Abstract
Rice (Oryza sativa L.) is an important food crop that belongs to family Gramineae and needs a larger amount of water to complete its life cycle as compared to other crops. Hence, rice production is severely affected by water stress. Drought is an important issue in rainfed areas across the globe which limits rice production. Several morphological characters like germination, plant height, plant biomass, number of tillers, various root and leaf traits, physiological characters like photosynthesis, stomatal conductance, transpiration, water use efficiency, relative water content, chlorophyll content, photosystem-II activity, carbon isotope discrimination, membrane stability, and abscisic acid content of rice are reduced under drought conditions. Drought also induces the accumulation of several biochemical osmoprotectants like proline, polyamines, sugars, antioxidants and alters the expression of several genes including transcription factors and defense-related proteins, hence thereby affects the yield of rice crop. Drought escape, drought avoidance, and drought tolerance are the mechanisms that prevent plant from harmful effects of drought. Thus, this review is focused mainly on recent information about the morphological, physio-biochemical, and molecular effects, responses, and adaptation mechanisms of rice under drought stress. Here we also discussed that how we can improve the rice for drought tolerance using various molecular tools and techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is an important food crop that belongs to the genus Oryza of Gramineae family. Globally, it provides 23% per capita energy which accounts for 65% of caloric intake (Khush 1996). It ranks second among cereal in the world (Farooq et al. 2009a, b) and most widely consumed staple food (Pirdashti et al. 2009). Rice occupied 197.59 million hectares area in the world with 996.07 million tons production (FAOSTAT 2018–19). India is second largest producer of rice with an annual production of over 172.58 million tons and has 44.5 million hectares area under rice cultivation (FAOSTAT 2018–19). Globally, with the increasing population, the demand for rice and other staple food grains is going to be increased day by day. Hence, to meet the global rice demand in the coming years, its production needs to be significantly increased from the current level. Globally, rice production is constrained by a number of biotic and abiotic factors. Among these abiotic stresses like drought, high temperatures, salinity, and oxidative stress are often interrelated, and may cause similar changes in plants (Szekeres 2003). In the past decades, drought has become frequent due to increasing aberrations in rainfall patterns. It limits crop production and is becoming a more severe problem in many regions of the globe (Passioura 1996, 2007). Drought refers to a situation where demand of water is more than water supply to plants and it becomes a limiting factor for biomass accumulation. Drought affects water relations both on the cellular level as well as a whole plant like other abiotic stresses (Beck et al. 2007). It influences several physiological, biochemical, and molecular processes in plants, like ion uptake, translocation, photosynthesis, respiration, carbohydrates, nutrient metabolism, harmonic balance, and expression level of several genes (Farooq et al. 2009c).
Since rice is an aquatic plant, it is very sensitive to drought stress for almost all its growth phases which cause poor expression of yield component traits (Hsiao et al. 1984; O’Toole 1982; Venuprasad et al. 2008, Bouman et al. 2005). Drought stress reduces cell division, cell elongation and thus restricts overall plant growth which is reflected from reduced height and lesser biomass. Drought at booting and seed setting stages caused decreased grain size and grain number (Zhang et al. 2018a, b). Breeding for drought tolerance has been a very challenging task, due to the complex genetic and molecular regulation of this trait. The production of varieties of drought tolerance will need to put together many features of drought tolerance along with high yield capacity under non-drought conditions, but progress in breeding for drought tolerance has been very slow (Kumar et al. 2008). Nevertheless, attempts by the International Rice Research Institute (IRRI) in this direction have contributed to the production of certain genotypes of drought-tolerant rice. These include IR 74371-46-1-1, IR 74371-54-1-1, and IR 74371-70-1-1. In addition, in various regions of South Asia, several drought-tolerant rice lines have been released (for example, DRR 42, 44 in India, SukhaDhan 4, 5 in Nepal, and BRRI Dhan 66, 71 in Bangladesh). In different countries, a conventionally bred line IR74371-70-1-1 was released with various names: in India as SahbhagiDhan, in Nepal as SukhaDhan 3, and in Bangladesh as BRRI 56 Dhan. This suggests the suitability of this line for a wide variety of environments to demonstrate improved results. But conventional breeding is costly, laborious, and takes a long time to develop a new variety for cultivation. Therefore, it is extremely important to have a comprehensive understanding of physiological and molecular basis of drought tolerance and associated morphological changes to facilitate designing of efficient breeding strategies for development of drought-tolerant varieties. In this review, we have discussed the morphological, physiological, and molecular responses of the rice plant under drought stress.
Drought-Induced Economic Losses
Droughts have a significant impact on agricultural production, but there is no agreement on how to measure and characterize them, which has ramifications for drought research and policy (Fontes et al. 2020). The cost–benefit analysis is frequently used to shape the right policy response to drought, particularly with respect to the costs of mitigation and climate adaptation in the agriculture sector (Mechler et al. 2008; Fontes et al. 2020). Recently, Kim et al. (2019) reported global patterns of drought-induced yield losses and associated national economic losses for the major cereals including rice, wheat and maize from 1983 to 2009. They applied empirical relationships among crop yields, a drought index, and annual precipitation to calculate production losses which was then translated into economic losses for these crops due to drought. The worldwide aggregated total economic loss of rice for the period 1983 to 2009 was estimated to be approximately $37Billion (Kim et al. 2019). More such studies are needed for evaluation of drought-induced economic losses in rice which will help design policy measures and technological development for effectively mitigating the drought in the most vulnerable regions.
Drought Categorization of Soils
Hsiao (1973) categorized drought stress based on the soil water potential (SWP) and reduction in leaf relative water content (RLWC) into three main categories; mild stress (SWP: − 0.1MPa; reduction in RLWC: 8-10%), moderate stress (SWP: − 1.2 to − 1.5; reduction in RLWC: 10-20%) and severe stress (SWP: < − 1.5; reduction in RLWC: > 20%). The US drought monitor map uses a range of indicators to measure intensity of drought and categorizes it into five categories; abnormally dry, moderate drought, severe drought, extreme drought and exceptional drought (https://droughtmonitor.unl.edu/About/AbouttheData/DroughtClassification.aspx). Although, drought is the most significant constraint to rainfed rice productivity; there are few papers which characterize the soil parameters in relation to the intensity of drought stress in the area. It has been reported that rice fields in similar locales that are totally rainfed are likely to have varying soil water potential and water table depth values (Singh et al. 2017). Other drought stresses that occur at the seedling and vegetative stages also provide considerable problems to rainfed rice growers, and can lead to varied crop performance and soil characteristics (Pandey and Bhandari 2008; Singh et al. 2017).
Evapotranspiration and Soil Moisture
Monitoring and modeling of land surface and vegetation processes is a critical component in determining the water and carbon dynamics of terrestrial ecosystems. In drought-related studies, the appropriate assessment of evapotranspiration (ET) and soil moisture content (SMC) is very crucial (Verstraeten et al. 2008). Controlling water stress levels and timing is critical for proper phenotyping for drought tolerance. Guimarães et al. (2016) determined the upland rice plant water use and its association with grain output during periods of irrigation withholding in order to improve the effectiveness of drought phenotyping. Such experiments are required for evaluating soil condition, which is directly linked with crop productivity.
Crop-Specific Drought Sensitivity Assessments
To combat drought stress, new rice cultivars with greater drought resistance should be developed. It is desirable to create a simple and accurate approach for evaluating rice drought tolerance for breeding reasons, particularly for producing highland rice (Zu et al. 2017). It is necessary to evaluate drought sensitivity of crop during their life in different time for understanding their need for water. Zu et al (2017) explained a new method that can be utilized to assess efficiently the drought tolerance degree (DTD) of upland rice varieties. The DTD is defined as the mean value of the relationships between the length of the green leaf and the total length of the three upper leaves in each rice shoot after the drought treatment and therefore takes values from zero to one. There is a need to develop such type of methods for crop-specific drought sensitivity assessments in near future.
Geographical Location of Drought Resistance
India and Bangladesh provided the majority of the reproductive stage drought-tolerant accessions (Rahman and Zhang 2018). Nearly 40,000 germplasms (accessions and breeding lines) were screened for the finest drought-tolerant germplasm between 1978 and 1985; nevertheless, breeding lines made up the bulk of the top 20 excellent germplasms. India has slightly more tolerant accessions than Bangladesh, although the majority of Indian accessions came from Bangladesh’s neighboring states. Furthermore, suggested cultivars for drought stress breeding such as Kataktara Da2, Dular, Shada Shaita, and DA 28 originated in Bangladesh, and the majority of these are still planted in large regions of the country (de Datta et al. 1988; Torres et al. 2013; Rahman and Zhang 2018).
Survival Mechanism of Plants Under Drought Stress
There are three ways by which plants can survive under drought conditions, i.e., drought escape, drought avoidance, and drought tolerance. The drought escape can be defined as the process in which a plant can complete its life cycle before developing the critical water deficiency in soil (Basu et al. 2016; Osmolovskaya et al. 2018). It consists of two different mechanisms like rapid phenological development and developmental plasticity. When the plants can produce flowers and seeds with minimum vegetative growth under limited water supply is known as rapid phenological development. When the plants can complete their proper vegetative growth, flowering and seed production during abundant rain seasons are known as developmental plasticity. This allows desert ephemerals to both survive long periods without rain and escape drought. Drought escape is an important process that allows rice to grow in a well manner and also to produce the grains while limited water availability (Kumar et al. 2008). Drought avoidance is referred as the ability of plants to maintain high water potential in tissues in spite of less moisture in soil. Rice genotype that shows the drought avoidance may follow ABA biosynthesis and other biochemical mechanisms or adaptation by a root system, to maintain the water status in plants. These drought avoidance rice genotypes can reduce the loss of yield which leads to drought stress (Singh et al. 2012). Drought avoidance can be also improved by improving water use efficiency (WUE) and minimizing the loss of water from plant. The rice genotypes that circumvent drought have several characteristics like, deep root system which has higher branching ability and soil penetration, high root–shoot ratio, less leaf rolling, early closure of stomata, and higher cuticle resistance (Blum et al. 1989; Samson et al. 2002; Wang et al. 2006). Likewise, drought tolerance is referred to as the ability of plants to survive under low water content in the tissues (Turner 1979; Delphine et al. 2010). Drought tolerance is one of the complex characters which is governed by polygenic effects and also includes complex morphological and physiological mechanisms (Li and Xu 2007), like maintenance of turgor pressure with the help of osmotic adjustment, increased cell elasticity, decreased cell size, and desiccation resistance with help of protoplasmic resistance (Sullivan and Ross 1979). The degree of drought tolerance can be determined by tissue water potential of plants and the traits involved in this phenomenon are appraised as secondary traits. Several secondary traits have been used for improvement of drought tolerance are leaf rolling, relative water content, osmotic adjustment and stomatal conductivity (Nguyen et al. 1997; Babu et al. 2001; Kato et al. 2006).
Effects of Drought on Rice Plants
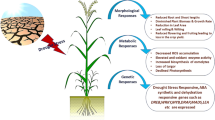
The effects of drought on the plants can appear at all phenological stages at any stage of growth and development of plant, when the water deficiency occurs in the field. Plants respond to drought by a range of changes at morphological, physiological, and molecular levels. A description of various changes in plants during the drought is given in Fig. 1.
Morphological Responses Under Drought
Growth
Growth is an important factor that is governed by several events (such as morphological, physiological, ecological, biochemical and genetic) and their complex interactions. It is established by cell division, cell elongation and cell differentiation and involves two main parts of a plant that participate in the event of growth, i.e., root and shoot system of the plant. Water stress critically impacts plant growth and development than any other environmental stress effects. The quality and quantity of the plant growth depends on the complex interactions of the events during the water stress. During the water stress, growth of the cell is inhibited due to reduction in the turgor pressure (Taiz and Zeiger 2006) and cell elongation can be inhibited because of the interruption of water flow from xylem to surrounded elongating cells (Nonami 1998; Farooq et al. 2009a, b). Drought impaired he cell division, cell elongation and cell expansion which ultimately resulted in a reduction of the growth and development of a plant (Nonami 1998; Kaya et al. 2006; Hussain et al. 2008) (Fig. 2). It also reduces the germination, establishment (Harris et al. 2002) and severely enabled the sprouting of the seeds (Kaya et al. 2006).
