuya S (2012) S-methylmethionine is involved in the salinity tolerance of Arabidopsis thaliana plants at germination and early growth stages. Physiol Plant 144:13–19. https://doi.org/10.1111/j.1399-3054.2011.01516.x
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49:157–165. https://doi.org/10.1007/s10725-006-9101-y
Pandey V, Bhatt ID, Nandi SK (2019) Role and regulation of auxin signaling in abiotic stress tolerance. Plant signaling molecules: role and regulation under stressful environments. Elsevier, Amsterdam, pp 319–331
Pavlů J, Novák J, Koukalová V et al (2018) Cytokinin at the crossroads of abiotic stress signalling pathways. Int J Mol Sci 19:2450
Peng J, Li Z, Wen X et al (2014) Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet 10:e1004664. https://doi.org/10.1371/journal.pgen.1004664
Pirasteh-Anosheh H, Emam Y, Rousta MJ, Ashraf M (2017) Salicylic acid induced salinity tolerance through manipulation of ion distribution rather than ion accumulation. J Plant Growth Regul 36:227–239. https://doi.org/10.1007/s00344-016-9633-y
Poór P, Tari I (2011) Ethylene-regulated reactive oxygen species and nitric oxide under salt stress in tomato cell suspension culture. Acta Biol Szegediensis 55:143–146
Qi J, Wang J, Gong Z, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38:92–100
Qiu Z, Zhu L, He L et al (2019) DNA damage and reactive oxygen species cause cell death in the rice local lesions 1 mutant under high light and high temperature. New Phytol 222:349–365. https://doi.org/10.1111/nph.15597
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129:232–237. https://doi.org/10.1016/j.scienta.2011.03.035
Rodríguez AA, Maiale SJ, Menéndez AB, Ruiz OA (2009) Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J Exp Bot 60:4249–4262. https://doi.org/10.1093/jxb/erp256
Roy S, Roy Choudhury S, Sengupta DN, Pada Das K (2013) Involvement of AtPolλ in the repair of high salt- and DNA cross-linking agent-induced double strand breaks in arabidopsis. Plant Physiol 162:1195–1210. https://doi.org/10.1104/pp.113.219022
Ryu H, Cho YG (2015) Plant hormones in salt stress tolerance. J Plant Biol 58:147–155. https://doi.org/10.1007/s12374-015-0103-z
Saddhe AA, Malvankar MR, Karle SB, Kumar K (2019) Reactive nitrogen species: paradigms of cellular signaling and regulation of salt stress in plants. Environ Exp Bot 161:86–97
Saeidi-Sar S, Abbaspour H, Afshari H, Yaghoobi SR (2013) Effects of ascorbic acid and gibberellin A3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol Plant 35:667–677. https://doi.org/10.1007/s11738-012-1107-7
Saha P, Mukherjee A, Biswas AK (2015) Modulation of NaCl induced DNA damage and oxidative stress in mungbean by pretreatment with sublethal dose. Biol Plant 59:139–146. https://doi.org/10.1007/s10535-014-0460-3
Samadi S, Habibi G, Vaziri A (2019) Effects of exogenous salicylic acid on antioxidative responses, phenolic metabolism and photochemical activity of strawberry under salt stress. Iran J Plant Physiol 9:2685–2694. https://doi.org/10.22034/ijpp.2019.545950
Sanower HM, Sultan ASJ (2019) Present scenario of global salt affected soils, its management and importance of salinity research. Int Res J Biol Sci Perspect 1:2663–5976
Santos MP, Zandonadi DB, de Sá AFL et al (2020) Abscisic acid-nitric oxide and auxin interaction modulates salt stress response in tomato roots. Theor Exp Plant Physiol 32:301–313. https://doi.org/10.1007/s40626-020-00187-6
Sanz L, Albertos P, Mateos I et al (2015) Nitric oxide (NO) and phytohormones crosstalk during early plant development. J Exp Bot 66:2857–2868
Sehar Z, Masood A, Khan NA (2019) Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ Exp Bot 161:277–289. https://doi.org/10.1016/j.envexpbot.2019.01.010
Seo SY, Park KY (2021) Translocation of chloroplast NPR1 to the nucleus in retrograde signaling for adaptive response to salt stress in tobacco. bioRxivxiv 2021.03.24.436779. https://doi.org/10.1101/2021.03.24.436779
Shabala S (2019) Linking ploidy level with salinity tolerance: NADPH-dependent ‘ROS–Ca2+ hub’in the spotlight. J Exp Bot 70:1063–1067
Shakar M, Yaseen M, Mahmood R, Ahmad I (2016) Calcium carbide induced ethylene modulate biochemical profile of Cucumis sativus at seed germination stage to alleviate salt stress. Sci Hortic 213:179–185. https://doi.org/10.1016/j.scienta.2016.10.037
Shaki F, Maboud HE, Niknam V (2018) Growth enhancement and salt tolerance of safflower (Carthamus tinctorius L.), by salicylic acid. Curr Plant Biol 13:16–22. https://doi.org/10.1016/j.cpb.2018.04.001
Shams M, Ekinci M, Ors S, Turan M, Agar G, Kul R, Yildirim E (2019) Nitric oxide mitigates salt stress effects of pepper seedlings by altering nutrient uptake, enzyme activity and osmolyte accumulation. Physiol Mol Biol Plants 25:1149–1161. https://doi.org/10.1007/s12298-019-00692-2
