Abstract
As the salt-affected areas are expected to increase substantially in subsequent years, the impact of salinity on plant growth and yield is likely to increase. One of the first consequences of plant exposure to high saline concentrations is the formation of reactive oxygen species (ROS). In order to allow adjustment of the cellular redox state, plant antioxidative system has to be activated. This system involves several enzymes and compounds, as the sulphur-containing metabolite glutathione (GSH). Therefore, our aim was to determine whether adequate sulphur nutrition might alleviate the adverse effects of salt stress on barley plants grown in the presence of different sulphate application rate and exposed to 100 mM NaCl, by studying differences in growth parameters, lipid peroxidation, sulphate and thiol accumulation and sulphur assimilation pathway. In salt-treated plants, an adequate sulphur supply allows adequate GSH synthesis (high-thiol concentration) thus avoiding the effects of ROS on photosynthetic functions (no effect on both chlorophyll and protein content), whereas in S-deficient plants, salt stress leads to excess ROS production that induces stress and plants showed reduction of photosynthetic efficiency (loss of chlorophyll and protein contents). As thiol levels are more abundant in S-sufficient plants than in those S-deficient, one might expect that S-sufficient plants are more able to remove the harmful effects of high salinity. The comparison of malondialdehyde levels between +S and −S salt-treated plants strongly supports this idea. In conclusion, we found that plant sulphur nutritional status plays a key role in the metabolic modifications necessary to cope with salt stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural soil pedogenetic processes in warm and dry regions could often result in saline soil formation with low agricultural potential. Cultivation practices, in particular an inadequate irrigation management, have also led to growing salinization of soil and groundwater resources (Mahajan and Tuteja 2005). To date, about one-third of land throughout the world is already affected by salt, but it is expected an increase of salt-affected areas and as a result the loss of up to 50 % fertile land by the middle of the 21st century (Manchanda and Garg 2008).
Salinity produces oxidative stress by means of enhanced occurrence of reactive oxygen species (ROS) (Zhu et al. 2007) acting both as the damaging toxic molecule and as the beneficial signal transduction molecule (Miller et al. 2010). In order to allow adjustment of the cellular redox state and to reduce toxic effect of salinity, plant antioxidant system has to be activated. Plants are provided with an efficient system for protection against ROS and peroxidation reactions that involves several enzymes and compounds, as the sulphur-containing metabolite glutathione (GSH). Glutathione is a major reservoir of non-protein thiols in most plant cells (Rennenberg and Brunold 1994) and plays a crucial role in plant ROS scavenging (Foyer and Noctor 2005).
Biosynthesis of GSH (γ-Glu-Cys-Gly; GSH) occurs in two steps: first, the synthesis of g-glutamylcysteine from l-glutamate, catalysed by g-glutamylcysteine synthetase (g-ECS), and then the addition of glycine to the C-terminus of g-glutamylcysteine to produce GSH catalysed by glutathione synthetase (GSHS) (Hell and Bergmann 1990).
Recent report demonstrated that in salt-tolerant tomato, enhanced ROS production is observed upon salt exposure when GSH synthesis is inhibited (Mittova et al. 2003). In this sense, salt tolerance in plants has been associated with their capability to synthesized GSH (Nazar et al. 2011).
Being cysteine, the donor of reduced sulphur (S) in the synthesis of GSH, an increased demand of S assimilation into cysteine is expected when environmental conditions induce increased synthesis of GSH. Recent studies indicate a potential role of the S nutrition on salt stress in plants. Ruiz and Blumwald (2002) reported that S assimilation rate and biosynthesis of cysteine and GSH greatly increased when Brassica plants were exposed to saline conditions. Furthermore, changes in S assimilation enzymes have been reported as a consequence of plant exposure to salinity stress (Lopez-Berenguera et al. 2007; Koprivova et al. 2008; Koprivova and Kopriva 2008; Khan et al. 2009a, b).
However, a better knowledge concerning the mechanisms of interaction between plant S nutritional status and salinity stress tolerance could provide information to estimate the impact of climate change on plant growth and yield. In addition, over recent years, S deficiency is becoming widespread in many regions in the world. Occurrence of S deficiency has been described in cereals as well as in other crops. The reasons behind this trend are mainly the strong decrease in the inputs of S from atmospheric deposition and the use of low-S fertilizers (Zhao et al. 1999).