Responses of Leaf, Root and Other Plant Characters
Water stress severely affects the growth and development of the leaves and roots and these are the important component which play important role in rice adaptation to drought stress. Since plants obtain the water and mineral nutrients from soil via, roots, so growth, development, proliferation, and size of the root are important factors for water stress resistance (Yoshida and Hasegawa 1982). Shallow rooting cultivars are more sensitive to drought than deep-rooting cultivars (Nemoto et al. 1998; Farooq et al. 2009e) because the deep root system of a plant allows obtaining the water from the depth of soil profile (Kondo et al. 2003). It has been found in several studies that rice varieties which have longer and thicker roots were found more drought resistant than other varieties those having short and thin root system (O’Toole and Chang 1979). In another study, the rice varieties which have deep root were found better adaptive during dry conditions (Boyer 1996). Drought reduces the number of leaves and new tillers decrease leaf elongation, promotes leaf rolling and increase leaf death(Cutler et al. 1980; Hsiao et al. 1984; Turner et al. 1986) in rice plants which results in reducing the photosynthetically active radiation (PAR) (Inthapan and Fukai 1988). The leaf expansion rate was decreased when 20 days of drought stress was given at vegetative phase in rice. Stomata are sensitive to water stress (Farooq et al. 2009d). When the leaf water potential decreases, it reduces the leaf conductance (O’Toole et al. 1984), which results in to decrease in the photosynthetic rate and radiation use efficiency (Inthapan and Fukai 1988). Several other responses of the plant have been observed during the water stress like reduction in height, leaf area and biomass production, decreasing the number of tillers and their abortion and changing in dry matter of roots and depth of root. According to recent studies water stress impact seedling and it significantly reduces the fresh and dry weight of seedlings (Farooq et al. 2008, 2009c).
Yield and Related Traits
Yield is the ultimate trait that is severely affected by water stress. There are several yield regulating factors that are affected by water stress. Many of these factors are responsible for the yield of a plant in several complex manners. Water stress effects yield by halting the leaf gas exchange which may adversely affect several processes and reduce the tissue size of source and sink, loading of phloem and assimilate translocation, etc. (Farooq et al. 2009a, b). In short, water stress decreases the growth and development of the crop plants that in turn causes inhibition of the grain filling process and flower developments. The reduction in grain filling process in plants occurs due to the decreasing of assimilate partitioning and starch and sucrose synthesizing enzymes. Under drought stress, the yield of rice plants depends on the drought timing and growth stages of the plant (Garrity and O’Toole 1994). Drought affects the yield component at different growth stages of plant, like vegetative stage (Guan et al. 2010; Sarvestani et al. 2008),Reproductive stage (Singh et al. 2018; Lafitte et al. 2007; Venuprasad et al. 2007; Dixit et al. 2012, 2014a, b; Guan et al. 2010; Swamy et al. 2017),flowering stage (Puteh et al. 2013; Sarvestani et al. 2008; Shamsudin et al. 2016a, b; Lanceras et al. 2004; Yang et al. 2019),anther dehiscence stage (Ekanayake et al. 1989) and Grain filling stage (Basnayake et al. 2006; Lanceras et al. 2004), Heading stage (Lafitte et al. 2006), panicle exertion stage (O’Toole and Namuco 1983). For example, water stress before 12 days of anthesis makes a severe impact on spikelet fertility with a higher reduction in the grain yield (Cruz and O’Toole 1984; Ekanayake et al. 1989) and water stress at reproductive phase leads to an increase in the number of unfilled grain on a spike and reduced grain weight thus ultimately reduce the grain yield of plants (Wopereis et al. 1996). Several other scientists also reported the reduction in yield of rice under drought stress condition (Jongdee et al. 2006; Kumar et al. 2008; Jian-Chang et al. 2008; Atlin et al. 2006; Carrijo et al. 2017; Daryanto et al. 2017; Zhang et al. 2018a, b) (Table 1).
Physiological Responses Under Drought
Drought induces various physiological changes in plants that may be critical for their survival under moisture deficit conditions. Some of the physiological responses to water stress in rice are described below.
Leaf Water Potential
During water stress, leaf water potential of rice plants decreases significantly in comparison to control plants (Hu et al. 2004; Farooq et al. 2009c; Parent et al. 2010). The net photosynthetic rate of a leaf is also decreased with the decreasing of leaf water potential when the water content or soil water potential reached at threshold level. In a study, the leaf water potential was decreased with the increased period of water stress induced by the supplying 20% PEG in all rice varieties. A significant difference was observed at 14 days after water stress treatment (Larkunthod et al. 2018). Liu et al. (2007a, b, c) found that upland rice cultivars decline the leaf water potential in comparison to lowland rice cultivars.
Stomatal Conductance
Stomatal conductance decreases the rate of transpiration and plays an important role to regulate the water balance of plants. The closing of stomata decreases the growth rate and expansion of cells thus significantly reduced the yield and biomass of the plants (Nemeskari et al. 2015; Rauf et al. 2015; Pirasteh-Anosheh et al. 2016). Several researchers believed that closing of the stomata is the first reaction given by the plants to prevent them during water stress for saving the water loss by transpiration (Torres-Ruiz et al. 2013; Clauw et al. 2015; Nemeskeri et al. 2015). Ouyang et al. (2017) reported the significant variation in stomatal conductance among species of rice. They observed that lowland rice showed much higher stomatal conductance than other cultivars. Stomatal conductance was found to decrease in all cultivars during the water stress treatments.
Phenology and Ontogeny or Leaf Area and Leaf Area Index
Leaf area also plays an important role during water stress. Rice plants reduce their leaf area under water stress to save themselves from drought (Kramer 1969). It has been observed that rice plants with reduced leaf areas use less water and are found less productive (Blum 2007). Plants with reduced leaf area can fight during water stress conditions but their growth rate and biomass production are relatively low than the normal irrigated plants (Henson 1985). Kamaruddin et al. (2018) observed that flag leaf area had a significant reduction in rice genotypes as compared to control plants under drought. Decreased flag leaf area results in small leaf size, senescence of leaf, and decreased emergence rate of a leaf (Solomon et al. 2007).
Effect of Drought on Root–Shoot Signaling
During stress environments, plants can regulate the signals positively or negatively between shoots and roots to maintain their growth and development. Root–shoot signaling reduced the growth and distribution of roots, growth, and functions of shoots during environmental stress (Novak and Lipiec 2012). Several molecules and factors act as signal molecules to regulate the physiological processes of a plant during the environmental stress such as Auxin, Cytokinin, Abscisic acid, Gibberellins, Ethylene and pH (Schachtman and Goodger 2008). Abscisic acid has been recognized as a stress hormone and plays an important role in root−shoot signaling during stress (Schachtman and Goodger 2008). During the water stress, plant roots synthesizes abscisic acid and transport it in the shoots via xylem, where it reduces the expansion of leaves and closes the stomata (Wang et al. 2000). Closing of stomata caused by the efflux of potassium ions from the guard cells resulting in loss of turgor pressure, which is promoted by the abscisic acid. Stomatal closure is an important process for plant adaptation under water stress conditions in the field. The level of abscisic acid has been found 50-folds increased due to loss of the turgor pressure of the cell (Guerrero and Mullet 1986). A series of signals from roots to shoots are responsible for the physiological changes in plants and determines the adaptation level of plants during the stress condition.
Photosynthesis
Photosynthesis is an important metabolic process that is directly altered by the drought. It determines the production of a crop. Plants respond to water stress by closing their stomata (Cornic and Massacci 1996; Hu et al. 2004; Farooq et al. 2009a, b), reducing the CO2 entrance into plant leaves, lipid peroxidation and in balancing of water status (Allen and Ort 2001). The amount of heat dissipated by the plant declines with the decreasing transpiration rate (Condon et al. 2002). Higher temperature and water pressure make a negative impact on plant and reduce the leaf photosynthesis and WUE thus ultimately reduce the photosynthesis rate and transpiration rate of the plant. These effects can be partially balanced by the increasing atmospheric CO2 concentration. The lower rate of transpiration for saving the water status can be managed by maintaining the optimal concentration of CO2 in sub stomatal chamber at a lower level of stomatal opening (Condon et al. 2002). Water stress inhibits the gas exchange in plants and this may have resulted in decreased leaf expansion, alteration in photosynthesis system, premature leaf senescence, chloroplast lipid oxidation and changed structure of proteins and pigments (Menconi et al. 1995). In several studies, the net photosynthetic rate of rice plants has been found to increase by 20–30% under the drought stress (Farooq et al. 2009c). It has been also found in several studies that stressful condition affects on photoassimilates (sink strength) (Sharkey 1990; Tezara et al. 1999; Paul and Foyer 2001; Von Caemmerer 2003; Zhou et al. 2007). Sink strength decreases the process of photosynthesis under a stressful environment. Photosynthetic capacity of rice plants has been found a decreased cause of early stomatal closure in response to drought stress resulted to decrease in the assimilation rate and yield of plants (Yang et al. 2019). It has been investigated that when the plants were in gradually stress condition, the distribution of assimilates between roots and shoots was not affected. However, roots were found to have additional biomass in such conditions.
Rice is a C3 cereal and has well defined grain (sink). Flag leaf of the plants has maximum contribution toward photosynthesis process and stores 62–90% carbon for grain development (Yoshida 1981). Tolerant plants maintain their assimilate export from source (leaves) to sink (storage organs) under the unfavorable environment and maintain their growth, development and yield during changed environments. Rubisco activity is reduced under drought conditions and it is responsible for decreasing the photosynthetic activity of a plant (Bota et al. 2004). During the drought, cell shrinks thus reduce the cell volume. At this time, the concentration of solutes in cell increased which may become toxic for the activity and function of the several important enzymes which are required for the photosynthetic process of the plants (Hoekstra et al. 2001).
Effect of Drought Stress on Pigment Composition
Photosynthetic pigments are an essential component of plants that plays an important role in light harvesting and in generating reducing power. Water stress affects both chlorophyll-A and chlorophyll-B (Farooq et al. 2009a, b). However, carotenoids may help to plant to stand against water stress. The changes in chlorophyll and carotenoids under the drought stress condition can be summarized as follows.
Chlorophyll Content
Chlorophyll is a major component of photosynthesis in green plants and has a positive relationship with photosynthetic rate. During the drought stress, chlorophyll degradation and oxidation of pigments occurs and this is considered as a typical symptom of oxidative stress and responsible for decreasing the chlorophyll content of the plant. Drought affects both chlorophyll-A and chlorophyll-B (Farooq et al. 2009a, b).The leaves of plant become old and yellow while losing chlorophyll and decreased the power of photosynthesis during the stress condition (Ahmadi 1985). Stress tolerance of plants is associated with high chlorophyll content (Kraus et al. 1995; Pastori and Trippi 1992). The measurement of chlorophyll fluorescence helps to understand the involvement of chlorophyll during the environmental stress (Brestic and Zivcak 2013; Jedmowski and Bruggemann 2015; Guidi et al. 2019). Stress has a negative impact and decreases chlorophyll content of plant. However, it has an important role in plant adaptation and maintaining the growth and development under the drought condition (Munne-Bosch and Alegre 1999; Sarani et al. 2014).Khan et al. (2017) observed the reduced chlorophyll content in drought stressed plants than normal plants (Nahakpam 2017).They reported the reduction in chlorophyll content of two rice cultivars under drought. The reduction was more pronounced in Super-7 (approx. 14.28%) in compare to PR-115 (approx. 7.04%) variety under drought. Whereas, Prasad et al. (2019) reported reduced chlorophyll content (SPAD value) in five rice genotypes Nagina 22 (approx 3% reduction), Swarna sub1 (approx 30% reduction), NDR 97(approx. 10% reduction), Shusk Samrat (approx 12% reduction) and NDR 9830102 (approx 20% reduction) at reproductive stage. Drought decreased chlorophyll-a content by 11.2 to 61.6% from 3 to 12 days of treatment and chlorophyll-b content by 21.4% at 6 days and it decreased the chlorophyll-a/b ratio by 18.2 to 58.5% from 3 to 12 days of treatment. It also decreased total chlorophyll content by 9.01 to 66.2% from 3 to 12 days of drought stressed condition (Nasrin et al. 2020).
Carotenoids
All photosynthetic and several non-photosynthetic organisms synthesize the carotenoids by de novo method of synthesis. Carotenoids are the molecules that belong to isoprenoid class(Andrew et al. 2008).Carotene plays an important role in the antioxidant defense system of plants but having much susceptibility to oxidative stress. Chloroplast is present in all green plants and contains Beta-carotene which is bound to the core of PSI and PS II complex (Havaux 1998). The concentration of both carotenoids and chlorophyll is decreased under the water stress condition (Kiani et al. 2008) because of ROS production in thylakoids(Reddy et al. 2004).Drought decreased the carotenoids content by 0.41 to 32.7% from 3 to 12 days of treatment (Nasrin et al. 2020). So the tolerance of the plants to drought can be improved by either inducing pigment synthesis or the modification in pathways that involves in pigment synthesis.
