Abstract
Nitric oxide (NO), a non-charged, small, gaseous free-radical, is a signaling molecule in all plant cells. Several studies have proposed multifarious physiological roles for NO, from seed germination to plant maturation and senescence. Nitric oxide is thought to act as an antioxidant, quenching ROS during oxidative stress and reducing lipid peroxidation. NO also mediates photosynthesis and stomatal conductance and regulates programmed cell death, thus providing tolerance to abiotic stress. In mitochondria, NO participates in the electron transport pathway. Nitric oxide synthase and nitrate reductase are the key enzymes involved in NO-biosynthesis in aerobic plants, but non-enzymatic pathways have been reported as well. Nitric oxide can interact with a broad range of molecules, leading to the modification of protein activity, GSH biosynthesis, S-nitrosylation, peroxynitrite formation, proline accumulation, etc., to sustain stress tolerance. In addition to these interactions, NO interacts with fatty acids to form nitro-fatty acids as signals for antioxidant defense. Polyamines and NO interact positively to increase polyamine content and activity. A large number of genes are reprogrammed by NO; among these genes, proline metabolism genes are upregulated. Exogenous NO application is also shown to be involved in salinity tolerance and/or resistance via growth promotion, reversing oxidative damage and maintaining ion homeostasis. This review highlights NO-mediated salinity-stress tolerance in plants, including NO biosynthesis, regulation, and signaling. Nitric oxide-mediated ROS metabolism, antioxidant defense, and gene expression and the interactions of NO with other bioactive molecules are also discussed. We conclude the review with a discussion of unsolved issues and suggestions for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, approximately 930 million hectares of arable land, representing more than 6% of the world’s total land area, are subjected to salt stress, and this percentage is increasing daily with climate change due to natural and anthropogenic activity (Munns and Tester 2008; Setia et al. 2013). At the physiological level, salt stress triggers osmotic stress and ionic stress. Salt-induced osmotic stress interrupts plant–water relations and hampers cell expansion and division. Due to ionic toxicity, nutrient homeostasis is disrupted, which leads to other stress factors associated with cellular functioning. Thus, plants under salt stress are affected in germination, growth, and developmental stages. Plant productivity with respect to biological or reproductive yield is diminished significantly under salt stress. Severe salt stress can cause plant death (Munns and Tester 2008). In a salty environment, plants cannot take up water due to osmotic stress; the dehydration stress perturbs stomatal conductance and the functioning of photosynthetic enzymes. This anomalous behavior leads to exacerbated ionic toxicity and nutrient imbalance in salt-affected plants. These physiological alterations lead to overproduction of reactive oxygen species (ROS), which severely damage cell membranes and other cellular components via oxidation of proteins and peroxidation of lipids. Nuclear material carrying genetic information, i.e., DNA, can be structurally altered due to this oxidative stress. Thus, oxidative stress has severe adverse effects in salt-affected plants (Hasanuzzaman et al. 2013a, b, c). Plants with antioxidant defense systems can combat ROS generation to an extent. This strategy is a good defense under normal growth conditions, but under conditions of stress, including salt stress, ROS are overproduced and cannot be overcome by the antioxidant defense system of the plant. Plants that can enhance the antioxidant defense system under conditions of stress are tolerant to ROS (Munns and Tester 2008; Hasanuzzaman et al. 2013a, b; Ahmad et al. 2016).
Nitric oxide (NO) is a gaseous, free-radical, redox-signaling molecule with diverse functions. NO acts as a messenger in various physiological processes and has been reported to affect plant responses to salt stress, excess light, low temperatures, high temperatures, ozone, UV-B, toxic-metal stress and various pathogens (Gaupels et al. 2011; Hasanuzzaman et al. 2012; Gill et al. 2013; Mur et al. 2013; Nahar et al. 2016). In salt-affected plants, seed vigor and germination are improved by NO (Hayat et al. 2012). Nitric oxide alleviates oxidative damage, improves the antioxidant defense mechanism, decreases methylglyoxal toxicity, and upregulates the glyoxalase system in salt-affected wheat plants (Hasanuzzaman et al. 2011). By adjusting the levels of osmolytes and antioxidant enzymes, NO alleviated salt-induced damage in chickpea plants (Ahmad et al. 2016). In salt-affected Bermuda grass, sodium nitroprusside (SNP, an NO donor) assuaged the damaging effects of NO on plant growth, ionic balance (K+:Na+, Mg2+:Na+, and Ca2+:Na+ ratios), water content, and chlorophyll (chl) content, and higher chl a fluorescence curves were observed. Additionally, decreased levels of electrolyte leakage, malondialdehyde (MDA), and hydrogen peroxide (H2O2) and improved antioxidant enzyme activities were observed (Liu et al. 2016).
Nitric oxide rapidly reacts and interacts with other radicals/ROS, phytohormones, and signaling molecules. NO can react with pro- and antioxidants (Hill et al. 2010). There is cross-talk between NO and other molecules that regulate vital physiological processes. Calcium (Ca)-calmodulin and NO in abscisic acid (ABA) and H2O2 signaling modulate antioxidant defense components in maize leaves (Sang et al. 2008). Interactions between ABA, H2O2, NO, and mitogen-activated protein kinase (MAPK)- induced stomatal closure and antioxidant defense in plants for survival under conditions of water stress (Hao and Zhang 2010). S-Nitrosoglutathione (GSNO), which is generated by the S-nitrosylation reaction of NO with glutathione, plays regulatory roles in the maintenance of the trans-nitrosation equilibrium between GSNO and S-nitrosylated proteins (Cheng et al. 2017). GSNO reductase (GSNOR) plays a regulatory role in post-translational modifications of cysteine residues (Tichá et al. 2017). GSNOR participates in activation of the antioxidant system, regulation of ROS and reactive nitrogen species (RNS) generation, the cellular redox status, stimulation of stress-responsive genes, and stress-induced programmed cell death (PCD) (Cheng et al. 2017; Tichá et al. 2017). All these features make NO a powerful molecule, the diverse roles of which are revealed daily in this era of complex environmental stress. In this review, NO-induced modulation of ROS metabolism and the signaling role and molecular interactions of NO with other molecules are discussed with respect to the development of salt tolerance in plants.
Salt stress in plants: a brief overview
Salinity is one of the most devastating abiotic stress factors in agriculture worldwide and is anticipated to be drastically enhanced by global climate change (da Silva et al. 2017; Reddy et al. 2017). Nearly 7% of the land in the world has high salt content, and approximately 20% of the cultivated land and 33% of the irrigated land area in the world are salt-affected and degraded (Kibria et al. 2017, Machado and; Serralheiro et al. 2017). Moreover, roughly three hectares of arable land is affected by soil salinization per minute, which leads to a 10% increase in salinity-affected area annually (Shrivastava and Kumar 2015; Bose et al. 2017). The most common soluble salts in soil are sodium chloride (NaCl) and sodium sulfate (Na2SO4). In addition, soil contains a considerable amount of calcium sulfate (CaSO4), magnesium sulfate (MgSO4), potassium nitrate (KNO3), sodium bicarbonate (NaHCO3), etc. However, most of these salts are not fully soluble in water (Hasanuzzaman et al. 2013a). Therefore, most widespread causes of soil salinity are Na+ and Cl− ions. Physiologically, salinity is considered one of the most limiting factors for plant growth and development due to the multifarious negative effects of salinity, including specific ion toxicity, osmotic stress, and oxidative damage in plants (Munns et al. 2006; Bose et al. 2017). Ion toxicity occurs due to disequilibrium in ion homeostasis. Salt in plant growth medium increases the Na+ levels, which competes with K+ uptake. Therefore, Na+ uptake increases, which causes K+ efflux and triggers K+ leakage from plant cells, ultimately resulting in K+ deficiency in the cytosol (Chokshi et al. 2017). Furthermore, Na+ replaces Ca2+ from the cell membrane, which also enhances the intracellular Na+ content. Consequently, during salinization, the Na+ content destroys the nutrient balance, particularly the Na+/K+ ratio (Rahman et al. 2016). The incidence of high salinity in soil or in any plant-growth medium decreases the osmotic potential, which reduces water uptake and even forces water out of the cell, resulting in closure of the stomata (Shabala and Cuin 2007; Chokshi et al. 2017; Rajput et al. 2017). Both ion toxicity and osmotic stress ultimately hinder the plant photosystem and lead to excess ROS and methylglyoxal (MG) production (Hasanuzzaman et al. 2017a). In addition to overproduction of ROS [singlet oxygens (1O2), superoxide anions (O2•−), H2O2, and hydroxyl radicals (OH•)] and MG in a plant cell, salt-stress perturbs the antioxidant defense and glyoxalase system, which leads to oxidative stress (Hasanuzzaman et al. 2013a, 2017a). On the other hand, Cl− is more dangerous than Na+ and causes many physiological disorders in plants. In addition to destroying nutrient balance, Cl− can induce chlorotic toxicity symptoms in plants due to impaired production of chl (Tavakkoli et al. 2010; Hasanuzzaman et al. 2013a).