In this study, barley plants grown in the presence of different S application rate were exposed to 100 mM NaCl to evaluate whether the requirement of S may be higher when plants are under salt stress and, on the other hand, plant responses to salinity are modified by S supply.
Barley is one of the world’s most extensively cultivated crops and best adapted to salt stress (Epstein et al. 1980), therefore it could represent an important alternative crop for agricultural use of the salt-affected environments.
In particular, we measured differences in growth parameters (shoot and root fresh weight, chlorophyll and protein content), sulphate and thiols accumulation and lipid peroxidation. Furthermore, we report about changes in extractable activities of ATPS, the first enzyme in S assimilation pathway and the key enzyme for cysteine biosynthesis (Pilon-Smits et al. 1999).
Materials and methods
Growing conditions
Barley (Hordeum vulgare L. cv. Europe) seeds were germinated in moistened paper in the dark at 20 °C for 4 days. Seedlings were then transferred to plastic pots (12 seedlings per pot) filled with 2.2 l of continuously aerated nutrient solutions (NS) (Zhang et al. 1991) with or without 1.2 mM sulphate. Previous experiments on barley plants (Astolfi et al. 2006) indicated that the amount of sulphate used here (1.2 mM) could be considered as sufficient S supply. The S-deficient NS was prepared by replacing sulphate salts (K+, Mn2+, Zn2+, Cu2+) with appropriate amounts of chloride salts (K+, Mn2+, Zn2+, Cu2+). Plants were grown in a climate chamber under 300 μmol m−2 s−1 PAR at leaf level and 14 h/10 h day/night regime (temperature 27 °C diurnal; 20 °C nocturnal; relative humidity 80 %). After 10 days from sowing, NaCl (final concentration 100 mM) was added to the NS and the plants were harvested 4 days after salt application. Seedlings were divided into root and shoot and then quickly frozen in N2 and stored at −80 °C until used.
Enzyme extraction and assays
Frozen tissue (ca 1 g of fresh weight) was ground to a fine powder in a pre-chilled mortar under liquid N2. Cold extraction buffer containing 50 mM HEPES–KOH (pH 7. 4), 5 mM MgCl2, 1 mM EDTA, 10 % (v/v) glycerol, 0.1 % (v/v) Triton X-100, 5 mM DTT, 1 mM PMSF and 1 % (w/v) PVP was added in a ratio of 1:7 (w/v). The brei was filtered through four layers of cheesecloth and the homogenate was centrifuged at 1,000×g for 5 min at 4 °C. The resulting supernatant was desalted at 4 °C on a Sephadex G-25 column (PD-10, Pharmacia, Uppsala, Sweden) pre-equilibrated with extraction buffer minus Triton X-100. The desalted extract was then centrifuged at 30,000g for 5 min at 4 °C. The supernatant was divided into 300 μl aliquots which were then frozen in liquid N2 and stored at −80 °C until analysis.
ATP sulphurylase (ATPS; EC 2.7.7.4) activity was assayed by the bioluminescence technique (Schmutz and Brunold 1982). The ATP production during the enzyme reaction is coupled to the light producing reaction catalysed by firefly luciferase (E.C. 1.13.12.7). The reaction mixture contained in a total volume of 0.25 ml: 16 mM Tris–acetate buffer pH 7.75, 8 μM APS, 68 μM Na4P2O7, 40 μl of firefly luciferase (ATP Monitoring Reagent, ThermoLabSystems) and 5 μl of sample. Light emission was measured with LKB 1250 luminometer.
Non-protein thiols content
Water soluble non-protein sulphydryl (SH) compounds were determined colorimetrically with DTNB following the procedure described in Astolfi et al. (2004). Briefly, roots were homogenized in a solution containing 80 mM TCA acid, 1 mM EDTA acid, 0.15 % (w/v) ascorbic acid and 10 % (w/v) PVPP using 3 ml buffer per gram fresh weight leaves. The DTNB-reactive compounds were measured spectrophotometrically at 415 nm.
Determination of malondialdehyde content
The level of lipid peroxidation was expressed as malondialdehyde (MDA) content and was determined as TBA-reactive metabolites as described in Astolfi et al. (2005). Plant fresh tissues (0.2 g) were homogenized in 10 ml of 0.25 % TBA made in 10 % TCA. Extract was heated at 95 °C for 30 min and then quickly cooled on ice. After centrifugation at 10,000×g for 10 min, the absorbance of the supernatant was measured at 532 nm. Correction of non-specific turbidity was made by subtracting the absorbance value taken at 600 nm. The level of lipid peroxidation was expressed as μmol g−1 fresh weight using an extinction coefficient of 155 mM cm−1.