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26. https://doi.org/10.1016/j.plaphy.2013.04.013
Sharma E, Sharma R, Borah P et al (2015) Emerging roles of auxin in abiotic stress responses. Elucidation of abiotic stress signaling in plants: functional genomics perspectives. Springer, New York, pp 299–328
Sharma A, Kapoor D, Wang J et al (2020) Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biol Plant 64:512–518
Shen Y, Shen L, Shen Z et al (2015) The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant, Cell Environ 38:2766–2779. https://doi.org/10.1111/pce.12586
Shi G, Guo X, Guo J et al (2015) Analyzing serial cDNA libraries revealed reactive oxygen species and gibberellins signaling pathways in the salt response of upland cotton (Gossypium hirsutum L.). Plant Cell Rep 34:1005–1023. https://doi.org/10.1007/s00299-015-1761-5
Soliman M, Elkelish A, Souad T, Alhaithloul H, Farooq M (2020) Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants 26:501–511. https://doi.org/10.1007/s12298-020-00765-7
Sripinyowanich S, Klomsakul P, Boonburapong B et al (2013) Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): the role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ Exp Bot 86:94–105. https://doi.org/10.1016/j.envexpbot.2010.01.009
Su LJ, Zhang JH, Gomez H et al (2019) Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. https://doi.org/10.1155/2019/5080843
Sun C, Liu L, Wang L et al (2021) Melatonin: a master regulator of plant development and stress responses. J Integr Plant Biol 63:126–145
Suo J, Zhao Q, David L et al (2017) Salinity response in chloroplasts: insights from gene characterization. Int J Mol Sci 18:1011
Suzuki N, Miller G, Morales J et al (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Tan DX, Manchester LC, Terron MP et al (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 42:28–42
Tanou G, Filippou P, Belghazi M et al (2012) Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J 72:585–599. https://doi.org/10.1111/j.1365-313X.2012.05100.x
Tanveer M, Ahmed HAI (2020) ROS signalling in modulating salinity stress tolerance in plants. Springer, Cham, pp 299–314
Tiwari RK, Kumar R, Sharma S et al (2020) Potato dry rot disease: current status, pathogenomics and management. 3 Biotech 10:503. https://doi.org/10.1007/s13205-020-02496-8
Tiwari RK, Lal MK, Naga KC et al (2020) Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci Hortic (Amsterdam) 272:109592. https://doi.org/10.1016/j.scienta.2020.109592
Tiwari RK, Bashyal BM, Shanmugam V et al (2021a) Impact of Fusarium dry rot on physicochemical attributes of potato tubers during postharvest storage. Postharvest Biol Technol 181:111638. https://doi.org/10.1016/J.POSTHARVBIO.2021.111638
Tiwari RK, Lal MK, Kumar R et al (2021b) Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol Plant 172:1212–1226. https://doi.org/10.1111/ppl.13307
Tiwari RK, Lal MK, Kumar R et al (2021c) Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01202-3
Tomar RS, Kataria S, Jajoo A (2021) Behind the scene: critical role of reactive oxygen species and reactive nitrogen species in salt stress tolerance. J Agron Crop Sci 207:577–588
Vetrano F, Moncada A, Miceli A (2020) Use of gibberellic acid to increase the salt tolerance of leaf lettuce and rocket grown in a floating system. Agronomy 10:505. https://doi.org/10.3390/agronomy10040505
Wang H, Liang X, Wan Q et al (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307. https://doi.org/10.1007/s00425-009-0946-y
Wang Y, Li L, Cui W et al (2011) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant and Soil 351:107–119. https://doi.org/10.1007/S11104-011-0936-2
Wang Y, Shen W, Chan Z, Wu Y (2015) Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis thaliana. Front Plant Sci 6:1004. https://doi.org/10.3389/fpls.2015.01004
Wang LY, Liu JL, Wang WX, Sun Y (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54:19–27
Wang YH, Zhang G, Chen Y, Gao J, Sun YR, Sun MF, Chen JP (2019) Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiol Plant. https://doi.org/10.1007/s11738-019-2869-y
Wang S, Guo T, Wang Z et al (2020) Expression of three related to ABI3/VP1 genes in Medicago truncatula caused increased stress resistance and branch increase in Arabidopsis thaliana. Front Plant Sci 11:611. https://doi.org/10.3389/fpls.2020.00611
Wei Z, Shi X, Wei F et al (2019) The cotton endocycle-involved protein SPO11-3 functions in salt stress via integrating leaf stomatal response, ROS scavenging and root growth. Physiol Plant 167:127–141. https://doi.org/10.1111/ppl.12875
Wiggins AT (2015) cr ip t Ac ce pt e an us cr ip t Ac ce pt e us. Aerosol Sci Technol 4723:1–17