Overall, major physiological and biochemical processes of plants, including photosynthesis, protein synthesis, and respiration, are perturbed upon the initiation of salt stress, which ultimately leads to growth inhibition or death of the plant (Fig. 1; Rahman et al. 2017). Germination, the most important phase of the plant life cycle, is severely hampered by salt stress (Hasanuzzaman et al. 2013a). Important processes involved in germination, such as imbibition, activation of different metabolic pathways, emergence of embryonic tissue, and seedling establishment, are severely affected by salinity (Hasanuzzaman et al. 2013b; Ibrahim 2016; Rahman et al. 2017). Rahman et al. (2016) experimented with rice plants and observed that salt stress caused water shortage and chlorosis in the plant, which ultimately reduced plant growth. They also reported that salt-induced stress perturbed ion homeostasis via Na+ influx and K+ efflux. Moreover, salt-induced stress reduced the uptake of different nutrients that were essential for the plant. Increased lipid peroxidation and loss of plasma membrane integrity were observed in salt-stressed rice plants due to over-generation of ROS and MG along with alteration of antioxidant defense and glyoxalase systems. Similar growth reductions via salt-induced oxidative stress were observed in many plants by several groups of scientists (Kibria et al. 2017; Chokshi et al. 2017). Salinity not only hinders germination and plant growth, but also reduces the yield and adversely affects yield-associated parameters and grain quality (Rahman et al. 2017; Reddy et al. 2017). Stavridou et al. (2017) observed that the biomass yield of bioenergy grass was notably hampered by salinity. Therefore, it is evident that salt-induced stress greatly hinders the growth and yield of plants by destroying the nutrient status of the plant-growth medium and perturbing the physiology and metabolism of the plant.
Nitric oxide biosynthesis in plants and its regulation
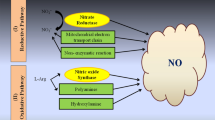
Since NO research began in animal systems, the biosynthetic pathway of NO in animals is well understood. Research in different plant species at different times has demonstrated various possible pathways of NO biosynthesis. Different researchers have shown NO-biosynthesis pathways in different plant species. There is no common or clear description of NO biosynthesis, which is why, in this review, we have attempted to summarize the information from available literature. An overview of the potential routes of plant NO production has been presented in Fig. 2. The nitrogen cycle represents the atmospheric nitrogen (N) that is incorporated via fixation (fixed by lightning strikes and symbiotic bacteria) and is then assimilated. Following ammonification, nitrification, and denitrification, the N is returned to the environment. Nitrification converts ammonia to nitrites (NO2−), and nitrites (NO2−) are oxidized to nitrates (NO3−) (Ghaly and Ramakrishnan 2015). Nitric oxide production may occur via enzymatic and non-enzymatic pathways. Oxidation of l-arginine via an NADPH-dependent pathway leads to the biosynthesis of NO. Here, NO synthase (NOS) is a catalytic agent (Foresi et al. 2010). Nitrite is converted to nitrate via nitrate reductase (NR) activity, and nitrite is subsequently reduced to NO by NR. Nitrite can be converted to NO via the mitochondrial electron transport chain (Yamasaki et al. 1999; Gupta et al. 2011). Xanthine oxidoreductase (XOR) has been reported to produce NO from nitrite using xanthine (Harrison 2002). Nitric oxide can be produced from hydroxylamine (HA) or salicylhydroxamate (SHAM). Production of NO was decreased by anoxia or by the addition of catalase (CAT), but was increased by conditions that induced reactive oxygen (ROS) or by the addition of H2O2. Addition of superoxide dismutase (SOD) stimulates H2O2 production and ultimately stimulates NO production (Rümer et al. 2009). Reduction of NO2 to NO by carotenoids can occur at low pH in the absence of enzymes (Crawford 2006). Phenolic compounds such as catechin can also reduce NO2 to NO (Bethke et al. 2004). Nitric oxide can be generated from N2O via oxidation (Schreiber et al. 2012). Polyamines have also been shown to be a source of NO, but the complete pathway has not been elucidated (Tun et al. 2006). When oxygen is available in normal conditions, NO biosynthesis occurs in peroxisomes, chloroplasts, and mitochondria (Wulff et al. 2009; Galatro et al. 2013; Corpas and Barroso 2014). In hypoxic or anoxic conditions, nitrite is reduced to NO by non-symbiotic hemoglobins, deoxy-heme proteins, and molybdo-cofactors or mitochondrial cytochrome c oxidase (Gupta et al. 2011). Further research is required to obtain a clear understanding of the NO biosynthesis pathway.
Nitric oxide improves plant physiology under salt-induced stress
Nitric oxide plays key roles in various physiological mechanisms required for growth and development of the plants and in biotic and abiotic stress response (Fig. 3; Siddiqui et al. 2011; Hasanuzzaman et al. 2013c, 2017b; Zuccarelli et al. 2017). However, the information available on salt-stress tolerance is controversial to some extent, and salt-stress tolerance is dependent on crop species, seedling age, and duration and severity of stress treatment (Hasanuzzaman et al. 2013c).
Seed priming with NO from exogenous sources, such as SNP, showed a promising effect on the control of germination under NaCl-induced stress. Wheat seeds (Triticum aestivum L., cv. Huaimai 17) were reported to have increased germination rates and radical and coleoptile weight under saline conditions (300 mM NaCl) upon treatment with NO (0.1 mM SNP) for 20 h. The seed respiration rate also increased. Furthermore, K+ and Na+ ion homeostasis and degradation of stored starch to yield soluble sugars increased with NO pretreatment (Zheng et al. 2009; Marvasi 2017). Exogenous application of NO has been proven to be advantageous for increasing the dry mass and shoot and root lengths of NaCl-stressed plants (Kausar et al. 2013). Decreased leaf area and decreased plant dry-matter production due to salt stress can be alleviated with NO application in several crops (Fatma and Khan 2014).