Other measurements and statistics
The concentration of chlorophyll content per unit area was estimated in attached leaves by a SPAD portable apparatus (Minolta Co., Osaka, Japan) using the first fully expanded leaf from the top of the plant.
Protein content was determined according to Bradford (1976) using BSA as standard.
To determine total S concentration, 1 g of each leaf or root sample was dried at 105 °C and then ashed in a muffle furnace at 600 °C. The ashes were dissolved in 10 mL of 3 N HCl and filtered through Whatman No. 42 paper. In contact with BaCl2, a BaSO4 precipitate is formed which is determined turbidimetrically (Bardsley and Lancaster 1962).
Each reported value represents the mean ± SD of data from four independent experiments on three measurements per experiment. Statistical analyses of data were carried out by ANOVA tests with the GraphPad InStat Program (version 3.06). Significant differences were established by posthoc comparisons (HSD test of Tukey) at P < 0.001, P < 0.01 or P < 0.05.
The relationship between ATPS activity and thiol concentration in the roots was calculated by linear regression analysis with the GraphPad InStat Program (version 3.06).
Results
At harvest, barley plants showed typical S starvation symptoms such as reduced plant fresh weight (Fig. 1) and decreased chlorophyll content (Fig. 2). On the other hand, salt treatment did not result in significant decreases in dry matter production of all barley plant parts (data not shown).
Shoot (a) and root (b) fresh weight of S-sufficient and S-deficient barley plants treated for 4 days with (salt) or without (control) 100 mM NaCl. Inset: root to shoot ratio. Data are mean ± SD of four independent replications run in triplicate. Different letters over the bars indicate significant differences (P < 0.01) (n = 4)
Figure 1 illustrates that plants grown under S starvation (−S) showed a significant decrease in the shoot fresh weight for an amount of 50 % in both control and salinity condition. Salt also inhibited the growth of barley shoots by 40 % at both +S and −S condition, compared to control (Fig. 1a). Noteworthy that neither S-deficiency nor salt application induced significant changes in root growth (Fig. 1b) and as a consequence root/shoot ratio was raised in both −S and salt-treated plants as compared to +S plants (inset in Fig. 1).
The chlorophyll content was determined by chlorophyll meter readings (SPAD) and relative data are showed in Fig. 2. Young developing leaves from 14-day-old barley plants were grown without sulphate in NS exhibited visible chlorosis (25 % lower SPAD units). With regard to salt treatment, no significant change in chlorophyll content was observed in leaves from +S salt-treated plants when compared to control (Fig. 2), whereas severe leaf chlorosis occurred in −S salt-treated plants with 30 % decrease in SPAD readings, compared to −S control (Fig. 2).
We demonstrated that imposed changes in S application rate were closely related to root protein concentration as evidenced by data in Fig. 3. There was a lower accumulation of protein in roots on exposure of barley seedlings to S starvation (30 and 35 % lower in control and salt-treated plants, respectively). We further found that salt-stressed barley seedlings showed a lower protein concentration than control ones (−15 %) in S-deficiency condition, while no significant difference in protein concentration was observed between control and salt-treated plants at optimal S supply (Fig. 3).
Protein concentrations in roots of S-sufficient and S-deficient barley plants treated for 4 days with (salt) or without (control) 100 mM NaCl. Data are mean ± SD of four independent replications run in triplicate. Statistics as in Fig. 2
Analysis of the root mineral composition showed that the S concentration in −S plants was very low compared to +S plants (−80 % in both control and salt-treated roots) (Fig. 4a). On the other hand, root S concentration remained unchanged following exposure to salt stress (Fig. 4a).
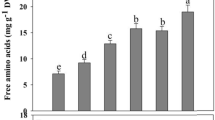
Total sulphur (a) and non-protein thiol (b) concentrations in roots of S-sufficient and S-deficient barley plants treated for 4 days with (salt) or without (control) 100 mM NaCl. Data are mean ± SD of four independent replications run in triplicate. Statistics as in Fig. 1
S starvation decreased by 30 % non-protein thiol concentration in both control and salt-treated roots (Fig. 4b). On the other hand, salt treatment favoured thiol accumulation into roots by 30 % in +S and by 40 % in −S plants over 4 days (Fig. 4b).