Osmolyte Accumulation
Under stressful conditions, different types of organic and inorganic substances are accumulated in the cytosol of the plants to reduce the osmotic potential by which they maintain their cell turgor (Rhodes and Samaras 1994). Several substances like Proline, Sucrose, soluble carbohydrates and others are accumulated under drought stress and improve the water uptake of plants from drying soil. The accumulation process of such substances under water stress conditions is called osmotic adjustment. The accumulation of proline occurs firstly in the plants under water stress condition to decrease the cells injury. Glycine betaine is also an important substance that improves the resistance of plants to several abiotic stresses. It has been suggested by several scientists to introduce the gene of glycine betaine in non-glycine betaine producing plants because it helps to improve the resistance of plant to abiotic stress (Chen and Murata 2002; Sakamoto and Murata 2002; Kumar et al. 2004). However, glycine betaine is not produced by rice plants in stress or non-stress condition (Rhodes and Hanson 1993). So, the transgenic plant of rice with overexpression of glycine betaine gene will increase the production of glycine betaine and shows resistance to water stress (Rhodes and Hanson 1993).
Seed Priming
When the metabolic processes for seed germination starts but the radical of seeds does not emerge cause of partial hydration of seeds is known as seed priming (Bradford 1986). It regulates the seed within lag phase (Taylor et al. 1998), so that seeds are not able to reach in log phase of growth. Primed seeds in rice usually show improved rate of germination, equal germination and percentage of total germination (Farooq et al. 2006; 2009d). It happens because of metabolic repair (Farooq et al. 2006; 2009d) during the process of seed imbibitions (Farooq et al. 2006).
Effects of Drought on Biochemical Characteristics
To maintain cell turgidity under water stressed conditions, plants accumulate several organic and inorganic compounds in the cytosol to maintain their osmotic potential (Rhodes and Samaras 1994). The process of biochemicals accumulation in cell is known as osmotic adjustment. Sucrose, glycine betaine, Proline, and several other solutes when accumulates in cytoplasm, osmotic adjustment is achieved which improves water uptake by a plant from drying soil. Among these compounds, due to its large importance in the stress tolerance, proline is most widely studied (Mostajeran and Rahimi-Eichi 2009; Chutia et al. 2012). The accumulation of soluble sugars is also induced by drought (Usman et al. 2013; Maisura et al. 2014). At molecular level, reactive oxygen species (ROS) is produced by the cell as a result of drought, which causes oxidative stress in cell. In terms of thiobarbituric acid (TBA) and thiobarbituric acid reactive substances (TBARS), the level of superoxide radical (O2) and lipid peroxidation (LPO) have been found increased, and on the other hand, the concentration of total soluble proteins and thiols have been found decreased in rice plants under drought. Several other experiments showed enhanced antioxidant enzymes like superoxide dismutase (SOD), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), glutathione reductase (GR) and dehydroascorbate reductase (DHAR) activity in the rice crop when subjected to drought stress (Sharma and Dubey 2005). Rice plants activate the antioxidants defense mechanism and increase the level of these enzymes during oxidative stress by drought. Better antioxidant production was observed in drought-tolerant varieties as compared to susceptible ones (Lum et al. 2014). Also, the drought tolerance varieties shows increased proline level and higher soluble sugars production under drought stress (Mostajeran and Rahimi-Eichi 2009; Chutia et al. 2012).
Role of Proline Under Drought
Proline plays an important role in plants when the plant suffered by various stress (Verbruggen and Hermans 2008). In rye grasses, Kemble and Mac-Phersonin (1954) firstly reported the accumulation of free proline due to water stress. Due to its osmolytic properties, proline accumulation contributes to drought tolerance and better performance to plant (Vajrabhaya et al. 2001). Under drought stress condition in rice, the variation in proline concentration has been observed (Sheela and Alexallder 1995; Mostajeran and Rahimi-Eichi 2009; Bunnag and Pongthai 2013; Kumar et al. 2014; Lum et al. 2014; Maisura et al. 2014).Proline accumulation in the leaf of rice was increased by 1.3- to 10.2-folds from 9 to 18 days of drought treatment (Nasrin et al. 2020).Likewise, Swapna and Shylaraj (2017) imposed the drought by15% PEG6000 with osmotic potential (OP) of − 2.35 to − 2.95 MPa for 5 days at (28 ± 2) °C and observed the increased proline content by 31.81-folds in Swarnaprabha variety under drought with compare to control plants. Proline has an osmolytic activity and plays three important roles, i.e., as a defense molecule (saves the cell by oxidative stress), as a metal chelator and a signaling molecule during drought stress (Hayat et al. 2012). The accumulation of proline under drought stress increases the antioxidants activity which improves the damage repairing ability of plants. Proline can be used as a biochemical marker for screening the varieties for drought-tolerant under drought stress condition (Fahramand et al. 2014).
Role of Polyamines Under Drought
Polyamines are the important molecule involved in plant response to drought (Calzadilla et al. 2014). Polyamines like Putrescine, spermine and spermidine are mostly present in plants. They act as antioxidant, help in membrane stabilization and controls ionic and osmotic homeostasis. Higher polymer content is seen by the water-tolerant plant and this is attributable to increased photosynthesis ability, decreased water loss and improved osmotic responses. Many scientists suggested the role of polyamines, like it improves the binding activity of TFs (Panagiotidis et al. 1995) to DNA thus regulates action of gene, ionic maintenance, senescence inhibition, preventing free radical formation and helps to stabilizing membranes (Bouchereau et al. 1999), involved in protein phosphorylation and conformational changes of DNA (Martin-Tanguy 2001). Under the drought condition in rice, the instant response of polyamines accumulation has been observed (Yang et al. 2007; Basu et al. 2010). It has been suggested in several studies that rice plants have higher capacity to biosynthesis of PAs especially spermidine and spermine under the drought condition (Yang et al. 2007). Thus rice plants can enhance their tolerance to drought. Several events, mechanisms and components such as photosynthesis, WUE, synthesis of Proline, phenolics and anthocyanins respectively can be improved by the application of polyamines (Farooq et al. 2009c). The foliar application has been found more efficient than seed priming in several studies and spermidine is found more effective among polyamines to enhance tolerance in rice plant under drought (Farooq et al. 2009c; Do et al. 2013).Genetic engineering technology can be used to modify the Polyamines level of a plant. A gene, Datura adc, encodes arginine decarboxylase has been introduced in rice. These transgenic rice plants produce higher level of putrescine under drought, enhance the synthesis of spermidine and spermine and resulted plants save themselves by drought stress (Capell et al. 2004).Thus, the drought-tolerant plants can be produced by overexpression of the polyamines synthesizing gene in transgenic plants (Calzadilla et al. 2014).
Role of Plant Growth Regulators Under Drought
Plant growth regulators are the substances that can regulate the growth and development of plants. They act as chemical messengers for intracellular communication (Fishel 2006). It has been found that PGRs improve the plant tolerance to drought. Rice plants accumulate ABA under drought (Wang et. al. 2007; Ye et al. 2011; Ashok et al. 2013). ABA reduces the water stress effects and helps to better growth and development of plants under the stress condition. ABA reduces loss of water through transpiration by stomatal closure (Zhu 2002; Wang and Song 2008) and induces the expression of several genes whose products are involved in providing strength to plant under drought. ABA significantly increases the level of antioxidant enzymes (Latif 2014; Li et al. 2014), improves protein transport, inducing the carbon metabolism and resistance proteins expression, resulted plant shows tolerance to drought (Zhou et al. 2014; Pandey and Shukla 2015). It has been found in several studies that cytokines regulates the cell differentiation, leaf senescence and other important processes of plant growth and development (Sakakibara et al. 2006; Reguera et al. 2013). It has been found to regulate the assimilate partitioning (Ronzhina et al. 1994), sink strength (Kuiper 1993) and relationship of source/sink (Roitsch 1999). The increased level of cytokines improves the survival rate of plants under drought (Rivero et al. 2007). It has been found that synthesis of cytokinins in rice improves the drought tolerance (Peleg and Blumwald 2011; Reguera et al. 2013).
Role of Antioxidant Under Drought
Drought stress imparts disturbance between the production and quenching of reacting oxygen species (ROS) (Smirnoff 1998; Faize et al. 2011). ROS cause lipid peroxidation, proteins denaturation, DNA mutation, disturbance in cellular homeostasis and several other types of oxidative damages in a cell by the production of hydrogen peroxide, singlet oxygen, superoxide radical and hydroxyl free radical. The plant cell saves themselves from harmful effect of ROS by the use of complex antioxidation defense system. The antioxidant defense system maybe non-enzymatic as well as enzymatic. In the cell, SOD, MDHAR, GPX, DHAR, APX and GR are enzymatic antioxidants whereas ascorbate and glutathione are non-enzymatic antioxidants (Bors et al. 1989; Noctor and Foyer 1998). These antioxidants play an important role in ROS scavenging system and presence of these antioxidants in cell can improve the drought tolerance of rice plants. In rice, the action of ASA, APX, GSH (Selote and Khanna-Chopra 2004), SOD, MDHAR, DHAR, GR (Sharma and Dubey 2005), Phenyl ammonia lyase and CAT (Shehab et al. 2010) has been found increased with the increased level of drought stress. Catalase activity was found to be increased in the leaf of rice by 74.70% and also increased superoxide dismutase activity in the leaf of rice by 1.1-folds at 15 days of drought treatment (Nasrin et al. 2020). Nahar et al. (2018) found the increased level of CAT in shoots (approx 25%) and roots (approx. 28.57%), increased level of SOD in shoots (approx. 100%) and roots (approx. 111%), increased level of GPX in shoots (approx.100%) and roots (approx. 78%), increased level of APX in shoots (approx. 100%) and roots (approx. 150%), increased level of GR in shoots (approx. 155%) and roots (approx 140%) by comparing with control plants under drought stress applied by using PEG6000 using different varieties. In the tolerant cultivar, SOD enzymatic activity increased significantly only in the root tissue in the reproductive stage (268.00 Un/Mg), whereas in the sensitive cultivar, SOD increased in the leaf (112.17 Un/Mg) and root (172.56 Un/Mg) tissues during the reproductive stage (Deus et al. 2015). The increased level of these antioxidant enzymes protects the plants from oxidative damages under the drought stress in rice. SOD, POD and CAT are found to effectively reducing the ROS production and thereby saving the plants from negative effect of drought stress (Lum et al. 2014; Yang et al. 2014).Wang et al. (2019) reported the activity of SOD, POD and CAT which maintains the ability to scavenge hydrogen peroxide under drought condition, was increased with accumulation of time and intensity of drought stress in rice. Therefore, increasing the production of naturally occurring antioxidant enzymes can be a strategy to save the plants from oxidative damage and enhancing the drought resistance of plants (Hasanuzzaman et al. 2014).
Molecular Responses and Mechanism of Drought
Underpinning molecular basis of drought tolerance is very crucial for development of drought-tolerant varieties. Over the past two decades, extensive studies have been conducted to understand molecular basis of drought tolerance in rice (McCouch et al. 2003; Yue et al. 2006; Pandey and Shukla 2015). Transcriptomics analyses of rice genotypes under drought stress have shown differential expression of large number of genes associated with physiological, biochemical and signaling pathways to be critical for drought tolerance response (Rabbani et al. 2003). Among the main categories of genes differentially expressed under drought are (1) genes associated with biosynthesis of osmolytes, (2) genes involved in water uptake and transport; water channels and transporters, (3) genes encoding for detoxification enzymes and protective function proteins, (4) transcriptional control/ signaling pathways associated genes (Yamaguchi-Shinozaki and Shinozaki 2005). Drought-induced intricate signaling network is a very critical component for activation molecular level responses that may determine adaptation of rice genotypes to low water regime. In rice, like any other plant species, drought stress is sensed at the level of plasma membrane level and transmitted through a range of signaling molecules (transducers) and thus finally regulate downstream genes encoding for protective proteins such as antioxidant enzymes, osmolyte biosynthesis pathway genes and stress associated proteins. Among the various transducers involved in drought signaling, receptor like kinases (RLKs) are the most important as these activate protein phosphorylation cascade needed for early stress responses (Tena et al. 2011; Ramegowda et al. 2014). RLKs are known to sense drought stress at the plasma membrane level by their extracellular domain which leads to dimerization followed by phosphorylation of the cytoplasmic domain that subsequently activates downstream signaling components. In rice two RLKs, GROWTH UNDER DROUGHT KINASE (GUDK) and STRESS INDUCED PROTEIN KINASE 1 (OsSIK1) are shown to be critical for drought tolerance response (Ouyang et al. 2010; Ramegowda et al. 2014). The GUDK phosphorylates a APETALA2/ETHYLENE RESPONSE FACTOR OsAP37 which then activates a range of drought stress responsive genes.
The drought responsive mechanisms in plant are broadly grouped into ABA-dependent and ABA-independent pathways (Liu et al. 2018). Water deficit causes enhanced ABA accumulation in vegetative parts leading to a range of morphological effects like stomata closure, accumulation of stress associated proteins and metabolites with protective function, and accumulation of H2O2 in guard cell. In a recent study, it was reported that early stage low water deficit treatment induced early flowering through ABA-dependent pathway and enabled rice plants escape drought (Du et al. 2020). Similarly, overexpression of LOS5/ABA3, which is involved in ABA biosynthesis was found to improve the grain filling and grain yield in rice under drought stress (Hu and Xiong 2014). The ABA-dependent responses essentially result from expression of ABA-responsive genes that contain a conserved ABA-responsive element (ABRE) in their promoter regions. On the other hand ABA- independent pathway involves drought-induced genes whose expression does not depend on accumulation of ABA and their promoter regions are.