Nitric oxide improves photosynthetic capacity by protecting photosynthetic pigments in leaves. Enhancement of photosynthetic capacity following NO treatment was observed with quenching of additional energy and quantum-yield enhancement of photosystem II (PSII) in Solanum melongena (Wu et al. 2013). Under salt-induced stress, NO treatment enhanced the photosynthetic machinery in B. juncea (Fatma and Khan 2014). Fatma et al. (2016a) found that under conditions of sufficient sulfur (S) supply, NO improves photosynthesis in salt-stressed B. juncea plants. They also reported that NO and S enhance the normal shape of thylakoids and increase chloroplast size, which facilitates higher chl content in salt-stressed plants. In conditions of stress, NO increased the uptake and translocation of several macro- and micronutrients, such as K, Mg, Zn and Fe, which enhance chl biosynthesis and respiration (Wang et al. 2013; Kong et al. 2014; Dong et al. 2015). Additionally, NO affects stomatal regulation. Reports show that exogenous NO increases stomatal conductance and ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) activity, thereby enhancing photosynthesis under conditions of salt-induced stress (Fatma and Khan 2014). Stomatal conductance is closely associated with the osmotic status of plant cells. Under conditions of salt-induced stress, plants close their stomata to cope with physiological drought. Increased ABA accumulation triggers stomatal closure, while exogenous NO and S induce stomatal opening. Moreover, NO induces the influx and efflux of Ca2+, thereby regulating stomatal movement and ABA concentration in guard cells (Wang 2014; Fatma and Khan 2014). Exogenous application of NO has been reported to slow down electron transport and inhibit the process of nonphotochemical and photochemical quenching (Wodala et al. 2008; Wu et al. 2010). Thus, NO helps in efficient energy dissipation.
Nitric oxide was reported to maintain ion homeostasis in plants exposed to saline conditions. NO helps the plants to enhance K+ uptake while reducing Na+ uptake and using a portion of the Na+ surplus to resist against salinity. In this compartmentalization process, the Na+/H+ antiporter enzyme is involved in the removal of cytosolic Na+ (Chen et al. 2010). Nitric oxide triggers the vacuolar H+-ATPase, and H+-PPase, which forces the Na+/H+ ion exchange to detoxify the cell. Several researchers have reported increased H+-ATPase activity during Na+ compartmentalization. In Zea mays, for example, exogenous NO increased the activity of H+-ATPase, leading to a high K+/Na+ ratio in the cytosol and inducing high salt tolerance in plants (Zhang et al. 2006). Exogenous use of an NO donor (50 µM SNP) is also a promising strategy allowing plants to cope with salt-induced stress by reducing ion leakage (Marvasi 2017).
Nitric oxide regulates ROS metabolism and antioxidant defense during salt-induced stress
Nitric oxide can directly or indirectly act on a broad range of targets, leading to the attenuation of oxidative stress by upregulating non-enzymatic and enzymatic antioxidants (Table 1; Fig. 4; Christou et al. 2014; Ahmad et al. 2016; Kong et al. 2016; da Silva et al. 2017). Nitric oxide is also recognized as a secondary antioxidant because NO has antioxidant properties that can counteract abiotic stress-induced ROS either by direct scavenging or by triggering the activity of various ROS-scavenging enzymes (Siddiqui et al. 2011; Hasanuzzaman et al. 2013c; Arora et al. 2016). Furthermore, NO is recognized as a powerful inhibitor of ROS that cause lipid peroxidation and protein oxidation (Fancy et al. 2017). Salt-induced stress increased the endogenous NO content in plants, which prevented salinity-induced damage to a certain extent (Fatma et al. 2016b; da Silva et al. 2017). In fact, in the last few years, exogenous application of NO to induce salt-stress tolerance in different plant species has gained popularity. Fan et al. (2007) carried out an experiment with the Cucumis sativus plant under salt-induced stress (50 mM NaCl) in combination with 10–400 µM SNP and found that, in contrast to salt-induced stress, 50 µM SNP enhanced the activity of antioxidant enzymes, including SOD, CAT, guaiacol peroxidase (POD), and ascorbate peroxidase (APX), which helped reduce membrane permeability, MDA content, H2O2 content and O2•− generation. subsequently, Sheokand et al. (2008) reported that 0.2 mM SNP increases the APX and peroxidase (POX) activity of salt-stressed Cicer arietinum. Accordingly, the MDA content of and relative injury to the plant due to overproduction of ROS were decreased in the presence of NO. At the same time, various reports indicated the protective role of NO in salt-stress tolerance due to the upregulation of antioxidant activity in various plant species, such as Kosteletzkya virginica (Guo et al. 2009), T. aestivum L. (Zheng et al. 2009), and C. arietinum L. (Sheokand et al. 2010). Hasanuzzaman et al. (2011) grew T. aestivum in 300 mM NaCl with 1 mM SNP and observed that NO contributed to the upregulation of both enzymatic (monodehydroascorbate reductase, MDHAR; dehydroascorbate reductase, DHAR; glutathione reductase, GR; glutathione S-transferase, GST; glutathione peroxidase, GPX; and CAT) and non-enzymatic (glutathione, GSH; and ascorbate, AsA) antioxidants. As a result, salt-induced enhancement of MDA and H2O2 levels decreased significantly. Similar SNP-induced salt-stress tolerance due to the positive effects of NO on the antioxidant defense system and ROS metabolism was observed in Lycopersicon esculentum (Wu et al. 2011), Aegiceras corniculatum (Chen et al. 2014), B. juncea (Gupta et al. 2017) and P. sativum (Yadu et al. 2017). Egbichi et al. (2014) used 2,2′(hydroxynitrosohydrazono) bis-ethanimine (DETA) as another NO-generating compound to combat salt-induced stress. When they used 10 µM DETA with 80 mM NaCl, they found that NO helped to increase APX activity, which leads to decreased H2O2 content. Recently, several types of studies have been conducted by many groups of scientists to confirm the positive role of NO against salt-induced stress. Ahmad et al. (2016) used 50 µM S-nitroso-N-acetylpenicillamine (SNAP) as a NO source and found that SNAP increased the activities of SOD, CAT, APX, and GR in the C. arietinum plant, which reduced the H2O2 and MDA content and decreased electrolyte leakage. In addition to co- or pretreatment of seedlings, seed priming with NO is also effective against salt-induced stress. Ali et al. (2017) and Gadelha et al. (2017) primed test seeds with 0.1 mM and 75 µM SNP, respectively, and observed positive results against 150 and 100 mM NaCl, respectively. Therefore, both endogenous and exogenous NO (seed priming, pretreatment or cotreatment) can play a positive role in the mitigation of salt-induced oxidative stress to a certain extent, but the effect is dose dependent and varies from plant to plant.
Nitric oxide signaling under salt-induced stress
Signaling is the conversion of certain stimuli to a series of intracellular events. Signaling is accomplished in plant cells in three steps, namely, reception (binding of the signal molecule to a specific receptor), transduction (release of the second messenger into the cytosol) and induction (alteration of cellular process). Being a free-radical, NO is involved in many physiological functions under adverse environmental conditions, especially salt-induced stress. However, to play a signaling role, a molecule should be structurally simple and highly diffusive and should be present in small amounts and should directly influence second messengers; NO possesses all of these properties (Hasanuzzaman et al. 2013c; Arasimowicz and Floryszak-Wieczorek 2007). Like other free radicals, the action of NO at the cellular level depends on the NO concentration in the cell. If the concentration increased markedly, nitro-oxidative stress is induced, but at a lower concentration, NO acts as a signal molecule (Arora et al. 2016). NO is highly reactive due to the presence of an unpaired electron and can form a variety of intermediates (Hasanuzzaman et al. 2011). Under conditions of salt-induced stress, plant signaling cascades are activated, triggering specific defense mechanisms and the production of a signaling molecule that acts as a second messenger to defend against salinity (Hasanuzzaman et al. 2011; Fancy et al. 2017).