Wu X, He J, Chen J, Yang S, Zha D (2014) Alleviation of exogenous 6-benzyladenine on two genotypes of eggplant (Solanum melongena Mill.) growth under salt stress. Protoplasma 251:169–176. https://doi.org/10.1007/s00709-013-0535-6
Xing Y, Chen WH, Jia W, Zhang J (2015) Mitogen-activated protein kinase kinase 5 (MKK5)-mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J Exp Bot 66:5971–5981. https://doi.org/10.1093/jxb/erv305
Xu L, Xiang G, Sun Q, Ni Y, Jin Z, Gao S, Yao Y (2019) Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic Res. https://doi.org/10.1038/s41438-019-0197-4
Wu XX (2012) Effects of 24-epibrassinolide on photosynthesis of eggplant (Solanum melongena L.) seedlings under salt stress. Afr J Biotechnol 11:8665–8671. https://doi.org/10.5897/ajb11.3416
Yadu S, Dewangan TL, Chandrakar V, Keshavkant S (2017) Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol Mol Biol Plants 23:43–58. https://doi.org/10.1007/s12298-016-0394-7
Yang L, Zu YG, Tang ZH (2013) Ethylene improves Arabidopsis salt tolerance mainly via retaining K+ in shoots and roots rather than decreasing tissue Na+ content. Environ Exp Bot 86:60–69. https://doi.org/10.1016/j.envexpbot.2010.08.006
Yang C, Ma B, He SJ et al (2015) MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol 169:148–165. https://doi.org/10.1104/pp.15.00353
Zagorchev L, Seal CE, Kranner I, Odjakova M (2012) Redox state of low-molecular-weight thiols and disulphides during somatic embryogenesis of salt-treated suspension cultures of Dactylis glomerata L. Free Radic Res 46:656–664. https://doi.org/10.3109/10715762.2012.667565
Zhang J, Liao W (2019) Protein S-nitrosylation in plant abiotic stresses. Funct Plant Biol 47:1–10. https://doi.org/10.1071/FP19071
Zhang JL, Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res 115:1–22. https://doi.org/10.1007/s11120-013-9813-6
Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, Cao YY, Weeda S, Zhao B, Ren S, Guo YD (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57:269–279
Zhang J, Zou D, Li Y, Sun X, Wang NN, Gong SY, Zheng Y, Li XB (2014) GhMPK17, a cotton mitogen-activated protein kinase, is involved in plant response to high salinity and osmotic stresses and ABA signaling. PLoS ONE. https://doi.org/10.1371/journal.pone.0095642
Zhao MG, Tian QY, Zhang WH (2007) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol 144:206–217. https://doi.org/10.1104/pp.107.096842
Zhao SP, Xu ZS, Zheng WJ et al (2017) Genome-wide analysis of the rav family in soybean and functional identification of GmRAV-03 involvement in salt and drought stresses and exogenous ABA treatment. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00905
Zhao G, Zhao Y, Yu X et al (2018) Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int J Mol Sci 19:1912. https://doi.org/10.3390/ijms19071912
Zhou Y, Wen Z, Zhang J, Chen X, Cui J, Xu W, Liu HY (2017) Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci Hortic (Amsterdam) 220:90–101. https://doi.org/10.1016/j.scienta.2017.02.021
Zhou Y, Wen Z, Zhang J et al (2017) Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci Hortic 220:90–101. https://doi.org/10.1016/j.scienta.2017.02.021
Zhou X, Joshi S, Khare T et al (2021) Nitric oxide, crosstalk with stress regulators and plant abiotic stress tolerance. Plant Cell Rep. https://doi.org/10.1007/s00299-021-02705-5
Zhu G, An L, Jiao X, Chen X, Zhou G, McLaughlin N (2019) Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chil J Agric Res 79:415–424. https://doi.org/10.4067/S0718-58392019000300415
Zvanarou S, Vágnerová R, Mackievic V et al (2020) Salt stress triggers generation of oxygen free radicals and DNA breaks in Physcomitrella patens protonema. Environ Exp Bot 180:104236. https://doi.org/10.1016/j.envexpbot.2020.104236
Funding
The study was supported by Indian Council of Agricultural Research.
Author information
Authors and Affiliations
Contributions
VM, MKL and RKT: Conceptualization and writing—original draft preparation; MAA, SS and DK: Writing—original draft preparation, figures and table; VB, BS, RKS and TA: Supervision, writing–review and editing. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
As the manuscript is a review article, ethical approval is not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling Editor: Durgesh kumar tripathi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mangal, V., Lal, M.K., Tiwari, R.K. et al. Molecular Insights into the Role of Reactive Oxygen, Nitrogen and Sulphur Species in Conferring Salinity Stress Tolerance in Plants. J Plant Growth Regul 42, 554–574 (2023). https://doi.org/10.1007/s00344-022-10591-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10591-8