Abstract
The application of brassinosteroids (BRs) has been reported to alleviate heat stress. This study investigated the effect of 7,8-dihydro-8α-20-hydroxyecdysone (DHECD)—a BR mimic—by comparison with 24-epibrassinolide (EBR) on the changes in photosynthetic performance, lipid peroxidation, and rice seed set. The results demonstrated that 10−8 M EBR and 10−7 M DHECD had the best actions to counteract the lethal heat temperature of 47 °C for 2 h indicated by a reduction in the number of wilted leaves and an increase in the relative water content and leaf greenness. Moreover, plants treated with EBR or DHECD were exposed to high day/night temperatures of 40/30 °C for 7 days. EBR-treated and DHECD-treated plants showed a high shoot fresh weight, leaf area, chlorophyll content, and carotenoid content. High temperature significantly decreased the leaf net CO2 assimilation rate as well as increased lipid peroxidation. The application of EBR and DHECD maintained the high level of the net CO2 assimilation rate by increasing the stomatal conductance and photochemical quenching. On the other hand, EBR and DHECD decreased the intracellular CO2 content and non-photochemical quenching leading to enhance photosynthesis under heat stress. EBR-treated and DHECD-treated plants significantly reduced their malondialdehyde and hydrogen peroxide contents as well as increasing their total soluble sugar contents. Moreover, BR treatments increased the filled seed of rice. This study confirmed that DHECD—a BR mimic—has activities of heat stress alleviation similar to EBR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heat stress is an important abiotic stress that limits the growth, development, and yield in many plants, such as rice, maize, soybean and, barley (Lobell and Asner 2003; Lobell and Field 2007). Global warming has caused an increase in temperature of about 0.6–0.8 °C from past levels (Hansen and others 2006). On average, the highest temperature in Thailand in the past 60 years was 40.04 °C and it has tended to increase every year. High temperature has decreased rice biomass, pollen germination, and seed set (Cao and Zhao 2008; Matsui and others 2001; Thussagunpanit and others 2013). Peng and others (2004) reported that rice grain yield was reduced by 10 % for each 1 °C increase in the optimal temperature.
Brassinosteroids (BRs) are naturally steroidal plant hormones that regulate plant growth and development (Clouse and Sasse 1998; Fujioka and Yokota 2003). Choe and others (2001) investigated the over-expression of BR-biosynthetically involved genes which enhanced the vegetative growth and increased the seed yield of Arabidopsis. Several studies reported that BRs alleviated photosynthetic inhibition which was induced by high temperature in rice (Cao and Zhao 2008), tomato (Ogweno and others 2008; Singh and Shono 2005), cucumber (Xia and others 2009), melon (Zhang and others 2013), and eggplant (Wu and others 2014). The changes in photosynthesis resulting from BR application might be caused by an increase in the stomatal conductance and the quantum efficiency of PSII as well as by decreased chlorophyll fluorescence (Hayat and others 2010; Wu and others 2014; Yu and others 2004). Furthermore, BRs could induce the antioxidant defensive system in stressed plants leading to reduction of reactive oxygen species such as hydrogen peroxide (H2O2), singlet oxygen (1O2), the superoxide radical (O •−2 ), and the hydroxyl radical (·OH) (Janeczko and others 2011; Khripach and others 2000; Ogweno and others 2008). Generally, BRs have been used in agriculture to ameliorate environmental stress and to increase plant yields (Khripach and others 2000; Zullo and Adam 2002). Natural BR extraction or BR synthesis is expensive so BR analogs have been produced to reduce the economic cost (Serna and others 2012; Zullo and Adam 2002).
In this study, we investigated the effects of 7,8-dihydro-8α-20-hydroxyecdysone (DHECD)—a BR mimic compound—compared with 24-epibrassinolide (EBR) on heat stress alleviation. DHECD was synthesized by the catalytic hydrogenation of 20-hydroxyecdysone obtained from Vitex glabrata stem bark (Suksamrarn and others 2002; Werawattanametin and others 1986). In a previous study, DHECD showed an active effect on the rice laminar inclination test but the effect was less active than brassinolide (Homvisasevongsa 2006). Moreover, DHECD has been reported to increase pollen viability, pollen germination, and the seed set of rice under heat stress conditions (Thussagunpanit and others 2013). The current study aimed to investigate the role of EBR (a commercial BR) and DHECD (a BR mimic) on the properties of photosynthesis, lipid peroxidation, and seed set in ‘Pathum Thani 1’ rice under heat stress conditions.
Materials and Methods
Chemical Preparation
24-Epibrassinolide (EBR) was purchased from Ruina International Co., Ltd., China. 7,8-Dihydro-8α-20-hydroxyecdysone (DHECD)—a BR mimic—was chemically modified from 20-hydroxyecdysone, which was extracted from Vitex glabrata (Suksamrarn and others 2002; Werawattanametin and others 1986). EBR and DHECD stock solutions each of 1 mM were prepared by dissolving each compound in 0.01 % ethanol and all solutions were stored at 4 °C. The various concentrations of EBR or DHECD were prepared from these stocks for foliar application to plants.