Nitric oxide can modulate a broad range of molecules, forming RNS and modifying protein activity, GSH biosynthesis, metal nitrosylation, tyrosine nitration/peroxynitrite formation, S-nitrosylation, S-glutathionylation, sulfhydration, etc., to sustain stress tolerance (Akter et al. 2015; Lamotte et al. 2015). Metal nitrosylation occurs when NO binds to transition metals in metalloproteins. The best example is the soluble guanylate cyclase enzyme, which undergoes such post-translational modifications (Arora et al. 2016). NO or the RNS target the cystine and tyrosine residues to modify the proteins by S-nitrosylation and nitration (Hasanuzzaman et al. 2013c). Nitration or tyrosine nitration are caused by the RNS peroxynitrite (ONOO−), generated from NO and O2•−. Previously, this nitration was thought to be harmful to plants, but studies have suggested that this nitration plays a signaling role. On the other hand, S-nitrosylation, the binding of NO to cysteine residues of target proteins, is the main process in signal transduction. Furthermore, S-nitrosylation can occur reversibly by trans-nitrosylation (Lamotte et al. 2015). Regardless of the mechanism of S-nitrosylation, these post-translational modifications (PTMs) have diverse roles in cellular processes and signaling cascades (Sevilla et al. 2015).
Nitric oxide signaling can be accomplished via inter- or intracellular interactions to generate the second messenger, such as, Ca2+, cGMP, cADP-ribose, phosphatidic acid, and ROS (Besson-Bard et al. 2009; Leitner et al. 2009). After the initial discovery of cGMP in Z. mays, this molecule was proven to be an intermediate in the NO-signaling pathway in plant and animal systems, increasing Ca2+ influx and reversible protein phosphorylation (Palavan-Unsal and Arisan 2009). Reports from different researchers have suggested that cGMP is a mediator of intracellular signaling, but to accomplishing this task, additional signals are necessary. For example, cGMP levels are increased by stress-induced ABA biosynthesis, which is essential for ABA-induced stomatal regulation (Hasanuzzaman et al. 2013c). Reports have also suggested that NO acts via cGMP and cADPR to alter Ca2+ channels an increase the Ca2+ content in the cytosol for various physiological functions under saline conditions (Arasimowicz and Floryszak-Wieczorek 2007). Increased cGMP levels can be lethal for plants and can cause cell destruction and PCD.
Both NO and NO-derived peroxynitrite combine with GSH to form GSNO with the involvement of the enzyme GSNO reductase (GSNOR), and the resulting GSNO further acts as a NO donor (Fancy et al. 2017). An important role played by GSNOR in plant cells under conditions of salt-induced stress is the limitation or alleviation of NO to combat stress (Hasanuzzaman et al. 2013c). Additionally, the stress is regulated by S-nitrosylation levels (Yu et al. 2014) and the absence of S-nitrosylation resulted in the accumulation of excess NO (Gong et al. 2014). Thus, GSNOR homeostasis is vital for stress tolerance (Corpas et al. 2013). The enzyme also catalyzed to formation of NH3 and GSSG from GSNO, which was further reduced by GR to form GSH (Leterrier et al. 2012) and is critical for regulating the downstream effects of RNS and ROS in plants (Xu et al. 2015).
Abiotic stress has been reported to boost nitrosative bursts (Camejo et al. 2013; Ziogas et al. 2013), changing the S-nitrosoproteome, which is integral for stress signaling (Tanou et al. 2009). The reaction of NO with protein thiols—S-nitrosylation—is important in many biological systems during abiotic stress (Romero-Puertas et al. 2013). Exposure to salt-induced stress, for example, results in the S-nitrosylation of proteins and enzymes associated with different physiological processes, such as respiration, photorespiration, and antioxidant activity (Camejo et al. 2013). Slight changes in S-nitrosylation have been reported in cultured Arabidopsis cell suspensions upon exposure to salinity (Fares et al. 2011). Additionally, both short-term and long-term salt-induced stress decrease S-nitrosylation (Ziogas et al. 2013), but long-term salinity was found to reduce S-nitrosylation to a greater extent (Camejo et al. 2013). Furthermore, S-nitrosylation has a potential role in ABA-dependent stomatal regulation in response to conditions of stress (Roychoudhury et al. 2013). Thus, NO-signaling improved photosynthetic efficiency by improving stomatal conductance and RuBisCO activity (Fatma and Khan 2014). Via nitration, NO also produces metal proteins with sulfhydryl and nitro groups to afford resistance in opposition to stress (Leterrier et al. 2012).
Redox balance is very important to cope with salinity and is maintained by the AsA–GSH cycle, which is very important for detoxification of H2O2 and toxic MG in plants. Reports have suggested that NO-signaling helps to regulate this cycle via S-nitrosylation. Under salt-induced stress, the activities of four vital enzymes of the AsA–GSH cycle, namely, APX, DHAR, MDHAR, and GR, are hampered (Hasanuzzaman et al. 2011; Rahman et al. 2016). However, NO was reported to modulate APX via S-nitrosylation under salt-induced stress and to increase APX activity to efficiently detoxify H2O2, leading to a strong antioxidative defense against salinity. In contrast, salinity leads to S-nitrosylation of MDHAR, which limits the activity of this enzyme (Begara-Morales et al. 2014; Yang et al. 2015).
Nitric oxide interacts with other signaling molecules to provide defense against salt-induced stress
Signaling networks in plants include a large number of receptors, messengers, transcription factors and enzymes that evolved to generating proper cellular responses (Memon and Durakovic 2014). Many biomolecules are involved in signal transduction in plants, such as plant hormones, ROS, RNS, MAPK, H2S, polyamines, plant nutrients, lipids, and peptides, and almost all of these biomolecules interact with NO (Fig. 5). For example, during salt-induced stress, plants are subjected to ion toxicity along with physiological drought, which leads to stomatal closure. Guard cells that regulate stomatal opening and closure are very sensitive to salt-induced stress; in other words, salt-induced stress alters the signaling components (ROS, NO, Ca2+) of guard cells. Therefore, interactions among these signaling molecules are vital for sustainable crop production.
Among signaling molecules, ROS and ROS intermediates are significant for signal transduction and participate actively during conditions of stress (Gill and Tuteja 2010). Among ROS, many researchers have suggested H2O2 to be a dynamic signaling molecule that participates in a variety of cellular responses and can induce several defense genes (Gill et al. 2016). In normal conditions, H2O2 can be generated in the cell from various sources. Experimental results have shown that there is a correlation between H2O2 generation and NO production (Delledonne et al. 2001). As NO is short-lived, it rapidly reacts with ROS to yield NO2, which is degraded to nitrate (Bellin et al. 2013). In the cell, H2O2 formation may occur via O2•− (Tanou et al. 2009). It is likely that NO reacts with O2•− to form highly reactive peroxynitrite anions (ONOO−), and subsequent cellular effects may then be induced by peroxynitrite (Bellin et al. 2013). However, under conditions of stress, H2O2 and NO can be generated simultaneously and can act together to accomplish many vital functions, such as cell death (Lin et al. 2012), senescence delay (Iakimova and Woltering 2015), root growth and development (Liao et al. 2011), and pollen-tube growth (Serrano et al. 2012). Moreover, these molecules interact closely with Ca2+ and ABA to form a complex signaling network to tolerate salinity (Huang et al. 2015; Shi et al. 2015, Niu and Laio 2016). Under salt-induced stress, NO interacts with Ca2+ and H2O2 to upregulating the Na+/H+ antiporter and reduce K+ efflux (Lang et al. 2014). When plants face physiological drought during salt-induced stress, NO induces increased cytosolic Ca2+ levels. This concentration gradient activates two channels (S-type and R-type) that release anions from the guard cell and cause depolarization. As a result, inward K+ channels are deactivated and outward K+ channels are activated. Therefore, there is a net loss in the K+ ion gradient, which causes a loss in the turgor pressure, leading to closure of the guard cells (Garcia-Mata et al. 2003). Therefore, it is clear that NO acts in Ca2+-mediated activation of cellular signaling (Palavan-Unsal and Arisan 2009; Gill et al. 2016).