Transcription factors (TFs) have also been implicated as major regulators of drought stress response in plants (Sahebi et al. 2018). TFs binds to the cis-elements located in the promoter region of their target downstream genes to regulate their expression and in turn responses to drought stress. Over the years many TF families such as AP2/ERF, MYB, NAC, WRKY, NF-Y and CAMTA have been demonstrated to have a role in abiotic stress tolerance (Mizoi et al. 2012, Puranik et al. 2012 and Lindemose et al. 2013). Notably, overexpression of some of the members of these TF genes under stress inducible or constitutive promoters conferred enhanced tolerance in rice against drought stress. Among these, AP2/ERF family TF, DREB that interacts with C-repeat/DRE (A/GCCGAC) elements have been most widely used to create drought-tolerant rice lines (Wang et al. 2008; Chen et al. 2008). Transgenic rice lines overexpressingOsDREB1G and OsDREB2B showed significantly higher level of tolerance to drought stress (Chen et al. 2008). Several members of NAC family also have role in drought tolerance. For example, a stress responsive NAC 1 (SNAC1), when over-expressed in rice, improved drought tolerance by regulating expression of its target gene OsSRO1c (You et al. 2013). Many other studies have demonstrated enhanced tolerance in rice lines expressing transcription factor genes such as HVCBF4 (Oh et al. 2007), Ap37 (Kim and Kim 2009, Oh et. al. 2009), OsNAC045 (Zheng et al. 2009), TSRF 1 (Quan et al. 2010), JERF 3 (Zhang et al. 2010a, b), OsDREB2A (Cui et al. 2011), OsDREB2A (Mallikarjuna et al. 2011), SbDREB 2 (Bihani et al. 2011), Os5DIRI (Datta et al. 2012), At DREB1A (Hussain et al. 2014; Ravikumar et al. 2014), OsNAC6 (Rachmat et al. 2014), and the BZIP family genes (Xiang et al. 2008; Liu et al. 2014a, b). Therefore, TFs have been considered as key targets to generate stress tolerance plants using genetic engineering approaches (Liu et al. 2014a, b).
Besides, signaling and transcription factor genes, there are many other proteins that play role in drought response. Accumulation of dehydrins and Late Embryogenesis Abundant (LEA) proteins in response to both low-temperature and water stress have been observed (Close 1997). LEA proteins are small proteins ranging from 10 to 30 kDa that are mostly present in plants that are produced during the embryo development maturation period and function as chaperons (Yadira and Reyes 2011). Overexpression of OsLEA3 in rice enhanced drought tolerance in response to water stress. Similarly, overexpression of HVA1 gene from barley which encodes LEA protein conferred enhanced tolerance in rice (Sivamani et al. 2000; Babu et al. 2004). Many studies reported changes in individual gene expression in rice under drought stress condition. These include glutathione reductase (Kaminaka et al. 1998), a translation elongation factor (Li and Chen 1999), an endo-1, 3-glucanase (Akiyama and Pillai 2001).
Drought Tolerance Associated Quantitative Trait Loci (QTLs)
A plant’s drought tolerance system is very complex, regulated by several genes known as Quantitative Trait Loci (QTLs) (Fleury et al. 2010). The use of genomic methods has contributed to the discovery of QTLs correlated with characteristics of drought tolerance. Some of the significant and consistent grain yield QTLs during reproductive stage have been successfully established and validated by the rice drought breeding program at IRRI (qDTYs) such as qDTY12.1 (Bernier et al. 2007), qDTY2.1 (Venuprasad et al. 2009), qDTY3.1(Venuprasad et al. 2009), qDTY1.1 (Vikram et al. 2011; Ghimire 2012), qDTY3.2 (Vikram et al. 2011), qDTY2.2 (Swamy et al. 2013), qDTY6.1 (Dixit et al. 2014a, b)using molecular markers. A variety of mapping experiments on drought stress in rice have been published. A list of QTLs listed for rice under drought is shown in Table 2.
By evolving different protein classes including transcriptional factors, molecular chaperones, enzymes, and other functional proteins, plants have evolved stable stress mechanisms or signaling chain processes (Usman et al. 2017). These proteins maximize plants’ tolerance or immunity to drought conditions. In fact, multiple genomic methods have detected a several number of these genes (regulatory part and protein). These genes have been introduced into the rice genome either by repression or by overexpressions (Table 3) to examine their effect on drought improvement.
miRNA and Drought Responses
miRNAs are 20–22 nt long molecules that serve as an important regulator of genes at the post-transcriptional level (Baldrich et al. 2015; Gebert and MacRae 2018). They have been implicated in multiple processes such as growth and development, photosynthesis, biotic and abiotic stress responses and hormone signaling. miRNAs work closely with target genes and TFs to regulate associated processes. Studies have shown the role for miRNA genes in shaping drought responses of plants. Differential expression of miRNA genes has been observed under stress. Under drought stress, miRNA genes act by regulation of ABA induced signaling (Samad et al. 2017). There are several families of TFs implicated under drought stress that regulate auxin-induced changes in tissues and organs, such as auxin response factors (ARFs), the NAC domain and AGO1(Sorin et al. 2005). miR160 targets ARF10 and ARF16, while ARF6 and ARF8 are controlled by miR167 in rice (Huang et al. 2014a, b; Li et al. 2016). Both miR160 and miR167 were downregulated in rice roots under drought stress. MiR164 targeted NAC genes and was shown to negatively regulate the resistance of drought in rice (Fang et al. 2014a, b).
On the other hand, miR160 negatively controls ARF10, resulting in sensitivity to ABA, which suggests cross-talk between the two hormones (Liu et al. 2007a, b, c). In rice, the miR167-ARF8-GH3 pathway is strongly reserved (Yang et al. 2006). In rice (Zhao et al. 2007), miR397 was also induced under drought conditions. Up regulation of Auxin responsive genes are occurred by suppressing miR396, where OsGRF6 positively controls auxin signaling in rice(Hu et al. 2018; Liu et al. 2014a, b).MiR396 and miR397 cross-talk and co-expression networks provide evidence that brassinosteroid (BR) and auxin is involved in controlling rice growth and yield (Zhang et al. 2014). BR cross talks with auxin and or GA to monitor rice yield (Niu et al. 2017). In Arabidopsis and rice, miR156 was dramatically induced, where it negatively controls SPL9 to improve tolerance of abiotic stress such as salinity and drought (Cui et al. 2014). The knockdown of miRNA166 in rice resulted in morphological changes related to the resistance to drought, such as leaf rolling and xylem constriction (Zhang et al. 2018a, b). In addition, miR167, miR394 and miR399 control physiological changes to the adventitious root growth in drought stress during mineral deficiency in rice (Grewal et al. 2018). MiR408 cleaves OsUCL8 (plastocyanin-like protein) to positively control photosynthesis and grain yield in rice (Zhang et al. 2017). MiR397 is downregulated in rice under drought (Zhou et al. 2010; Fahad et al. 2017) while miR397b is upregulated in Arabidopsis by ABA and drought. Another modulator, miR398, controls rice respiration by targeting subunit V (COX5b) of cytochrome C oxidase, which functions as an electron transporter in respiration (Ding et al. 2013; Cheah et al. 2015).
Two zinc finger TFs are regulated by miR159 such as OsTZF1 (CCCH-tandem zinc finger protein 1) and OsDOS (delay of senescence protein onset) that negatively control senescence and thus provide immunity to abiotic stresses such as drought in rice (Kong et al. 2006; Pomeranz et al. 2010). MiR159, along with miR167 and miR172, mediates resistance to senescence in rice (Lim et al. 2003). In addition, miR164 was strongly expressed in rice leaves, where SIP19 (salicylic acid-induced protein 19) was negatively regulated by it, hence delayed senescence (Xu et al. 2014). NAC genes are also targeted to negatively control drought resistance by miR164 in rice (Fang et al. 2014a, b).
Seven miRNAs were identified by Li et al. (2011a, b) in rice during oxidative stress which was responsible for the regulation of H2O2 (miR169, miR319a.2, miR397, miR408-5p, miR528, miR827, miR1425). Many others like miR164 and miR169 (Andres-Colas et al. 2018), downregulation ofmiR159f, miR397a, miR398b, miR408-3p, miR528-5p, miR1871, and miR2878-5p was found to involve in antioxidant defense system.
Fertilizer Management
Fertilizers input play vital role in increasing crop productivity. Over the past five decades, productivity of rice in irrigated ecosystems has increased due to the combined use of modern semi-dwarf varieties and high inputs of nitrogen fertilizers (Banayo et al. 2021a, b). The improved fertilizer management system, known as “site-specific nutrient management,” has been developed for use in rice cultivation in tropical Asia, where fertilizer requirements of rice crop are estimated from the nutrient supply of the soil and the needs of plants (Dobermann et al. 2002). This approach has been extensively evaluated in farm studies over the past two decades (Buresh et al. 2019; Pampolino et al. 2007). Among the major plant nutrients, appropriately higher levels of nitrogen have been shown to alleviate drought stress tolerance and reduce grain yield losses in rice as well as many other species. The plant supplied with appropriate level of nitrogen have better stomatal dynamic, maintain photosynthetic integrity, elasticity in root system development and have strong antioxidant capacity. A recent study by Du et al. (2020) suggests that heavy nitrogen application before start of the drought may be an important approach to improve grain yield in rice. However, too much of nitrogen application in rice nursery can produce seedling with weak stem that are easily damaged during transplanting. Further, rice varieties are also known to differ in their response to N fertilizer, therefore choosing fertilizer-efficient varieties in drought conditions appears to be the key to increasing rice productivity through better fertilizer management in the rainfed lowlands (Haefele et al. 2016; Banayo et al. 2021a, b).
Conclusions
Abiotic stress signaling is a significant area for augmenting plant productivity. Among the abiotic stress, drought is a major global problem, which severely reduced quality and crop production worldwide. Due of advances in omic-based integrated approaches and rice genome sequencing project provides the genetic and genomic information to the researchers for improving the trait against stress condition. Several novel molecular markers were investigated after genome sequencing project and being utilized for crop improvement. The responses of rice at genomic, transcriptomic and phenomics levels under drought stress are also decoded and this information can be utilized for improving drought tolerance in rice using integrated approaches. The integration of the multidisciplinary fields like genomics, transcriptomics and other omics with precise phenotyping can be used for the improvement of rice against drought stress in future and this can result in the higher production of rice for global food security.