In order to evaluate if changes in the level of thiols required an adjustment in plant S assimilation capacity, we measured changes in ATPS activity, the first enzyme of S assimilation pathway (Fig. 5). ATPS activity increased in response to S starvation in both control and salt-treated roots (+25 and +70 %, respectively); on the other hand, salt stress significantly reduced ATPS activity. In particular, in plants under the optimal S dosage, NaCl treatment severely decreased the root ATPS activity (−40 % of the control), whereas in S starved plants induced, however, a decrease in enzyme activity but to a lesser extent (−20 % of the control) (Fig. 5).
Changes in ATPS (nmol ATP min−1 mg−1 prot) activity in roots of S-sufficient and S-deficient barley plants treated for 4 days with (salt) or without (control) 100 mM NaCl. Data are mean ± SD of four independent replications run in triplicate. Statistics as in Fig. 2
Oxidative stress due to the presence of NaCl in the nutrient solution could generally be demonstrated by enhanced MDA content. As shown in Fig. 6, salt exposure increased MDA levels (+55 %) in the roots of barley seedlings grown in −S condition, while it is noteworthy that MDA levels were similar in control and salt-treated roots from plants in +S condition (Fig. 6).
MDA levels in roots of S-sufficient and S-deficient barley plants treated for 4 days with (salt) or without (control) 100 mM NaCl. Data are mean ± SD of four independent replications run in triplicate. Statistics as in Fig. 2
Discussion
In the last few years, sulphur availability in soil has declined dramatically in many areas of western Europe (Zhao et al. 1999) mainly due to reduction of SO2 emissions to the atmosphere and changes in fertilizer practices. On the other hand, soil salinity is one of the main factors which affects crop productivity and is increasing progressively all around the world (Manchanda and Garg 2008). In this way, it is reasonable to suppose that plants could be exposed to sulphur deficiency and salt stress at the same time and at the same place. As a consequence, plant responses are more complicated and it often hinders to find the solution to stress symptoms.
In the present study, we aimed to characterise barley response to salt exposure at adequate and limited S availability.
The adverse effect of salt on growth results from the reduction in shoot biomass of treated barley plants (Fig. 1). The degree of salt-induced growth inhibition was generally similar in plants from both S conditions; however, considering the increase in root to shoot ratio as a stress index, S-deficient plants was shown to be more susceptible than those S-sufficient to salt ions, at least within the first 4 days of treatment (inset in Fig. 1). The increase in the root to shoot ratio is considered to be a classical response to S supply, as extensively reported in the literature (Robinson 1994).
Chlorophyll content is frequently used to evaluate stress-induced effects on plants photosynthetic efficiency. As expected, S-deficient condition significantly reduced the SPAD values in barley leaves (Fig. 2). Furthermore, in −S plants, the addition of 100 mM NaCl resulted in the lowest SPAD value compared to all other treatments, most likely due to excess ROS generation and oxidative stress (Kato and Shimizu 1985).
Optimal plant growth requires an adequate S supply for protein production (Zhao et al. 1999), and a decrease in this amount is the most likely cause of protein synthesis decline in the −S plants (Fig. 3). On the other hand, root protein concentration of barley plants seems to be less or not affected by the NaCl treatment as has been previously demonstrated (Dluzniewska et al. 2007).
It is well known that salinity triggering oxidative stress in plant tissues induces the production of ROS (Foyer and Noctor 2003). Plants are able to scavenge/detoxify ROS by producing different types of antioxidants, which include glutathione (Noctor and Foyer 1998).
Glutathione (γ-Glu-Cys-Gly; GSH) is a major reservoir of non-protein reduced S and a multifunctional metabolite in plants. Its synthesis is assumed to be linked to the provision of cysteine and thus it is regulated by the S supply of the plants. Therefore, we first measured in root the content of total sulphate, which is required for the biosynthesis of thiol compounds through S assimilation pathway, and of total non-protein thiols, which in its turn reflects the GSH production.
It was found that exposure of barley seedlings to 100 mM NaCl significantly increased the production of the non-protein thiols in roots (Fig. 4b), but total sulphur contents were not affected by the treatment (Fig. 4a).