Nitric oxide together with H2S upregulates several genes to increase H+-ATPase activity in the plasma membrane and to maintain a high K+/Na+ ratio in salt-stressed seedlings (Shi et al. 2014; de Silva et al. 2017). In this process, the Na+/H+ antiporter enzyme is also activated, which compartmentalizes and removes cytosolic Na+ (Chen et al. 2015). Hence, NO activates H2S to maintain redox homeostasis. In contrast, H2S hinders NO during stomatal regulation. Furthermore, NO induces reduction in total free PA, free putrescine (Put), spermidine (Spd), and polyamine oxidase (PAO) activity, as reported by Fan et al. (2007) in cucumber seedlings exposed to NaCl stress.
Interaction of nitric oxide (NO) with other signaling molecules in signal transduction pathways to provide a defense against salt-induced stress. AsA ascorbate, APX ascorbate peroxidase, AUX auxin, cADPR cyclic adenine dinucleotide phosphate ribose, cGMP cyclic guanosine monophosphate, DHA dehydroascorbate, DHAR dehydroascorbate reductase, ET ethylene, ABA abscisic acid, GTP guanosine triphosphate, GC guanylate cyclase, RNS reactive nitrogen species, ROS reactive oxygen species, GR glutathione reductase, GSH reduced glutathione, GSSG oxidized glutathione, GSNO S-nitrosoglutathione, GSNOR S-nitrosoglutathione reductase, JA jasmonates, MDA malondialdehyde, MDHAR monodehydroascorbate reductase, MAPKs mitogen-activated protein kinases, NADPH nicotinamide adenine dinucleotide phosphate (reduced), PCD programmed cell death, SA salicylic acid. Dotted lines represent activation/enhancement
In plant systems, NO also activates MAPK signaling pathways (Baudouin 2011). Controversial reports have suggested that H2O2 or NO stimulate the activation of a MAPK, which can also be activated by salicylic acid (SA), jasmonic acid (JA) and ethylene (ETH) (Kumar and Klessig 2000; Hasanuzzaman et al. 2013c). Therefore, it remains unknown whether NO activates MAPK by itself or with the help of other messengers (Wrzaczek et al. 2010; Liu and He 2017). It has been reported that a NO-mediated cGMP-independent pathway is involved in activation of the MAPK signaling cascade induced by indole acetic acid (IAA) during the process of adventitious root formation in cucurbits (Pagnussat et al. 2004). Then, another study reported that H2O2 and NO activate MAPK leaf tissue with increased antioxidant activity (Zhang et al. 2007). Reports have also suggested that MAPK cascades act upstream or downstream of cell organelles such as chloroplasts, mitochondria, or peroxisomes to regulate ROS homeostasis or ROS signaling (Liu and He 2017). It has been suggested that several defense mechanisms against biotic and abiotic stress are triggered when NO and ROS are in equilibrium (Fancy et al. 2017). Furthermore, ROS and MAPK cascades interact with other signaling pathways to fine-tune the system response.
Studies have suggested that NO can form nitro-fatty acids (NO2-FAs) via interactions with fatty acids. NO2-FAs are produced by reactions of RNS with unsaturated fatty acids. These NO2-FAs can participate in cellular signaling. Although scarcely studied, it has been suggested that NO2-FAs are present in extra-virgin olive oil (EVOO). Mata-Pérez et al. (2017) reported the presence of endogenous nitrolinolenic acid (NO2-Ln) in A. thaliana and showed the modulation of NO2-Ln in the development of this plant in response to conditions of stress. Thus, the level of NO2-Ln was found to be significantly high during stress, which proved that this molecule plays a role in signaling by directly releasing NO for further nitration or S-nitrosylation.
Nitric oxide is a major player in signaling networks, interacting with hormonal signaling pathways in plants. Reports have shown that NO interacts with auxins (AUX), gibberellins, ABA, ETH, jasmonic acid, brassinosteroids, and other plant hormones to regulate cellular metabolism as well as growth and development of plants. NO participates in the upstream and downstream regulation of plant hormones and modulates the biosynthesis, degradation, distribution of these hormones along with transportation and signaling (Hancock et al. 2011; Feng et al. 2013).
Nitric oxide, together with phytohormones, plays a vital role in improving the tolerance and acclimatization of plants in saline conditions. Plant hormones, such as ABA, ETH, and AUX, are transported from the roots to leaves of salt-stressed plants, thus triggering NO biosynthesis and/or distribution throughout the plant body (Molassiotis et al. 2010). It has been suggested that ABA stimulates H2O2 accumulation cells, which boosts NO biosynthesis, leading to MAPK activation and gene upregulation for the synthesis of antioxidant enzymes (Lu et al. 2009; Nawaz et al. 2017).
Another phytohormone, ETH, also acts as a signaling hormone and interacts with signaling cascades to enhance resistance during abiotic stress (Fatma et al. 2016b). Studies using Arabidopsis calli have reported that 100 mM NaCl triggered NO accumulation, resulting in increased ETH emission and plasma membrane H+-ATPase activity to improve salt tolerance (Wang et al. 2010). Fatma et al. (2016b) also reported that S assimilation is promoted by NO, which is associated with ETH biosynthesis from the cystine-synthesis pathway. Therefore, it can be hypothesized that NO modulates ABA and ETH levels and regulates photosynthesis under conditions of salt-induced stress. Liu et al. (2015) reported that the growth of the root meristem in salt-stressed Arabidopsis was due to AUX and NO, whereas SA promoted NO generation in guard cells, leading to stomatal closure (Khokon et al. 2011). SA alleviated the toxicity of NaCl-induced salt stress by increasing proline accumulation. SA also interacts with NO-signaling cascades to modulate photosynthesis, decrease the accumulation of H2O2, improve Ca2+/Mg2+ uptake and reduced the uptake of Na2+ under saline conditions (Dong et al. 2015). Kong et al. (2016) reported that NO upregulated CK biosynthesis and increased the longevity of leaves in cotton by reducing senescence.
Nitric oxide-regulated gene expression during salt-induced stress
Studies with NO in plants have demonstrated the signaling functions of this molecule, which were seen to be correlated with plant developmental processes and stress defense mechanisms. Several studies have recently provided genetic evidence for the involvement of NO in gene induction and gene expression. Activation of the plasma membrane (PM) H+-ATPase and the vacuolar H+-ATPase enzymes and of the Na+/H+ antiporter by the action of the NO signal upregulated the K+/Na+ ratio (Zhao et al. 2004). Association of H2S and NO modulated the expression of K+/Na+ transporters in NaCl-affected roots of barley. H2S and NO amplified PM H+-ATPase, the transcriptional levels of PM H+-ATPase (HvHA1) and the Na+/H+ antiporter (HvSOS1), which decreased the cellular Na+ levels. Interaction of H2S and NO augmented the transcriptional levels of the vacuolar Na+/H+ antiporter (HvVNHX2) and H+-ATPase subunit β (HvVHA-β) and the protein expression of the vacuolar Na+/H+ antiporter (NHE1). Increased expression of these genes enhanced the vacuolar compartmentalization of Na+, which decreased the cytoplasmic Na+ levels (Chen et al. 2015). Foliar spraying with SNP increased the chlorophyll content, photosynthetic rate and expression of the LHCB gene in salt-affected cotton plants (after 22 days of stress). Expression of the SOS1 and NHX1 genes by SNP supplementation increased the K+ levels and decreased the Na+ levels in leaves. Decreased expression of ABA biosynthesis genes, NCED2 and NCED9, led to downregulation of ABA content and delayed leaf senescence. Increased expression of cytokinin biosynthesis genes, IPT1 and ZR, and iPA content was induced by exogenous SNP supplementation (Kong et al. 2016). Enhanced expression of the SOD, CAT and APX genes were documented in S-nitroso-N-acetylpenicillamine (NO donor)-treated salt-affected chickpea plants, contributing to decreased electrolyte leakage and H2O2, and MDA levels. Improved growth parameters, leaf relative water content, photosynthetic pigment production and osmolyte accumulation were demonstrated as being caused by NO treatment during salt-induced stress (Ahmad et al. 2016). Nitric oxide-mediated modulation of auxin and signaling decreased the root meristem size in salt-affected Arabidopsis. The mechanism of salt-induced root meristem growth inhibition was the repression of PIN expression (which was responsible for decreasing AUX levels) and stabilization of IAA17 (which suppressed AUX signaling) via increased NO levels (Liu et al. 2015). Published research reports regarding NO-mediated regulation of gene expression in plants under salt-induced stress are limited, and further research is required.