References
Akiyama T, Pillai MA (2001) Molecular cloning, characterization and in vitro expression of a novel endo-1,3-beta-glucanase up-regulated by ABA and drought stress in rice (Oryza sativa L.). Plant Sci 161:1089–1098
Ali ML, Pathan MS, Zhang J, Bai G, Sarkarung S, Nguyen HT (2000) Mapping QTLs for root traits in a recombinant inbred population from two indica ecotypes in rice. Theor Appl Genet 101:756–766
Allen DJ, Ort D (2001) Impact of chilling temperatures on photosynthesis in warm climate plants. Trends Plant Sci 6:36–42
Andres-Colas N, Carrió-Seguí A, Abdel-Ghany SE, Pilon M, Peñarrubia L (2018) Expression of the intracellular COPT3-mediated Cu transport is temporally regulated by the TCP16 transcription factor. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00910
Andrew JS, Moreau H, Kuntz M, Pagny G, Lin C, Tanksley S, McCarthy J (2008) An investigation of carotenoid biosynthesis in Coffea canephora and Coffea arabica. J Plant Physiol 165:1087–1106
Ashok K, Kumar SM, Sudha M, Vijayalakshmi D, Vellaikumar S, Senthil N, Raveendran M (2013) Identification of genes controlling ABA accumulation in rice during drought stress and seed maturation. Int J Adv Biotechnol Res 4(4):481–487
Atlin GN, Lafitte HR, Tao D, Laza M, Amante M, Courtois B (2006) Developing rice cultivars for high-fertility upland systems in the Asian tropics. Field Crops Res 97:43–52
Babu RC, Shashidhar HE, Lilley JM, Thanh ND, Ray JD, Sadasivam S, Sarkarung S, O’Toole JC, Nguyen HT (2001) Variation in root penetration ability, osmotic adjustment and dehydration tolerance among accessions of rice adapted to rainfed lowland and upland ecosystems. Plant Breed 120:233–238
Babu RC, Zhang J, Blum A, Ho TH, Wu R, Nguyen HT (2004a) HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Plant Sci 166:855–862
Bae H, Kim SK, Cho SK, Kang BG, Kim WT (2011) Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.). Plant Sci 180:775–782
Baisakh N, Yabes J, Gutierrez A, Mangu V, Peiyong Ma, Famoso A, Pereira A (2020) Genetic mapping identifies consistent quantitative trait loci for yield traits of rice under greenhouse drought conditions. Genes 11:62. https://doi.org/10.3390/genes11010062
Baldrich P, Campo S, Wu MT, Liu TT, Hsing YIC, Segundo BS (2015) MicroRNA-mediated regulation of gene expression in the response of rice plants to fungal elicitors. RNA Biol 12:847–863
Banayo NP, Rahon RE, Cruz PS, Kato Y (2021) Fertilizer responsiveness of high-yielding drought-tolerant rice in rainfed lowlands. Plant Prod Sci 24(3):279–286
Banayo NP, Rahon RE, Cruz PS, Kato Y (2021) Fertilizer responsiveness of high-yielding drought-tolerant rice in rainfed lowlands. Plant Prod Sci 24(3):279–286
Basu S, Roychoudhury A, Saha PP, Sengupta DN (2010) Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. Acta Physiol Plant 32(3):551–563
Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. F1000 Res 5:1554
Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T (2007) Specific and unspecific responses of plants to cold and drought stress. J Biosci 32:501–510
Bernier J, Kumar A, Venuprasad R, Spaner D, Atlin GN (2007) A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Sci 47:507–516
Bihani P, Char B, Bhargava S (2011) Transgenic expression ofsorghum DREB2 in rice improves tolerance and yield under water limitation. J Agric Sci 149(1):95–101
Blum A, Shpiler L, Golan G, Mayer J (1989) Yield stability and canopy temperature of wheat genotypes under drought stress. Field Crop Res 22:28996
Bors W, Langebartels C, Michel C, Sandermann JH (1989) Polyamine as radical scavengers and protectants against ozone damage. Phytochemistry 28:1589–1595
Bota J, Flexas J, Medrano H (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681
Bouchereau A, Aziz A, Larher F, Tanguy M (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bouman BAM, Peng S, Castaneda AR, Visperas RM (2005) Yield and water use of irrigated tropical aerobic rice systems. Agric Water Manag 74:87–105
Boyer JS (1996) Advances in drought tolerance in plants. Adv Agron 56:187–218
Bradford KJ (1986) Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Hort Sci 21:1105–1112
Brestic M, Zivcak M (2013) PS II Fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: protocols and applications. Molecular stress physiology of plants. Springer, New Delhi, pp 87–131
Bunnag S, Pongthai P (2013) Selection of rice (Oryza sativa L.) cultivars tolerant to drought stress at the vegetative stage under field conditions. Am J Plant Sci 4(9):1701–1708
Buresh RJ, Castillo RL, Dela Torre JC, Laureles EV, Samson MI, Sinohin PJ, Guerra M (2019) Site-specific nutrient management for rice in the Philippines: calculation of field-specific fertilizer requirements by Rice Crop Manager. Field Crop Res 239:56–70
Calzadilla PI, Gazquez A, Maiale SJ, Rodriguez AA, Ruiz OA, Bernardina MA (2014) Polyamines as indicators and modulators of the abiotic stress in plants. In: Anjum NA, Gill SS, Gill R (eds) Plant adaptation to environmental change: significance of amino acids and their derivatives. CABI, Wallingford, pp 109–128
Capell T, Escobar C, Liu H, Burtin D, Lepri O, Christou P (1998) Over-expression of the oat arginine decarboxylase cDNA in transgenic rice (Oryza sativa L.) affects normal development patterns in vitro and results in putrescine accumulation in transgenic plants. Theor Appl Genet 97:246–254
Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101(26):9909–9914
Carrijo DR, Lundy ME, Linquist BA (2017) Rice yields and water use under alternate wetting and drying irrigation: a meta-analysis. Field Crop Res 203:173–180
Cheah BH, Nadarajah K, Divate MD, Wickneswari R (2015) Identification of four functionally important microRNA families with contrasting differential expression profiles between drought-tolerant and susceptible rice leaf at vegetative stage. BMC Genom 16:692
Chen THH, Murata N (2002) Enhancement of tolerance to abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257
Chen JQ, Meng XP, Zhang Y, Xia M, Wang XP (2008) Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol Lett 30:2191–2198. https://doi.org/10.1007/s10529-008-9811-5
Chutia J, Borah SP, Tanti B (2012) Effect of drought stress on protein and proline metabolism in seven traditional rice (Oryza sativa L.) genotypes of Assam, India. J Res Biol 2(3):206–214
Clauw P, Coppens F, De Beuf K, Dhondt S, Van Daele T, Maleux K, Inze D (2015) Leaf responses to mild drought stress in natural variants of Arabidopsis thaliana. Plant Physiol 167(3):800–816
Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol Plant 100:291–296
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water use efficiency and crop yield. Crop Sci 42:122–131
Cornic G, Massacci A (1996) Leaf photosynthesis under drought stress. In: Baker NR (ed) Photosynthesis and the environment. Kluwer Academic Publishers, Dordrecht
Cruz RT, O’Toole JC (1984) Dry land rice to an irrigation gradient at flowering stage. Agron J 76:178–183
Cui M, Zhang WJ, Zhang Q, Xu ZQ, Zhu ZG, Duan FP, Wu R (2011) Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol Biochem 49(12):1384–1391
Cui LG, Shan JX, Shi M, Gao JP, Lin HX (2014) The miR156-SPL 9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J 80:1108–1117
Cui Y, Zhang W, Lin X, Xu S, Xu J, Li Z (2018) Simultaneous improvement and genetic dissection of drought tolerance using selected breeding populations of rice. Front Plant Sci 9:320
Cutler JM, Steponkus PL, Wach MJ, Shahan KW (1980) Dynamic aspects and enhancement of leaf elongation in rice. Plant Physiol 66:147–152
Daryanto S, Wang L, Jacinthe PA (2017) Global synthesis of drought effects on cereal, legume, tuber and root crops production: a review. Agric Watermanag 179:18–33
Datta K, Baisakh N, Ganguly M, Krishnan S, Shinozaki KY, Datta SK (2012) Overexpression of Arabidopsis and rice stress genes inducible transcription factor confers drought and salinity tolerance to rice. Plant Biotechnol J 10(5):579–586
de Datta SK, Malabuyoc JA, Aragon EL (1988) A field screening technique for evaluating rice germplasm for drought tolerance during the vegetative stage. F Crop Res 19:123–134
Delphine F, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot 61(12):3211–3222
Deus KE, Lanna AC, Abreu FRM, Silveira RDD, Pereira WJ, Brondani C, Vianello RP (2015) Molecular and biochemical characterization of superoxide dismutase (SOD) in upland rice under drought. Aust J Crop Sci 9(8):744–753
Ding Y, Tao Y, Zhu C (2013) Emerging roles of microRNAs in the mediation of drought stress response in plants. J Exp Bot 64:3077–3086
Dixit S, Swamy BPM, Vikram P, Ahmed HU, Sta Cruz MT, Amante M, Atri D, Leung H, Kumar A (2012) Fine mapping of QTLs for rice grain yield under drought reveals sub-QTLs conferring a response to variable drought severities. Theor Appl Genet 125:155–169
Dixit S, Singh A, Cruz MTS, Maturan PT, Amante M, Kumar A (2014a) Multiple major QTL lead to stable yield performance of rice cultivars across varying drought intensities. BMC Genet 15:16
Dixit S, Singh A, Kumar A (2014) Rice breeding for high grain yield under drought: a strategic solution to a complex problem. Int J Agron 2014:1–15
Do PT, Degenkolbe T, Erban A, Heyer AG, Kopka J, Kohl KL, Hincha DK, Zuther E (2013) Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS One 8(4):e60325
Dobermann A, Witt C, Dawe D, Gines GC, Nagarajan R, Satawathananont S, Satawathananont S, Son TT, Tan PS, Wang GH, Chien NV, Thoa VTK, Phung CV, Stalin P, Muthukrishnan P, Ravi V, Babu M, Chatuporn S, Sookthongsa J, Fu R et al (2002) Site-specific nutrient management for intensive rice cropping systems in Asia. Field Crop Res 74(1):37–66. https://doi.org/10.1016/S0378-4290(01)00197-6
Du H, Wang N, Cui F, Li X, Xiao J, Xiong L (2010) Characterization of carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and ABA synthesis in rice. Plant Physiol 154:1304–1318
Du J, Shen T, Xiong Q et al (2020a) Combined proteomics, metabolomics and physiological analyses of rice growth and grain yield with heavy nitrogen application before and after drought. BMC Plant Biol 20:556. https://doi.org/10.1186/s12870-020-02772-y
Duan J, Cai W (2012) OsLEA3–2, an abiotic stress induced gene of rice plays a key role in salt and drought tolerance. PLoS ONE 7:e45117
Ekanayake IJ, Steponkus PL, DeDatta SK (1989) Spikelet sterility and flowering response of rice to water stress at anthesis. Ann Bot 63:257–264
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Fahramand M, MahmoodyM KA, Noori M, Rigi K (2014) Influence of abiotic stress on proline, photosynthetic enzymes and growth. Int Res J Appl Basic Sci 8(3):257–265
Faize M, Burgos L, FaizeL PA, Nicolas E, Barba-Espin G, Clemente-Moreno MJ, Alcobendas R, Artlip T, Hernandez JA (2011) Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot 62:2599–2613
Fang Y, Xie K, Xiong L (2014a) Conserved miR164-targeted NAC genes negatively regulate drought resistance inrice. J Exp Bot 65:2119–2135
Fang Y, Xie K, Xiong L (2014b) Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J Exp Bot 65:2119–2135
Farooq M, Basra SMA, Khalid M, Tabassum R, Mehmood T (2006) Nutrient homeostasis, reserves metabolism and seedling vigor as affected by seed priming in coarse rice. Can J Bot 84:1196–1202
Farooq M, Basra SMA, Wahid A, Cheema ZA, Cheema MA, Khaliq A (2008) Physiological role of exogenously applied glycine betaine in improving drought tolerance of fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 194:325–333
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009a) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Farooq M, Wahid A, Lee D, Ito O, Siddique KHM (2009b) Advances in drought resistance of rice. Crit Rev Plant Sci 28(4):199–217
Farooq M, Basra SMA, Wahid A, Ahmad N, Saleem BA (2009) Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J Agron Crop Sci 195(4):237–246
Farooq M, Basra SMA, Wahid A, Khaliq A, Kobayashi N (2009) Rice seed invigoration. In: Lichtfouse E (ed) Sustainable agriculture reviews. Springer, Dordrecht
Farooq M, Kobayashi N, Wahid A, Ito O, Basra SMA (2009e) Strategies to produce more rice with less water. Adv Agron 10:351–387
Fleury D, Jeferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot 61:3211–3222
Fontes F, Gorst A, Palmer C (2020) Does choice of drought index influence estimates of drought-induced rice losses in India? Environ Dev Econ 25(5):459–481
Gao T, Wu Y, Zhang Y, Liu L, Ning Y, Wang D, Xie Q (2011) OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol Biol 76:145–156
Garrity DP, O’Toole JC (1994) Screening rice for drought resistance at the reproductive phase. Field Crops Res 39:99–110
Gebert LF, MacRae IJ (2018) Regulation of microRNA function in animals. Nat Rev Mol Cell Biol 20:21–37
Ghimire KH (2012) Identification and mapping of QTL (qDTY1.1) with a consistent effect on GY under RS. Field Crops Res 131:88–96
Grewal RK, Saraf S, Deb A, Kundu S (2018) Differentially expressed microRNAs link cellular physiology tophenotypic changes in rice under stress conditions. Plant Cell Physiol 59:2143–2154