As salt-stress affects root thiols content, one might expect salinity to affect the rate of S assimilation. Thus, it was investigated the influence of salt stress on S metabolism through evaluation of changes in ATPS activity, the first enzyme of S assimilation pathway (Fig. 5). Sulphur is available to plants mainly in the form of sulphate from the soil. Sulphur, taken up mainly as sulphate, is transported to the shoot via xylem transport. There sulphate can be reduced in a light-dependent reaction and can be used for protein synthesis and other S metabolites. Increased activities of ATPS in response to S starvation have been extensively reported (Saito 2000; Davidian and Kopriva 2010). Also in our experimental conditions, ATPS activity was inversely related to the S supply and it is also worthy of note that the highest effect of S starvation on induction percentage was found in salt-treated roots as compared to their respective untreated controls (70 vs. 25 %). On the other hand, ATPS activity showed to be modulated also by salt exposure: enzyme activity was reduced already in the first 4 days after salt addition to nutrient solution and the reduction was more pronounced in S-sufficient plants as compared to their respective untreated controls (40 vs. 15 %).
The reported effect of salinity stress on ATPS activity varies in the literature, including increases (Khan et al. 2009a, b) and decreases (Fei et al. 2008) in enzyme activity in response to salt treatment. It was previously shown (Fig. 4b) that salt treatment determined a significant rise in thiols content and most likely increased amounts of reduced S-containing compounds might act negatively on ATPS activity. This is in agreement with data reported by other authors (Reuveny et al. 1980; Haller et al. 1986; Hesse et al. 2004; Zuchi et al. 2012), who found that ATPS activity is repressed by availability of readily assimilated S sources. However, as the level of thiols decreased, ATPS activity progressively increased, as evidenced by the inversely linear relationship found for enzyme activity and thiol concentration in the roots (R = −0.977 with P < 0.05, as by linear regression analysis) (Fig. 7).
Since thiol levels are more abundant in S-sufficient plants than in those S-deficient, it is reasonable to expect that in these plants the effects of ROS are less deleterious and also that S-sufficient plants are more able to remove the harmful effects of high salinity. The comparison of MDA levels between +S and −S salt-treated plants strongly supports this idea (Fig. 6), and even allows to finally recognize a major role for S in alleviating salinity-induced oxidative damage.
In conclusion, in salt-treated plants, an adequate S supply allows adequate GSH synthesis (high-thiol concentration), thus avoiding the effects of ROS on photosynthetic functions (no effect on both chlorophyll and protein content), whereas in S-deficient plants, salt stress leads to excess ROS production that induces stress and plants showed reduction of photosynthetic efficiency (loss of chlorophyll and protein contents). However, NaCl-induced increase in thiol concentration, as earlier suggested, could as well be responsible for the observed reduction in ATPS activity in salt-treated roots.
As the salt-affected areas are expected to increase substantially in subsequent years, the impact of salinity on plant growth and yield is likely to increase and our findings could provide the opportunity to develop an agronomic practice to reduce the negative impact of soil salinization. Our results support the view that an adequate S nutrition can contribute to protect plants against salinity by alleviating the salt induced oxidative stress.
Author contribution
SA participated in the project’s design, data analysis and manuscript writing and revision. SZ made a substantial contribution to data collection and interpretation and manuscript drafting. All authors read and approved the final manuscript.
Abbreviations
- DTNB:
-
5,5′-Dithio-bis(2-nitrobenzoic acid)
- DW:
-
Dry weight
- FW:
-
Fresh weight
- EDTA:
-
Ethylene diamine tetraacetic acid
- NS:
-
Nutrient solution
References
Astolfi S, Zuchi S, Cesco S, Varanini Z, Pinton R (2004) Influence of iron nutrition on sulphur uptake and metabolism in maize (Zea mays L.) eoots. Soil Sci Plant Nutr 50(7):1079–1083
Astolfi S, Zuchi S, Passera C (2005) Effect of cadmium on H+ATPase activity of plasma membrane vesicles isolated from roots of different S-supplied maize (Zea mays L.) plants. Plant Sci 169:361–368. doi:10.1016/j.plantsci.2005.03.025
Astolfi S, Zuchi S, Cesco S, Sanità di Toppi L, Pirazzi D, Badiani M, Varanini Z, Pinton R (2006) Fe deficiency induces sulphate uptake and modulates redistribution of reduced sulphur pool in barley plants. Func Plant Biol 33:1055–1061