Conclusions and future perspectives
The advancement of research focusing on NO in plants revealed various functions of NO. Nitric oxide rapidly reacts and interacts with other radicals/ROS, phytohormones and signaling molecules, including ABA, H2O2, NO, MAPK, and Ca, which not only modulate developmental processes, but also adaptive physiological responses under conditions of stress. The roles of PM H+-ATPase and vacuolar H+-ATPase and the effect of NO on these enzymes are vital for the regulation of the cellular Na+/K+ ratio and is one of the most important features in salt adaptation and tolerance of plants; we could not find much literature on this subject. The capacity to metabolize GSNO provides new perspective on the metabolism of NO in plants. Nitric oxide interacts with ROS and RNS, and the activity of GSNOR has been shown to regulate stress responses and PCD, but the mechanism is not defined. GSNOR plays a regulatory role in the post-translational modification of cysteine residues and thus may have roles in plant developmental processes and stress adaptation that are unknown to NO researchers. However, further in-depth studies are needed to elucidate the signaling behavior of NO in signal transduction in addition to transcriptional regulation and ion detoxification. The signaling function of NO is an indication of the effect of NO on gene expression. Several studies have recently provided genetic evidence for the involvement of NO in gene induction and gene expression, but resources and researchers are limited. The implementation of research plans to elucidate the unclear/unknown roles of NO and is necessary to understand the mechanism of NO-induced salt-stress tolerance in plants.
Abbreviations
- ABA:
-
Abscisic acid
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- ATP:
-
Adenosine triphosphate
- CAT:
-
Catalase
- chl:
-
Chlorophyll
- DHAR:
-
Dehydroascorbate reductase
- ETH:
-
Ethylene
- GPX:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- GR:
-
Glutathione reductase
- GSNO:
-
S-Nitrosoglutathione
- GSNOR:
-
GSNO reductase
- GST:
-
Glutathione S-transferase
- H2S:
-
Hydrogen sulfide
- MAPK:
-
Mitogen-activated protein kinase
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase
- MG:
-
Methylglyoxal
- NO2-FAs:
-
Nitro-fatty acids
- NO:
-
Nitric oxide
- NOS:
-
NO synthase
- NR:
-
Nitrate reductase
- PA:
-
Polyamine
- PCD:
-
Programmed cell death
- PM:
-
Plasma membrane
- POD:
-
Guiacol peroxidase
- PTM:
-
Post-translational modification
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- SNAP:
-
S-Nitroso-N-acetylpenicillamine
- SNP:
-
Sodium nitroprusside
- SOD:
-
Superoxide dismutase
- XOR:
-
Xanthine oxidoreductase
References
Ahmad P, Latef AAA, Hashem A, Abd_Allah EF, Gucel S, Tran L-SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00347
Akter S, Huang J, Waszczak C, Jacques S, Gevaert K, Van Breusegem F, Messens J (2015) Cysteines under ROS attack in plants: a proteomics view. J Exp Bot 66:2935–2944
Ali Q, Daud MK, Haider MZ, Ali S, Aslam N, Noman A, Iqbal N, Shahzad F, Rizwan M, Deeba F, Ali I, Jin ZS (2017) Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol Biochem 119:50–58
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887
Arora D, Jain P, Singh N, Kaur H, Bhatla SC (2016) Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic Res 50:291–303
Baudouin E (2011) The language of nitric oxide signalling. Plant Biol 13:233–242
Begara-Morales JC, Sánchez-Calvo B, Luque F, Leyva-Pérez MO, Leterrier M, Corpas FJ, Barroso JB (2014) Differential transcriptomic analysis by RNA-Seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant Cell Physiol 55:1080–1095
Bellin D, Asai S, Delledonne M, Yoshioka H (2013) Nitric oxide as a mediator for defense responses. Mol Plant Microbe Interact 26:271–277
Besson-Bard A, Astier J, Rasul S, Wawer I, Dubreuil-Maurizi C, Jeandroz S, Wendehenne D (2009) Current view of nitric oxide-responsive genes in plants. Plant Sci 177:302–309
Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Bose J, Munns R, Shabala S, Gilliham M, Pogson B, Tyerman SD (2017) Chloroplast function and ion regulation in plants growing on saline soils: lessons from halophytes. J Exp Bot 68:3129–3143
Camejo D, Romero-Puertas MC, Rodríguez-Serrano M, Sandalio LM, Lázaro JJ, Jiménez A, Sevilla F (2013) Salinity-induced changes in S-nitrosylation of pea mitochondrial proteins. J Proteom 79:87–99
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819
Chen J, Xiao Q, Wang C, Wang WH, Wu FH, Chen J, He BY, Zhu Z, Ru QM, Zhang LL, Zheng HL (2014) Nitric oxide alleviates oxidative stress caused by salt in leaves of a mangrove species, Aegiceras corniculatum. Aquat Bot 117:41–47
Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL (2015) Hydrogen sulfide enhances salt tolerance through nitric oxide mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 5:12516
Cheng T, Shi J, Dong Y, Ma Y, Peng Y, Hu X, Chen J (2017) Hydrogen sulfide enhances poplar tolerance to high-temperature stress by increasing S-nitrosoglutathione reductase (GSNOR) activity and reducing reactive oxygen/nitrogen damage. Plant Growth Regul. https://doi.org/10.1007/s10725-017-0316-x
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour Technol 244:1376–1383
Christou A, Manganaris GA, Fotopoulos V (2014) Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ Exper Bot 107:46–54
Corpas FJ, Barroso JB (2014) Peroxynitrite (ONOO–) is endogenously produced in arabidopsis peroxisomes and is overproduced under cadmium stress. Ann Bot 113:87–96
Corpas FJ, Alché JD, Barroso JB (2013) Current overview of S-nitrosoglutathione (GSNO) in higher plants. Front Plant Sci 4:126. https://doi.org/10.3389/fpls.2013.00126
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
da Silva CJ, Fontes EPB, Modolo LV (2017) Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana Plant Sci 256:148–159
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Dong F, Simon J, Rienks M, Lindermayr C, Rennenberg H (2015) Effects of rhizopheric nitric oxide (NO) on N uptake in Fagus sylvatica seedlings depend on soil CO2 concentration, soil N availability and N source. Tree physiol 35:910–920
Egbichi I, Keyster M, Ludidi N (2014) Effect of exogenous application of nitric oxide on salt stress responses of soybean. South Afr J Bot 90:131–136
Fan H, Guo S, Jiao Y, Zhang R, Li J (2007) Effects of exogenous nitric oxide on growth, active oxygen species metabolism, and photosynthetic characteristics in cucumber seedlings under NaCl stress. Front Agric China 1:308–314
Fancy NN, Bahlmann A-K, Loake GJ (2017) Nitric oxide function in plant abiotic stress. Plant Cell Environ 40:462–472
Fares A, Rossignol M, Peltier JB (2011) Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem Biophys Res Commun 416:331–336
Fatma M, Khan NA (2014) Nitric oxide protects photosynthetic capacity inhibition by salinity in Indian mustard. J Funct Environ Bot 4:106–116
Fatma M, Masood A, Per TS, Rasheed F, Khan NA (2016a) Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. Crop J 4:153–161
Fatma M, Masood A, Per TS, Khan NA (2016b) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00521
Feng J, Wang C, Chen Q, Chen H, Ren B, Li X, Zuo J (2013) S-Nitrosylation of phosphotransfer proteins represses cytokinin signaling. Nat Commun 4:1529. https://doi.org/10.1038/ncomms2541
Foresi N, Correa-Aragunde N, Parisi G, Caló G, Salerno G, Lamattina L (2010) Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 22:3816–3830
Gadelha CG, Miranda RS, Alencar NLM, Costa JH, Prisco JT, Gomes-Filhoa E (2017) Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J Plant Physiol 212:69–79