Guan YS, Serraj R, Liu SH, Xu JL, Ali J, Wang WS, Venus E, Zhu LH, Li ZK (2010) Simultaneously improving yield under drought stress and non-stress conditions: a case study of rice (Oryza sativa L.). J Exp Bot 61:4145–4156
Guerrero F, Mullet JE (1986) Increased abscisic acid biosynthesis during plant dehydration requires transcription. Plant Physiol 80:588–591
Guidi L, Piccolo E, Landi M (2019) Chlorophyll fluorescence, photoinhibition and abiotic stress: does it make any difference the fact to be a C3 or C4 species. Frontiers 10(174):1–11
Guimarães CM, Stone LF, Silva ACDL (2016) Evapotranspiration and grain yield of upland rice as affected by water deficit. Rev Bras Eng Agríc Ambient 20:441–446
Haefele SM, Kato Y, Singh S (2016) Climate ready rice: Augmenting drought tolerance with best management practices. Field Crop Res 190:60–69
Harris D, Tripathi RS, Joshi A (2002) On-farm seed priming to improve crop establishment and yield in dry direct-seeded rice. Direct seeding: research strategies and opportunities. International Research Institute, Manila, pp 231–240
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int 2014:1–17
Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3:147–151
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
Hemamalini GS, Shashidhar HE, Hittalmani S (2000) Molecular marker assisted tagging of morphological and physiological traits under two contrasting moisture regimes at peak vegetative stage in rice (Oryza sativa L.). Euphytica 112:69–78
Henson IE (1985) Modification of leaf size in rice (Oryza sativa L.) and its effects on water stress-induced abscisic acid accumulation. Ann Bot 56:481–487
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6:431–438
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Hsiao TC, O’Toole JC, Yambao EB, Turner NC (1984) Influence of osmotic adjustment on leaf rolling and tissue death in rice (Oryza sativa L.). Plant Physiol 75:338–341
Hu H, Xiong L (2014) Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol 65:715–741
Hu J, Jiang D, Cao W, Luo W (2004) Effect of short-term drought on leaf water potential, photosynthesis and dry matter partitioning in paddy rice. Chin J App Ecol 15:63–67
Hu J, Zeng T, Xia Q, Qian Q, Yang C, Ding Y, Chen L, Wang W (2018) Unravelling miRNA regulation in yield of rice (Oryza sativa) based on differential network model. Sci Rep 8:8498
Huang L, Zhang F, Wang W, Zhou Y, Fu B, Li Z (2014) Comparative transcriptome sequencing of tolerant rice introgression line and its parents in response to drought stress. BMC Genom 15(1):1–6
Huang J, Si W, Deng Q, Li P, Yang S (2014b) Rapid evolution of avirulence genes in rice blast fungus Magnaportheoryzae. BMC Genet 15:45
Hussain M, Malik MA, Farooq M, Ashraf MY, Cheema MA (2008) Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J Agron Crop Sci 194:193–199
Hussain Z, Ali S, Hayat Z, Zia MA, Iqbal A, Ali GM (2014) Agrobacterium mediated transformation of DREB1A gene for improved drought tolerance in rice cultivars (Oryza sativa L.). Aust J Crop Sci 8(7):1114–1123
Inthapan P, Fukai S (1988) Growth and yield of rice cultivars under sprinkler irrigation in south-eastern Queensland. 2. Comparison with maize and grain sorghum under wet and dry conditions. Aust J Exp Agric 28:243–248
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47(1):141–153
Jang IC, Oh SJ, Seo JS, Choi WB, Song SI, Kim CH, Kim JK (2003) Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131:516–524
Jedmowski C, Bruggemann W (2015) Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. J Photochem Photobiol, B 151:153–160
Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Do Choi Y, Kim M, Reuzeau C, Kim JK (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153:185–197
Jian-Chang Y, Liu K, Zhang SF, Wang XM, Wang ZQ, Liu LJ (2008) Hormones in rice spikelets in responses to water stress during meiosis. Acta Agron Sin 34:111–118
Jongdee B, Pantuwan G, Fukai S, Fischer K (2006) Improving drought tolerance in rainfed lowland rice: an example from Thailand. Agric Water Manag 80:225–240
Kamarudin ZS, Yusop MR, Tengku M, Mohamed M, Ismail MR, Harun AR (2018) Growth performance and antioxidant enzyme activities of advanced mutant rice genotypes under drought stress condition. Agronomy 8(279):1–15
Kaminaka H, Morita S, Nakajima M, Masumura T, Tanaka K (1998) Gene cloning and expression of cytosolic glutathione reductase in rice (Oryza sativa L.). Plant Cell Physiol 39:1269–1280
Kanneganti V, Gupta AK (2008) Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol Biol 66:445–462
Kato Y, Abe J, Kamoshita A, Yamagishi J (2006) Genotypic variation in root growth angle in rice (Oryza sativa L.) and its association with deep root development in upland fields with different water regimes. Plant Soil 287:117–129
Kaya MD, Okçub G, Ataka M, Cikilic KO (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur J Agron 24:291–295
Kemble AR, Macpherson HT (1954) Liberation of amino acids in perennial rye grass during wilting. Biochem J 58(1):46–49
Khan F, Upreti P, Singh R, Shukla PK, Shirke PA (2017) Physiological performance of two contrasting rice varieties under water stress. Physiol Mol Biol Plants 23(1):85–97
Khowaja FS, Price AH (2008) QTL mapping rolling, stomata conductance and dimension traits of excised leaves in the Bala-Azucena recombinant inbred population of rice. Field Crop Res 106:248–257
Khush GS (1996) Prospects and approaches to increasing the genetic yield potential of rice. In: Evenson RE, Herdt RW, Hossain M (eds) Rice research in Asia: progress and priorities. CABI/IRRI, Manila
Kiani SP, Maury P, Sarrafi A, Grieu P (2008) QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci 175:565–573
Kim YS, Kim JK (2009) Rice transcription factor AP37 involved in grain yield increase under drought stress. Plant Signal Behav 4(8):735–736
Kim W, Iizumi T, Nishimori M (2019) Global patterns of crop production losses associated with droughts from 1983 to 2009. J Appl Meteorol Climatol 58:1233–1244
Kondo M, Pablico PP, Aragones DV, Agbisit R, Abe J, Morita S (2003) Genotypic and environmental variations in root morphology in rice genotypes under upland field conditions. Plant Soil 255:189–200
Kong Z, Li M, Yang W, Xu W, Xue Y (2006) A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol 141:1376–1388
Kramer PJ (1969) Plant and soil water relationship. TATA McGraw-Hill, Bombey, New Delhi
Kraus TE, Mckersie BD, Fletcher RA (1995) Paclobutrazole induced tolerance of wheat leaves to paraquat may involve antioxidant enzyme activity. J Plant Physiol 145:570–576
Kuiper D (1993) Sink strength: Established and regulated by plant growth regulators. Plant Cell Environ 16(9):1025–1026
Kumar S, Dhingra A, Daniell H (2004) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol 136:2843–2854
Kumar R, Venuprasad R, Atlin GN (2007) Genetic analysis of rainfed lowland rice drought tolerance under naturally-occurring stress in eastern India: Heritability and QTL effects. Field Crop Res 103:42–52
Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN (2008) Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crops Res 107(3):221–231
Kumar S, Dwivedi SK, Singh SS, Bhatt BP, Mehta P, Elanchezhian R, Singh VP, Singh ON (2014) Morpho-physiological traits associated with reproductive stage drought tolerance of rice (Oryza sativa L.) genotypes under rain-fed condition of eastern Indo-Gangetic Plain. Ind J Plant Physiol 19(2):87–93
Lafitte HR, Li ZK, Vijayakumar CHM, Gao YM, Shi Y, Xu JL, Fu BY, Yu SB, Ali AJ, Domingo J, Maghirang R, Torres R, Mackill D (2006) Improvements of rice drought tolerance through backcross breeding: evaluation of donors and selection in drought nurseries. Field Crops Res 97:77–86
Lafitte HR, Yongsheng G, Yan S, Li ZK (2007) Whole plant responses, key processes, and adaptation to drought stress: the case of rice. J Exp Bot 58:169–175
Lanceras J, Pantuwan G, Jongdee B, Toojinda T (2004) Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiol 135:384–399
Larkunthod P, Nounjan N, Siangliw JL, Toojinda T, Sanitchon J, Jongdee B, eTherakulpisut P (2018) Physiological responses under drought stress of improved drought-tolerant rice lines and their parents. Not Bot Horti Agrobo 46:679–687
Latif HH (2014) Physiological responses of (Pisum sativum) plant to exogenous ABA application under drought condition. Pak J Bot 46(3):973–982
Li ZY, Chen SY (1999) Inducible expression of translation elongation factor 1A gene in rice seedlings in response to environmental stresses. Acta Bot Sin 41:800–806
Li ZK, Xu JL (2007) Breeding for drought and salt tolerant rice (Oryza sativa L.): progress and perspective. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in molecular breeding towards salinity and drought tolerance. Springer, Dordrecht, pp 531–564
Li HW, Zang BS, Deng XW, Wang XP (2011a) Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234:1007–1018
Li T, Li H, Zhang YX, Liu JY (2011b) Identification and analysis of seven H2O2-responsive miRNAs and 32new miRNAs in the seedlings of rice (Oryza sativa L. ssp. indica). Nucleic Acids Res 39:2821–2833
Li CH, Wang G, Zhao JL, Zhang LQ, Ai LF, Han YF, Sun DY, Zhang SW, Sun Y (2014) The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 26:2538–2553
Li SB, Xie ZZ, Hu CG, Zhang JZ (2016) A review of auxin response factors (ARFs) in plants. Front Plant Sci 7:47
Lilley JM, Ludlow MM, McCouch SR, O’toole JC (1996) Locating QTL for osmotic adjustment and dehydration tolerance in rice. J Exp Bot 47:1427–1436
Lim PO, Woo HR, Nam HG (2003) Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci 8:272–278
Lindemose S, O’Shea C, Jensen MK, Skriver K (2013) Structure, function and networks of transcription factors involved in abiotic stress responses. Int J Mol Sci 14:5842–5878
Liu GL, Mei HW, Yu XQ, Zou GH, Liu HY, Li MS, Chen L, Wu JH, Luo LJ (2007a) Panicle water potential, a physiological trait to identify drought tolerance in rice. J Integr Plant Biol 49:1464–1469
Liu K, Wang L, Xu Y, Chen N, Ma Q, Li F, Chong K (2007b) Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta 226:1007–1016
Liu PP, Montgomery TA, Fahlgren N, Kasschau KD, Nonogaki H, Carrington JC (2007c) Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J 52:133–146
Liu CT, Mao BG, Ou SJ, Wang W, Liu LC, Wu YB, Chu CC, Wang XP (2014a) OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol Biol 84(1/2):19–36
Liu H, Guo S, Xu Y, Li C, Zhang Z, Zhang D, Xu S, Zhang C, Chong K (2014b) OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol 165:160–174
Liu S, Lv Z, Liu Y, Li L, Zhang L (2018) Network analysis of ABA-dependent and ABA-independent drought responsive genes in Arabidopsis thaliana. Genet Mol Biol 41(3):624–637
Lou Q, Chen L, Mei H, Wei H, Feng F, Wang P, Luo L (2015) Quantitative trait locus mapping of deep rooting by linkage and association analysis in rice. J Exp Bot 66:4749–4757
Lu G, Gao C, Zheng X, Han B (2009) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229:605–615
Lum MS, Hanafi MM, Rafii YM, Akmar ASN (2014) Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J Anim Plant Sci 24(5):1487–1493
Maisura MA, Chozin IL, Junaedinand A, Ehara H (2014) Some physiological character responses of rice under drought conditions in a paddy system. J Int Soc Southeast Asian Agric Sci 20(1):104–114
Mallikarjuna G, Mallikarjuna K, Reddy MK, Kaul T (2011) Expression of OsDREB2A transcription factor confers enhanced dehydration and salt stress tolerance in rice (Oryza sativa L.). Biotechnol Lett 33:1689–1697
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul 34(1):135–148
McCouch S, Teytelman L, Xu Y, Lobos K, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2003) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Mechler R, Hochrainer S, Kull D, Chopde S, Singh P, Wajih S (2008) Uttar Pradesh drought costbenefit analysis, from risk to resilience. Working Paper No. 5. In: Moench M, Caspari E, Pokhrel A (eds) ISET-Nepal and ProVention. ISET, Kathmandu
Menconi M, Sgherri CLM, Pinzino C, NavariIzzo F (1995) Activated oxygen production and detoxification in wheat plants subjected to a water deficit programme. J Exp Bot 46:1123–1130
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819:86–96
Mostajeran A, Rahimi-Eichi V (2009) Effects of drought stress on growth and yield of rice (Oryza sativa L.) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different ages leaves. Am–Eurasian J Agric Environ Sci 5(2):264–272
Munne-Bosch S, Alegre L (1999) Role of dew on the recovery of water-stressed Melissa officinalis L. plants. J Plant Physiol 154:759–766
Nahakpam S (2017) Effectual tolerant traits for grain yield in rice genotypes grown under drought. J Pharmacogn Phytochem 1:890–897
Nahar S, Vemireddy LR, Sahoo L, Tanti B (2018) Antioxidant protection mechanisms reveal significant response in drought-induced oxidative stress in some traditional rice of Assam. India Rice Sci 25(4):185–196