Bardsley CE, Lancaster JD (1962) Determination of reserve sulphur and soluble sulphate in soils. Soil Sci Soc Am Proc 24:265–268
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
Davidian JC, Kopriva S (2010) Regulation of sulfate uptake and assimilation—the same or not the same? Mol Plant 3(2):314–325
Dluzniewska P, Gessler A, Dietrich H, Schnitzler J-P, Teuber M, Rennenberg H (2007) Nitrogen uptake and metabolism in Populus × canescens as affected by salinity. New Phytol 173:279–293
Epstein E, Norlyn JD, Rush DW, Kingsbury RW (1980) Saline culture of crops: a genetic approach. Science 210:399–404
Fei G, YiJun Z, LingYun H, DaCheng HE, GenFa Z (2008) Proteomic analysis of long-term salinity stress responsive proteins in Thellungiella halophila leaves. Chinese Sci Bull 53:3530–3537
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Haller E, Suter M, Brunold C (1986) Regulation of ATP-sulfurylase and adenosine 5′-phosphosulfate sulfotransferase by the sulfur and the nitrogen source in heterotrophic cell suspension cultures of Paul’s scarlet rose. J Plant Physiol 125:275–286
Hell R, Bergmann L (1990) γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta 180:603–612
Hesse H, Nikiforova V, Gakiere B, Hoefgen R (2004) Molecular analysis and control of cysteine biosynthesis: integration of nitrogen and sulphur metabolism. J Exp Bot 55(401):1283–1292
Kato M, Shimizu S (1985) Chlorophyll metabolism in higher plants VI. Involvement of peroxidase in chlorophyll degradation. Plant Cell Physiol 26:1291–1301
Khan NA, Anjum NA, Nazar R, Iqbal N (2009a) Increased activity of ATPsulfurylase, contents of cysteine and glutathione reduce high cadmium-induced oxidative stress in high photosynthetic potential mustard (Brassica juncea L.) cultivar. Russ J Plant Physiol 56:670–677
Khan NA, Nazar R, Anjum NA (2009b) Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci Hort 122:455–460
Koprivova A, Kopriva S (2008) Lessons from investigation of regulation of APS reductase by salt stress. Plant Signal Behav 8:567–569
Koprivova A, North KA, Kopriva S (2008) Complex signaling network in regulation of adenosine 5-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol 146:1408–1420
Lopez-Berenguera C, Carvajala M, Garcea-Viguerab C, Alcaraz CF (2007) Nitrogen, phosphorus, and sulfur nutrition in Broccoli plants grown under salinity. J Plant Nutr 30:1855–1870
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Manchanda G, Garg N (2008) Salinity and its effect on the functional biology of legumes. Acta Physiol Plant 30:595–618
Miller G, Suzuki N, Ciftci-Yilmazi N, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittova V, Theodoulou FL, Kiddle G, Gómez L, Volokita M, Tal M, Foyer C, Guy M (2003) Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett 554:417–421
Nazar R, Iqbal N, Syeed S, Khan NA (2011) Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol 168:807–815
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Pilon-Smits EAH, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N (1999) Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction and tolerance. Plant Physiol 119:123–132
Rennenberg H, Brunold C (1994) Significance of glutathione in plants under stress. Progr Bot 55:142–156
Reuveny Z, Dougall DK, Trinity PM (1980) Regulatory coupling of nitrate and sulfate assimilation pathways in cultured tobacco cells. Proc Natl Acad Sci USA 77:6670–6672
Robinson D (1994) The responses of plants to non-uniform supplies of nutrients. New Phytol 127:635–674
Ruiz JM, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Saito K (2000) Regulation of sulphate transport and synthesis of sulphur-containing amino acids. Curr Opin Plant Biol 3:188–195
Schmutz D, Brunold C (1982) Rapid and simple measurement of ATP sulphurylase activity in crude plant extracts using an ATP meter for bioluminescence determination. Anal Biochem 121:151–155
Zhang FS, Römheld V, Marschner H (1991) Role of the root apoplasm for iron acquisition by wheat plants. Plant Physiol 97:1302–1305
Zhao FJ, Hawkesford MJ, McGrath SP (1999) Sulphur assimilation and effects on yield and quality of wheat. J Cereal Sci 30:1–17
Zhu J, Fu X, Koo YD, Zhu JK et al (2007) An enhancer mutant of Arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol Cell Biol 27:5214–5224
Zuchi S, Cesco S, Astolfi S (2012) High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Env Exp Bot 77:25–32. doi:10.1016/j.envexpbot.2011.11.001
Acknowledgments
Research was financially supported by grants from Italian M.I.U.R.-PRIN 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Astolfi, S., Zuchi, S. Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol Plant 35, 175–181 (2013). https://doi.org/10.1007/s11738-012-1060-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1060-5