Galatro A, Puntarulo S, Guiamet JJ, Simontacchi M (2013) Chloroplast functionality has a positive effect on nitric oxide level in soybean cotyledons. Plant Physiol Biochem 66:26–33
Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl– channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100:11116–11121
Gaupels F, Kuruthukulangarakoola GT, Durner J (2011) Upstream and down stream signals of nitric oxide in pathogen defence. Curr Opin Plant Biol 14:707–714
Ghaly AE, Ramakrishnan VV (2015) Nitrogen sources and cycling in the ecosystem and its role in air, water and soil pollution: a critical review. J Pollut Eff Cont 3:136
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63:254–261
Gill SS, Anjum NA, Gill R, Tuteja N (2016) Abiotic stress signaling in plants—an overview. In: Gill SS, Anjum NA, Gill R, Tuteja N (eds) Abiotic Stress Response in Plants Vol. 1. Wiley, Weinheim. pp 3–12
Gong B, Wen D, Wang X, Wei M, Yang F, Li Y, Shi Q (2014) S-nitrosoglutathione reductase-modulated redox signaling controls sodic alkaline stress responses in Solanum lycopersicum L. Plant Cell Physiol 56:790–802
Guo Y, Tian Z, Yan D, Zhang J, Qin P (2009) Effects of nitric oxide on salt stress tolerance in Kosteletzkya virginica. Life Sci J 6:67–75
Gupta KJ, Fernie AR, KaiserWM, van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16:160–168
Gupta P, Srivastava S, Seth CS (2017) 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 411:483–498
Hancock JT, Neill SJ, Wilson ID (2011) Nitric oxide and ABA in the control of plant function. Plant Sci 181:555–559
Hao GP, Zhang JH (2010) The role of nitric oxide as a bioactive signaling molecule in plants under abiotic stress. In: Hayat S, Mori M, Pichtel J, Ahmad A (eds) Nitric oxide in plant physiology. Wiley, Weinheim, pp 115–138
Harrison R (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33:774–797
Hasanuzzaman M, Hossain MA, Fujita M (2011) Nitric oxide modulates antioxidant defense and methylglyoxal detoxification system and reduces salinity induced damage in wheat seedling. Plant Biotechnol Rep 5:353–365
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2012) Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 6:1314–1323
Hasanuzzaman M, Nahar K, Fujita M (2013a) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmed P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 25–87
Hasanuzzaman M, Nahar K, Fujita M, Ahmad P, Chandna R, Prasad MNV, Ozturk M (2013b) Enhancing plant productivity under salt stress: relevance of poly-omics. In: Ahmad P, Azooz MM, Prasad MNV (eds) Salt stress in plants: signaling, omics and adaptations. Springer, New York, pp 113–156
Hasanuzzaman M, Gill SS, Fujita M (2013c) Physiological role of nitric oxide in plants grown under adverse environmental conditions. In: Tuteja N, Singh Gill S (eds) Plant acclimation to environmental stress. Springer, New York, pp 269–322
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H. Fujita M (2017a) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18:200
Hasanuzzaman M, Nahar K, Hossain MS, Anee TI, Parvin K, Fujita M (2017b) Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. J Plant Interact 12:323–331
Hayat S, Yadav S, Wani AS, Irfan M, Alyemini MN, Ahmad A (2012) Impact of sodium nitroprusside on nitrate reductase, proline content, and antioxidant system in tomato under salinity stress. Hortic Environ Biotechnol 53:362–367
Hill BG, Dranka BP, Bailey SM, Lancaster JRJ, Darley-Usmar VM (2010) What part of NO don’t you understand. Some answers to the cardinal questions in nitric oxide biology. J Biol Chem 285:19699–19704
Huang X, Chen MH, Yang LT, Li YR, Wu JM (2015) Effects of exogenous abscisic acid on cell membrane and endogenous hormone contents in leaves of sugarcane seedlings under cold stress. Sugar Technol 17:59–64
Iakimova ET, Woltering EJ (2015) Nitric oxide prevents wound-induced browning and delays senescence through inhibition of hydrogen peroxide accumulation in fresh-cut lettuce. Innovative Food Sci Emerg Technol 30:157–169
Ibrahim EA (2016) Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol 192:38–46
Kausar F, Shahbaz M, Ashraf M (2013) Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turkish J Bot 37:1155–1165
Khokon MD, Okuma EIJI., Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34:434–443
Kibria MG, Hossain M, Murata Y, Hoque MA (2017) Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci 24:155–162
Kong J, Dong Y, Xu L, Liu S, Bai X (2014) Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. Bot Stud 55:9. https://doi.org/10.1186/1999-3110-55-9
Kong X, Wang T, Li W, Tang W, Zhang D, Dong H (2016) Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38:61
Kumar D, Klessig DF (2000) Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Mol Plant Microb Interact 13:347–351
Lamotte O, Bertoldo JB, Besson-Bard A, Rosnoblet C, Aimé S, Hichami S, Wendehenne D (2015) Protein S-nitrosylation: specificity and identification strategies in plants. Front Chem 2:114. https://doi.org/10.3389/fchem.2014.00114
Lang T, Sun H, Li N, Lu Y, Shen Z, Jing X, Xiang M, Shen X, Chen S (2014) Multiple signaling networks of extracellular ATP, hydrogen peroxide, calcium, and nitric oxide in the mediation of root ion fluxes in secretor and non-secretor mangroves under salt stress. Aquat Bot 119:33–43
Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M (2009) NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol 12:451–458
Leterrier M, Airaki M, Palma JM, Chaki M, Barroso JB, Corpas FJ (2012) Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ Pollut 166:136–143
Liao W, Huang G, Yu J, Zhang M, Shi X (2011) Nitric oxide and hydrogen peroxide are involved in indole-3-butyric acid-induced adventitious root development in marigold. J Hortic Sci Biotechnol 86:159–165
Lin A, Wang Y, Tang J, Xue P, Li C, Liu L, Hu B, Yang F, Loake GJ, Chu C (2012) Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol 158:451–464
Liu Y, He C (2017) A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol 11:192–204
Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT (2015) Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168:343–356
Liu A, Fan J, Gitau MM, Chen L, Fu J (2016) Nitric oxide involvement in bermuda grass response to salt stress. J Am Soc Hortic Sci 141:425–433
Lu S, Su W, Li H, Guo Z (2009) Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2 and NO-induced antioxidant enzyme activities. Plant Physiol Biochem 47:132–138
Machado RMA, Serralheiro RP (2017) Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulture 3:30. https://doi.org/10.3390/horticulturae3020030
Marvasi M (2017) Potential use and perspectives of nitric oxide donors in agriculture. J Sci Food Agric 97:1065–1072
Mata-Pérez C, Sánchez-Calvo B, Padilla MN, Begara-Morales JC, Valderrama R, Corpas FJ, Barroso JB (2017) Nitro-fatty acids in plant signaling: new key mediators of nitric oxide metabolism. Redox Biol 11:554–561
Memon AR, Durakovic C (2014) Signal perception and transduction in plants. Period Eng Nat Sci 2:15–29
Molassiotis A, Tanou G, Diamantidis G (2010) NO says more than ‘YES’ to salt tolerance: salt priming and systemic nitric oxide signaling in plants. Plant Signal Behav 5:209–212
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Munns R, James RA, Läuchli (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJ, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5:pls052. https://doi.org/10.1093/aobpla/pls052
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense, and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255