Nasrin S, Saha S, Begum HH, Samad R (2020) Impacts of drought stress on growth, protein, proline, pigment content and antioxidant enzyme activities in rice (Oryza sativa L. Var. Brri dhan-24) Dhaka University. J Biol Sci 29(1):117–123
Nemeskeri E, Molnar K, Vigh R, Nagy J, Dobos A (2015) Relationships between stomatal behaviour, spectral traits and water use and productivity of green peas (Pisum sativum L.) in dry seasons. Acta Physiol Plant 37:1–16
Nemoto H, Suga R, Ishihara M, Okutsu Y (1998) Deep rooted rice varieties detected through observation of root characteristics using the trench method. Breed Sci 48:321–324
Nguyen HT, Babu RC, Blum A (1997) Breeding for drought tolerance in rice: physiology and molecular genetics considerations. Crop Sci 37:1426–1434
Niu Y, Zhao T, Xu X, Li J (2017) Genome-wide identification and characterization of GRAS transcription factors in tomato (Solanum lycopersicum). Peer J 5:3955
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Nonami H (1998) Plant water relations and control of cell elongation at low water potentials. J Plant Res 111:373–382
Novak V, Lipiec J (2012) Water extraction by roots under environmental stresses. In: Halasi-Kun J, Stekauerova V, Fodor I, Nagy V, Sinoros-Szabo B, Lo Pinto R (eds) Pollution and water resources, Columbia University seminar proceedings: impact of anthropogenic activity and climate changes on the environment of Central Europe and USA. Slovak Academy of Sciences—Hungarian Academy of Sciences—Columbia University, New York
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim JK (2005a) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005b) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138(1):341–351
Oh SJ, Kwon CW, Choi DW, Song SI, Kim JK (2007) Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnol J 5(5):646–656
Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK (2009) Over-expression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol 150(3):1368–1379
Osmolovskaya N, Shumilina J, Kim A, Didio A, Grishina T, Bilova T, Keltsieva OA, Zhukov V, Tikhonovich I, Tarakhovskaya E, Frolov A, Wessjohann L (2018) Methodology of drought stress research: experimental setup and physiological characterization. Int J Mol Sci 19(4089):1–28
O’Toole JC (1982) Adaptation of rice to drought-prone environments. Drought resistance in crops with emphasis on rice. International Rice Research Institute, Los Bafios, pp 195–213
O’Toole JC, Chang TT (1979) Drought resistance in cereal. Rice: a case study. Stress physiology in crop plants. Wiley, New York
O’Toole JC, Namuco OS (1983) Role of panicle exsertion in water stress induced sterility. Crop Sci 23:1093–1097
O’Toole JC, Hsiao TC, Namuco OS (1984) Panicle water relations during water stress. Plant Sci 33:111–114
Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B et al (2010) Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J 62:316–329. https://doi.org/10.1111/j.1365-313X.2010.04146.x
Ouyang W, Struik PC, Yin X, Yang J (2017) Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J Exp Bot 68(18):5191–5205
Palanog AD, Swamy BM, Shamsudin NAA, Dixit S, Hernandez JE, Boromeo TH, Kumar A (2014) Grain yield QTLs with consistent-effect under reproductive-stage drought stress in rice. Field Crop Res 161:46–54
Pampolino MF, Manguiat IJ, Ramanathan S, Gines HC, Tan PS, Chi TTN, Rajendran R, Buresh RJ (2007) Environmental impact and economic benefits of site-specific nutrient management (SSNM) in irrigated rice systems. Agric Syst 93(1–3):1–24
Panagiotidis CA, Artandi S, Calame K, Silverstein SJ (1995) Polyamines alter sequence-specific DNA-protein interactions. Nucl Acids Res 23(10):1800–1809
Pandey S, Bhandari H (2008) Drought: economic costs and research implications. In: Serraj R, Bennett J, Hardy B (eds) Drought Frontiers in Rice: crop improvement for increased Rainfed production. World Scientific Publishing, Singapore, pp 3–17
Pandey V, Shukla A (2015) Acclimation and tolerance strategies of rice under drought stress. Rice Sci 22(4):147–161
Parent B, Suard B, Serraj R, Tardieu F (2010) Rice leaf growth and water potential are resilient to evaporative demand and soil water deficit once the effects of root system are neutralized. Plant Cell Environ 33:1256–1267
Passioura JB (1996) Drought and drought tolerance. Plant Growth Regul 20(2):79–83
Passioura JB (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58:113–117
Pastori GM, Trippi VS (1992) Antioxidative protection in a drought-resistant maize strain during leaf senescence. Physiol Plant S7:227–231
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Phung T, Jung HI, Park J, Kim JG, Back K, Jung S (2011) Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol 157:1746–1764
Pirasteh-Anosheh H, Saed-Moucheshi A, Pakniyat H, Pessarakli M (2016) Stomatal responses to drought stress. Water stress and crop plants: a sustainable approach. Wiley, Hoboken, pp 24–40
Pirdashti H, Sarvestani Z, Bahmanyar M (2009) Comparison of physiological responses among four contrast rice cultivars under drought stress conditions. World Acad Sci Eng Technol 49:52–53
Pomeranz MC, Hah C, Lin PC, Kang SG, Finer JJ, Blackshear PJ, Jang JC (2010) The Arabidopsis tandem zinc finger protein AtTZF1 tra_cs between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol 152:151–165
Prasad S, Kumar A, Kumar A, Mishra V, Kumar K, Dwivedi R, Kumar A, Ram C, Nandan R, Singh MP, Dwivedi DK (2019) Effect of drought stress at reproductive stage of rice (Oryza sativa L.) genotypes. Bull Env Pharmacol Life Sci 83:91–95
Price AH, Steele KA, Moore BJ, Barraclough PP, Clark LJ (2000) A combined RFLP and AFLP linkage map of upland rice (Oryza sativa L.) used to identify QTLs for root-penetration ability. Theor Appl Genet 100:49–56
Price AH, Townend J, Jones MP, Audebert A, Courtois B (2002) Mapping QTLs associated with drought avoidance in upland rice grown in the Philippines and West Africa. Plant Mol Biol 48:683–695
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381
Puteh AB, Saragih AA, Ismail MR, Mondal MMA (2013) Grain yield of cultivated and weedy rice to water stress at reproductive stage. J Food Agric Environ 11:742–746
Quan RD, Hu SJ, Zhang ZL, Zhang HW, Zhang ZJ, Huang RF (2010) Over expression of an ERF transcription factor TSRF1improves rice drought tolerance. Plant Biotechnol J 8(4):476–488
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133(4):1755–1767
Rachmat A, Nugroho S, Sukma D, Aswidinnoor H, Sudarsono S (2014) Overexpression of OsNAC6 transcription factor from Indonesia rice cultivar enhances drought and salt tolerance. Emir J Food Agric 26(6):497–507
Rahman ARB, Zhang J (2018) Preferential geographic distribution pattern of abiotic stress tolerant rice. Rice 11(1):1–16
Ramegowda V, Basu S, Krishnan A, Pereira A (2014) Rice GROWTH UNDER DROUGHT KINASE is required for drought tolerance and grain yield under normal and drought stress conditions. Plant Physiol 166(3):1634–1645
Rauf S, Al-Khayri JM, Zaharieva M, Monneveux P, Khalil F (2015) Breeding strategies to enhance drought tolerance in crops. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Advances in plant breeding strategies. Agronomic, abiotic and biotic stress traits. Springer, Cham
Ravikumar G, Manimaran P, Voleti SR, Subrahmanyam D, Sundaram RM, Bansal KC, Viraktamath BC, Balachandran SM (2014) Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res 23(3):421–439
Ray JD, Yu L, McCouch SR, Champoux MC, Wang G, Nguyen HT (1996) Mapping quantitative trait loci associated with root penetration ability in rice (Oryza sativa L.). Theor Appl Genet 92:627–636
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:189–1202
Redillas MCFR, Jeong JS, Kim YS, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim J (2012) The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol J 10:792–805
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013) Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol 163(4):1609–1622
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulphonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Rhodes D, Samaras Y (1994) Genetic control of osmoregulation in plants. In: Strange SK (ed) Cellular and molecular physiology of cell volume regulation. CRC Press, Boca Raton, pp 347–361
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104:19631–19636
Rohila JS, Jain RK, Wu R (2002) Genetic improvement of Basmati rice for salt and drought tolerance by regulated expression of a barley HVA1 cDNA. Plant Sci 163:525–532
Roitsch T (1999) Source-sink regulation by sugars and stress. Curr Opin Plant Biol 2:198–206
Ronzhina ES, Mokronosov AT, Sokolova SV (1994) The action of cytokinins on transport and partitioning of substances in detached leaves: 1. Sink and Retention Effects of Cytokinin in Plants of Different Types of Terminal Phloem. Fiziol Rast (Moscow) 41:707–717
Sabar M, Shabir G, Shah SM, Aslam K, Naveed SA, Arif M (2019) Identification and mapping of QTLs associated with drought tolerance traits in rice by a cross between Super Basmati and IR55419-04. Breed Sci Mar 69(1):169–178
Sahebi M, Hanafi MM, Rafii MY, Mahmud TMM, Azizi P, Osman M, Miah G (2018) Improvement of drought tolerance in rice (Oryza sativa L.): genetics, genomic tools, and the WRKY gene family. Bio Med Res Int. https://doi.org/10.1155/2018/3158474
Saikumar S, Gouda PK, Saiharini A, Varma CMK, Vineesha O, Padmavathi G, Shenoy VV (2014) Major QTL for enhancing rice grain yield under lowland reproductive drought stress identified using an O. sativa/ O. glaberrimaintrogression line. Field Crop Res 163:119–131
Sakakibara H, Takei K, Hirose N (2006) Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci 11:440–448
Sakamoto A, Murata N (2002) The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25(2):163–171
Samad AF, Sajad M, Nazaruddin N, Fauzi IA, Murad A, Zainal Z, Ismail I (2017) MicroRNA and transcription factor: key players in plant regulatory network. Front Plant Sci 8:565
Samson BK, Hasan M, Wade LJ (2002) Penetration of hardpans by rice lines in the rainfed lowlands. Field Crop Res 76(2–3):175–188
Sarani M, Namrudi M, Hashemi SM, Raoofi MM (2014) The effect of drought stress on chlorophyll content, root growth, glucosinolate and proline in crop plants. Intl J Farm Alli Sci 3(9):994–997
Sarvestani ZT, Pirdashti H, Sanavy SA, Balouchi H (2008) Study of water stress effects in different growth stages on yield and yield components of different rice (Oryza sativa L.) cultivars. Pak J Biol Sci 11(10):1303–1309
Schachtman DP, Goodger JQD (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Selote DS, Khanna-Chopra R (2004) Drought-induced spikelet sterility is associated with an inefficient antioxidant defence in rice panicles. Physiol Plant 121:462–471
Shamsudin NA, Swamy BPM, Ratnam W, Cruz MTS, Sandhu N, Raman AK, Kumar A (2016a) Pyramiding of drought yield QTLs into a high quality Malaysian rice cultivar MRQ74 improves yield under reproductive stage drought. Rice 9:21
Shamsudin NAA, Swamy BM, Ratnam W, Cruz MTS, Sandhu N, Raman AK, Kumar A (2016b) Pyramiding of drought yield QTLs into a high quality Malaysian rice cultivar MRQ74 improves yield under reproductive stage drought. Rice (NY) 9:21
Sharkey TD (1990) Water stress effects on photosynthesis. Photosynthetica 24:651
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzyme in growing rice seedling. Plant Growth Regul 46:209–221
Sheela KR, Alexallder VT (1995) Physiological response of rice varieties as influenced by soil moisture and seed hardening. Ind J Plant Physiol 38(3):269–271
Shehab GG, Ahmed OK, El-Beltagi HS (2010) Effects of various chemical agents for alleviation of drought stress in rice plants (Oryza sativa L.). Not Bot Horti Agrobo Cluj-Napoca 38:139–148
Shim JS, Oh N, Chung PJ, Youn SK, Choi YD, Kim JK (2018) Overexpression of OsNAC14 improves drought tolerance in rice. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00310
Singh S, Pradhan S, Singh A, Singh O (2012) Marker validation in recombinant inbred lines and random varieties of rice for drought tolerance. Aust J Crop Sci 6:606–612
Singh SP, Jain A, Anantha MS, Tripathi S, Sharma S, Kumar S, Prasad A, Sharma B, Karmakar B, Bhattarai R, Das SP (2017) Depth of soil compaction predominantly affects rice yield reduction by reproductive-stage drought at varietal screening sites in Bangladesh, India, and Nepal. Plant Soil 417(1):377–392
Singh S, Prasad S, Yadav V, Kumar A, Jaiswal B, Kumar A, Khan NA, Dwivedi DK (2018) Effect of drought stress on yield and yield components of rice (Oryza sativa L.) genotypes. Int J Curr Microbiol App Sci 7:2752–2759
Sivamani E, Bahieldin A, Wraith JM, Al-Niemi T, Dyer WE, Ho THD, Qu R (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci 155:1–9
Smirnoff N (1998) Plant resistance to environmental stress. Curr Opin Biotechnol 9:214–219
Solomon S, Qin D, Manning M, Marquis M, Marquis M, Averyt K, Tignor MMB, Miller HL, Chen Z (2007) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Inter govermental Panel on Climate Change. Cambridge University Press, New York
Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G (2005) Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 17:1343–1359
Su J, Wu R (2004) Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci 166:941–948
Sullivan CY, Ross WM (1979) Stress physiology in crop plants. Wiley, New York, pp 263–281
Swamy BPM, Ahmed HU, Henry A, Mauleon R, Dixit S, Vikram P, Tilatto R, Verulkar SB, Perraju P, Mandal NP, Variar M (2013) Genetic, physiological, and gene expression analyses reveal multiple QTL enhance the yield of rice mega-variety IR64 under drought. PLoS One 8:e62795
Swamy BPM, Shamsudin NAA, Rahman SNA, Mauleon R, Ratnam W, Sta Cruz MT, Kumar A (2017) Association mapping of yield and yield-related traits under reproductive stage drought stress in rice (Oryza sativa L.). Rice 10:21
Swapna S, Shylaraj KS (2017) Screening for osmotic stress responses in rice varieties under drought condition. Rice Sci 24(5):253–263
Szekeres M (2003) Brassinosteroid and system in: two hormones perceived by the samereceptor. Trends Plant Sci 8:102–104
Taiz L, Zeiger E (2006) Plant physiology, 4th edn. Sinauer Associates, Sunderland
Taylor AG, Allen PS, Bennett MA, Bradford JK, Burris JS, Misra MK (1998) Seed enhancements. Seed Sci Res 8:245–256
Tena G, Boudsocq M, Sheen J (2011) Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol 14:519–529
Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Torres RO, McNally KL, Cruz CV et al (2013) Screening of rice Genebank germplasm for yield and selection of new drought tolerance donors. Field Crop Res 147:12–22
Torres-Ruiz JM, Diaz-Espejo A, Morales-Sillero A, Martín-Palomo MJ, Mayr S, Beikircher B, Fernández JE (2013) Shoot hydraulic characteristics, plant water status and stomatal response in olive trees under different soil water conditions. Plant Soil 373:77–87
Trijatmiko KR, Prasetiyono J, Thomson MJ, Cruz CMV, Moeljopawiro S, Pereira A (2014) Meta-analysis of quantitative trait loci for grain yield and component traits under reproductive-stage drought stress in an upland rice population. Mol Breed 34:283–295
Tripathy JN, Zhang J, Robin S, Nguyen TT, Nguyen HT (2000) QTLs for cell-membrane stability mapped in rice (Oryza sativa L.) under drought stress. Theor Appl Genet 100:1197–1202
Turner NC (1979) Drought resistance and adaptation to water deficits in crop plants. In: Mussell H, Staples CR (eds) Stress physiology in crop plants. Wiley, New York, pp 343–372
Turner NC, O’Toole JC, Cruz RT, Namuco OS, Ahmad S (1986) Response of seven diverse rice cultivars to water deficits. I. Stress development, canopy temperature, leaf rolling and growth. Field Crops Res 13:257–271
Usman M, Raheem ZF, Ahsan T, Iqbal A, Sarfaraz ZN, Haq Z (2013) Morphological, physiological and biochemical attributes as indicators for drought tolerance in rice (Oryza sativa L.). Eur J Biol Sci 5(1):23–28
Usman MG, Rafii MY, Martini MY, Yusuf OA, Ismail MR, Miah G (2017) Molecular analysis of Hsp70 mechanisms in plants and their function in response to stress. Biotechnol Genet Eng Rev 33:26–39
Vajrabhaya M, Kumpun W, Chadchawan S (2001) The solute accumulation: The mechanism for drought tolerance in RD23 rice (Oryza sativa L.) lines. Sci Asia 27:93–97
Venuprasad R, Lafitte HR, Atlin GN (2007) Response to direct selection for grain yield under drought stress in rice. Crop Sci 47:285–293
Venuprasad R, Cruz MT, Amante M, Magbanua R, Kumar A, Atlin GN (2008) Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crop Res 107:232–244
Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, Cruz MS, Atlin GN (2009) Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theor Appl Genet 120:177–190
Venuprasad R, Bool ME, Quiatchon L, Atlin GNA (2012) QTL for rice grain yield in aerobic environments with large effects in three genetic backgrounds. Theor Appl Genet 124:323–332
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35(4):753–759
Verstraeten WW, Veroustraete F, Feyen J (2008) Assessment of evapotranspiration and soil moisture content across different scales of observation. Sensors 8(1):70–117
Vikram P, Swamy BM, Dixit S, Ahmed HU, Cruz MTS, Singh AK, Kumar A (2011) qDTY1.1, a major QTL for Rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet 12(1):89
Vikram P, Swamy BM, Dixit S, Ahmed H, Cruz MS, Singh AK, Kumar A (2012) Bulk segregant analysis:”An effective approach for mapping consistent-effect drought grain yield QTLs in rice”. Field Crop Res 134:185–192
Von Caemmerer S (2003) C4 photosynthesis in a single C3 cell is theoretically inefficient but may ameliorate internal CO2 diffusion limitations of C3 leaves. Plant Cell Environ 26:1191–1197
Wang P, Song CP (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178:703–718
Wang Z, Xiong Y, Wang M (2000) Study on expert system of irrigation forecast and decision making for water saving. Institute of Agricultural Soil Water Engineering of North West Science and Technology University of Agriculture Forestry, Yangling
Wang H, Inukai Y, Yamauchi A (2006) Root development and nutrient uptake. Crit Rev Plant Sci 25:279–301
Wang S, Xia S, Peng K, Kuang F, Cao Y, Xiao L (2007) Effects of formulated fertilizer synergist on abscisic acid accumulation, proline content and photosynthetic characteristics of rice under drought. Rice Sci 14:42–48
Wang Q, Guan Y, Wu Y, Chen H, Chen F, Chu C (2008) Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol 67(6):589–602
Wang Y, Zhang Q, Zheng T, Cui Y, Zhang W, Xu J, Li Z (2014) Drought-tolerance QTLs commonly detected in two sets of reciprocal introgression lines in rice. Crop Pasture Sci 65:171–184
Wang X, Liu H, Yu F, Hu B, Jia Y, Sha H, Zhao H (2019) Differential activity of the antioxidant defense system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci Rep 9:8543
Wopereis MCS, Kropff MJ, Maligaya AR, Tuong TP (1996) Drought-stress responses of two lowland rice cultivars to soil water status. Field Crops Res 46:21–39
Xiang Y, Tang N, Du H, Ye H, Xiong L (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148:1938–1952
Xiao B, Huang Y, Tang N, Xiong L (2007) Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor Appl Genet 115:35–46
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996) Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110:249–257
Xu X, Bai H, Liu C, Chen E, Chen Q, Zhuang J, Shen B (2014) Genome-wide analysis of microRNAs andtheir target genes related to leaf senescence of rice. PLoS ONE 9:e114313
Yadav R, Courtois B, Huang N, McLaren G (1997) Mapping genes controlling root morphology and root distribution in a doubled-haploid population of rice. Theor Appl Genet 94:619–632
Yadav RB, Dixit S, Raman A, Mishra KK, Vikram P, Swamy BM, Kumar A (2013) A QTL for high grain yield under lowland drought in the background of popular rice variety Sabitri from Nepal. Field Crop Res 144:281–287
Yadira OC, Reyes JL, Alejandra AC (2011) Late embryogenesis abundant proteins. Plant Signal Behav 6:586–589
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10(2):88–94
Yang JH, Han SJ, Yoon EK, Lee WS (2006) Evidence of an auxin signal pathway, microRNA167-ARF8-GH3, and its response to exogenous auxin in cultured rice cells. Nucleic Acids Res 34:1892–1899
Yang J, Zhang J, Liu K, Wang Z, Liu L (2007) Involvement of polyamines in the drought resistance of rice. J Exp Bot 58:1545–1555
Yang PM, Huang QC, Qin GY, Zhao SP, Zhou JG (2014) Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 52(2):193–202
Yang X, Wang B, Chen L, Li P, Cao C (2019) The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci Rep 9:3742
Ye N, Zhu G, Liu Y, Li Y, Zhang J (2011) ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol 5:689–698
Yoshida S (1981) Fundamentals of rice crop science. The International Rice Research Institute, Los Banos
Yoshida S, Hasegawa S (1982) Drought resistance in crops with emphasis on rice. International Rice Research Institute, Manila
You J, Hu H, Xiong L (2012) An ornithine_-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci 197:59–69
You J, Zong W, Li X, Ning J, Hu H, Li X, Xiong L (2013) The SNAC1-targeted gene OsSRO1c modulates stomata closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J Exp Bot 64:569–583
Yue B, Xue W, Xiong L, Yu X, Luo L, Cui K, Jin D, Xing Y, Zhang Q (2006) Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172(2):1213–1228
Zhang J, Zheng HG, Aarti A, Pantuwan G, Nguyen TT, Tripathy JN, Sarkarung S (2001) Locating genomic regions associated with components of drought resistance in rice: comparative mapping within and across species. Theor Appl Genet 103:19–29
Zhang Z, Li F, Li D, Zhang H, Huang R (2010a) Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 232:765–774
Zhang ZJ, Li F, Li DJ, Zhang HW, Huang RF (2010b) Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 232(3):765–774
Zhang L, Xiao S, Li W, Feng W, Li J, Wu Z, Shao M (2011) Overexpression of a Harpin-encoding gene hrf1 in rice enhances drought tolerance. J Exp Bot 62:4229–4238
Zhang C, Bai MY, Chong K (2014) Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep 33:683–696
Zhang H, Zhang J, Yan J, Gou F, Mao Y, Tang G, Botella JR, Zhu JK (2017) Short tandem target mimic rice lines uncover functions of miRNAs in regulating important agronomic traits. Proc Natl Acad Sci USA 114:5277–5282
Zhang J, Zhang S, Cheng M, Jiang H, Zhang X, Peng C, Lu X, Zhang M, Jin J (2018a) Effect of drought on agronomic traits of rice and wheat: a meta-analysis. Int J Environ Res Public Health 15(839):1–14
Zhang J, Zhang H, Srivastava AK, Pan Y, Bai J, Fang J, Shi H, Zhu JK (2018b) Knockdown of rice MicroRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol 176:2082–2094
Zhao B, Liang R, Ge L, Li W, Xiao H, Lin H et al (2007) Identification of drought-induced microRNAs in rice. Biochem Bioph Res Commun 354:585–590. https://doi.org/10.1016/j.bbrc.2007.01.022
Zheng BS, Yang L, Zhang WP, Mao CZ, Wu YR, Yi KK, Wu P (2003) Mapping QTLs and candidate genes for rice root traits under different water-supply conditions and comparative analysis across three populations. TheorAppl Genet 107:1505–1515
Zheng BS, Yang L, Mao CZ, Huang YJ, Wu P (2008) Mapping QTLs for morphological traits under two water supply conditions at the young seedling stage in rice. Plant Sci 175:767–776
Zheng XN, Chen B, Lu GJ, Han B (2009) Overexpression of aNAC transcription factor enhances rice drought and salt tolerance. Biochem Biophys Res Commun 379(4):985–989
Zhou Y, Lam HM, Zhang J (2007) Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J Exp Bot 58:1207–1217
Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L (2010) Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61:4157–4168
Zhou L, Xu H, Mischke S, Meinhardt LW, Zhang DP, Zhu XJ, Li XH, Fang WP (2014) Exogenous abscisic acid significantly affects proteome in tea plant (Camellia sinensis) exposed to drought stress. Hort Res 1:14029
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu B, Su J, Chang M, Verma DPS, Fan YL, Wu R (1998) Overexpression of a D1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water-and salt-stress in transgenic rice. Plant Sci 139:41–48
Zu X, Lu Y, Wang Q, Chu P, Miao W, Wang H, La H (2017) A new method for evaluating the drought tolerance of upland rice cultivars. Crop J 5(6):488–498
Ahmadi N (1985) Plant physiology (photosynthesis and nutrition). 1st Edn. Center for Academic Publication. pp 14–16
Basnayake J, Fukai S, Ouk M (2006) Contribution of potential yield, drought tolerance and escape to adaptation of 15 rice varieties in rainfed lowlands in Cambodia. In: Proceedings of the Australian Agronomy Conference, Australian Society of Agronomy, Birsbane, Australia
Blum A (2007). The mitigation of drought stress. Available at http://www.plantstress.com/articles/droughtm/droughtm.htm.
FAOSTAT (2018–19). Available at http://www.fao.org/faostat/en/#data/QC
Fishel FM (2006) Plant growth regulators. Document PI-139, Pesticide Information Office, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida
Acknowledgements
This study was supported by Sardar Vallabhbhai Patel University of Agriculture and Technology, Modipuram, Meerut. The author, Arun Kumar is highly grateful to Indian Council of Agricultural Research, New Delhi, India for financial support in the form of Senior Research Fellowship (ICAR-SRF).
Author information
Authors and Affiliations
Contributions
RSS and AKS conceptualized the manuscript. AK wrote the manuscript. RKP and AKS edited the manuscript and contributed in critically revising the draft and updating the manuscript for publication. All authors read and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Handling Editor: Mikihisa Umehara.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Sengar, R.S., Pathak, R.K. et al. Integrated Approaches to Develop Drought-Tolerant Rice: Demand of Era for Global Food Security. J Plant Growth Regul 42, 96–120 (2023). https://doi.org/10.1007/s00344-021-10561-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10561-6