Nawaz F, Shabbir RN, Shahbaz M, Majeed S, Raheel M, Hassan W, Sohail MA (2017) Cross talk between nitric oxide and phytohormones regulate plant development during abiotic stresses. In: El-Esawi M (ed) Phytohormones-signaling mechanisms and crosstalk in plant development and stress responses. InTech, Rijeka, pp 117–141
Niu L, Liao W (2016) Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front Plant Sci 7:230. https://doi.org/10.3389/fpls.2016.00230
Palavan-Unsal N, Arisan D (2009) Nitric oxide signaling in plants. Bot Rev 75:203–229
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide Mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–286
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci 7:609
Rahman A, Nahar K, Mahmud JA, Hasanuzzaman M, Hossain MS, Fujita M (2017) Salt stress tolerance in rice: emerging role of exogenous phytoprotectants. In: Li J (ed) Advances in international rice research. InTech, Rijeka, pp 139–174
Rajput VD, Yaning C, Ayup M, Minkina T, Sushkova S, Mandzhieva S (2017) Physiological and hydrological changes in Populus euphratica seedlings under salinity stress. Acta Ecol Sin 37:229–235
Reddy INBL., Kim B-K, Yoon I-N, Kim KH, Kwon TR (2017) Salt tolerance in rice: focus on mechanisms and approaches. Rice Sci 24:123–144
Romero-Puertas MC, Rodríguez-Serrano M, Sandalio LM (2013) Protein S-nitrosylation in plants under abiotic stress: an overview. Front Plant Sci 4:373. https://doi.org/10.3389/fpls.2013.00373
Roychoudhury A, Paul S, Basu S (2013) Cross-talk between abscisic acid dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep 32:985–1006
Rümer S, Gupta KJ, Kaiser WM (2009) Plant cells oxidize hydroxylamines to NO. J Exp Bot 60:2065–2072
Sang J, Zhang A, Lin F, Tan M, Jiang M (2008) Cross-talk between calcium-calmodulin and nitric oxide in abscisic acid signaling in leaves of maize plants. Cell Res 18:577–588
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:372. https://doi.org/10.3389/fmicb.2012.00372
Serrano I, Romero-Puertas MC, Rodríguez Serrano M, Sandalio LM, Olmedilla A (2012) Role of peroxynitrite in programmed cell death induced in self-incompatible pollen. Plant Signal Behav 7:779–781
Setia R, Gottschalk P, Smith P, Marschner P, Baldock J, Setia D, Smith J (2013) Soil salinity decreases global soil organic carbon stocks. Sci Total Environ 465:267–272
Sevilla F, Camejo D, Ortiz-Espín A, Calderón A, Lázaro JJ, Jiménez A (2015) The thioredoxin/peroxiredoxin/sulfiredoxin system: current overview on its redox function in plants and regulation by reactive oxygen and nitrogen species. J Exp Bot 66:2945–2955
Shabala S, Cuin TA (2007) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Sheokand S, Kumari A, Sawhney V (2008) Effect of nitric oxide and putrescine on antioxidative responses under NaCl stress in chickpea plants. Physiol Mol Biol Plants 14:355–362
Sheokand S, Bhankar V, Sawhney V (2010) Ameliorative effect of exogenous nitric oxide on oxidative metabolism in NaCl treated chickpea plants. Braz J Plant Physiol 22:81–90
Shi H, Ye T, Zhu JK, Chan Z (2014) Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J Exp Bot 65(15):4119–4131
Shi K, Li X, Zhang H, Zhang G, Liu Y, Zhou Y et al (2015) Guard cell hydrogen peroxide and nitric oxide mediate elevated CO2-induced stomatal movement in tomato. New Phytol 208:342–353
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Stavridou E, Hastings A, Webster RJ, Robson PRH (2017) The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. Gcb Bioenergy 9:92–104
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G et al (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60(5):795–804
Tavakkoli E, Rengasamy P, McDonald GK (2010) High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 61:4449–4459
Tichá T, Lochman J, Činčalová L, Luhová L, Petřivalský M (2017) Redox regulation of plant S-nitrosoglutathione reductase activity through post-translational modifications of cysteine residues. Biochem Biophys Res Commun 494:27–33
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EIS, Scherer GF (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Wang P, Du Y, Li Y, Ren D, Song CP (2010) Hydrogen peroxide–mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22:2981–2998
Wang X, Hou C, Liu J, He W, Nan W, Gong H, Bi Y (2013) Hydrogen peroxide is involved in the regulation of rice (Oryza sativa L.) tolerance to salt stress. Acta Physiol Plant 35:891–900
Wang C, El-Shetehy M, Shine MB, Yu K, Navarre D, Wendehenne D, Kachroo P (2014) Free radicals mediate systemic acquired resistance. Cell Rep 7:348–355
Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F (2008) In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol 146:1920–1927
Wrzaczek M, Brosché M, Salojärvi J, Kangasjärvi S, Idänheimo N, Mersmann S, Robatzek S, Karpiński S, Karpińska B, Kangasjärvi J (2010) Transcriptional regulation of the CRK/DUF26 group of receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol 10:95. https://doi.org/10.1186/1471-2229-10-95
Wu XX, Ding HD, Chen JL, Zhang HJ, Zhu WM (2010) Attenuation of salt-induced changes in photosynthesis by exogenous nitric oxide in tomato (Lycopersicon esculentum Mill. L.) seedlings. Afr J Biotechnol 9:7837–7846
Wu X, Zhu W, Zhang H, Ding H, Zhang HJ (2011) Exogenous nitric oxide protects against salt induced oxidative stress in the leaves from two genotypes of tomato (Lycopersicom esculentum Mill.). Acta Physiol Plant 33:1199–1209
Wu P, Shou H, Xu G, Lian X (2013) Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Curr Opin Plant Biol 16:205–212
Wulff A, Oliveira HC, Saviani EE, Salgado I (2009) Nitrite reduction and superoxide-dependent nitric oxide degradation by Arabidopsis mitochondria: influence of external NAD(P)H dehydrogenases and alternative oxidase in the control of nitric oxide levels. Nitric Oxide 21:132–139
Xu LL, Fan ZY, Dong YJ, Kong J, Bai XY (2015) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol Plant 59:171–182
Yadu S, Dewangan TL, Chandrakar V, Keshavkant S (2017) Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol Mol Biol Plants 23:43–58
Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 4:128–129
Yang H, Mu J, Chen L, Feng J, Hu J, Li L, Zhou JM, Zuo J (2015) S-Nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol 167:1604–1615
Yu M, Lamattina L, Spoel SH, Loake GJ (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol 202:1142–1156
Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224:545–555
Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M (2007) Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol 175:36–50
Zhao L, Zhang F, Guo J, Yang Y, Li B, Zhang L (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134:849–857
Zheng C, Jiang D, Liu F, Dai T, Liu W, Jing Q, Cao W (2009) Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot 67:222–227
Ziogas V, Tanou G, Filippou P, Diamantidis G, Vasilakakis M, Fotopoulos V, Molassiotis A (2013) Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiol Biochem 68:118–126
Zuccarelli R, Coelho AC, Peres LE, Freschi L (2017) Shedding light on NO homeostasis: light as a key regulator of glutathione and nitric oxide metabolisms during seedling deetiolation. Nitric Oxide 68:77–90
Acknowledgements
We thank Ms. Khursheda Parvin and Abdul Awal Chowdhury Masud for their critical reading of the manuscript. The first author acknowledges Japan Society for the Promotion of Sciences (JSPS) for providing research grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasanuzzaman, M., Oku, H., Nahar, K. et al. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol Rep 12, 77–92 (2018). https://doi.org/10.1007/s11816-018-0480-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0480-0