Preparation of Rice Plants
Seeds of rice (Oryza sativa L.) cv. Pathum Thani 1 were sown in 500 cm2 plastic pots containing soil which had a pH of 6.5 and contained 2.5 % organic matter. Plants were grown in a greenhouse at the Department of Botany, Kasetsart University, Bangkok, Thailand (13°50′41.6″N, 100°34′14.7″E).
High Temperature Conditions
Experiment I: Appropriate Brassinosteroid Concentrations
EBR and DHECD at various concentrations of 10−9, 10−8, 10−7, and 10−6 M were prepared to investigate the appropriate concentration. The EBR solutions, DHECD solutions, and 0.01 % ethanol as the control were mixed with 0.025 % Tween-20 prior to use and 10 ml of each solution was separately sprayed as a foliar application onto rice plants at 35 days after sowing (DAS). Rice seedlings were sprayed with different concentrations of BRs using ten replications per treatment and with a completely randomized design applied. One week after application with various concentrations of EBR or DHECD, the rice plants were exposed to a lethal heat temperature of 47 °C for 2 h. The wilted leaves, the relative water content (RWC) in the leaves, and leaf greenness were investigated. The concentrations of EBR and DHECD which had the best effect on lethal temperature alleviation were selected to use in experiment II.
Experiment II: Effects of Brassinosteroids on Photosynthesis, Lipid Peroxidation and Seed Set
The 35 DAS rice plants were treated with 10 ml of 10−8 M EBR, 10−7 M DHECD, or 0.01 % ethanol as the control. All solutions were mixed with 0.025 % Tween-20 prior to use. One week after the BR application, the control plants were divided into two groups: (1) normal temperature conditions of growth in a greenhouse (30/25 °C day/night); and (2) heat stress conditions (40/30 °C day/night). The stress control plants, EBR-treated plants, and DHECD-treated plants were exposed to high day/night temperatures of 40/30 °C for 7 days in a growth chamber under 300 µmol m−2 s−1 light irradiance and 75 % relative humidity. After 7 days of heat stress treatment, the plants were transferred to normal temperature conditions at average day/night temperatures of 30/25 °C for recovery. Each treatment involved five replications with one plant per pot. The pots were arranged in a completely randomized design.
Estimation of Wilted Leaves and the Relative Water Content
The rice plants showed wilting symptoms after exposure of the plants to a lethal heat temperature (47 °C for 2 h). The wilted leaves were calculated from the percentage ratio of the number of wilted leaves per total number of leaves on the plant. The relative water content (RWC) in leaves was estimated by the measurement of leaf fresh weight (FW) and maintaining those leaves in distilled water for 24 h to measure the turgid weight (TW). Finally, the leaves were dried at 80 °C for 48 h, and the leaf dry weight (DW) was measured. The relative water content was determined from the formula RWC (%) = [(FM − DM)/(TM − DM)] × 100. The RWC in the leaves was evaluated in the lethal heat temperature experiment and in the experiment involving the high temperatures of 40/30 °C day/night on days 0, 1, 3, 5, and 7 of the heat stress condition and on day 7 after recovery (re-7).
Measurement of Leaf Greenness Index
The leaf greenness index under the lethal heat temperature was taken on the highest, fully expanded rice leaves. Each leaf was measured 10 times using a chlorophyll meter (SPAD-502, Konica-Minolta, Osaka, Japan).
Plant Growth Parameters
The plant growth parameters were estimated on days 0, 1, 3, 5, and 7 of the heat stress conditions and on day 7 after recovery (re-7) in terms of the shoot and root fresh weight. The leaf area was estimated using an image processing program (Pukpao Co., Ltd., Thailand). The total leaf area per plant was calculated by the multiplication of the leaf area by the total number of leaves.
Measurement of Chlorophyll and Carotenoid Contents
The highest, fully expanded rice leaves were collected. The chlorophyll a, chlorophyll b, chlorophyll (a + b), and total carotenoid contents in these leaves were estimated using the methods described by Lichtenthaler and Buschmann (2001). The chlorophyll and carotenoid contents were measured on days 0, 1, 3, 5, and 7 of the heat stress conditions and on day 7 after recovery (re-7).
Determination of Photosynthetic Efficiency
The photosynthetic efficiency was measured on the highest, fully expanded leaves. Photosynthetic gas exchange and chlorophyll fluorescence were measured on days 0, 1, 3, 5, and 7 of the heat stress conditions and on day 7 after recovery (re-7).
Leaf gas exchange was measured using a gas exchange analyzer (LI-6400, Licor Inc., Lincoln, NE, USA). The leaf net CO2 assimilation rate (A), stomatal conductance (G s), transpiration rate (E), and intracellular CO2 content (C i) were measured on 0.7 × 3.0 cm2 of leaf area using an external CO2 concentration of 400 ppm, a photon flux density (PPFD) of 1,000 µmol m−2 s−1, relative air humidity at about 65–70 %, a flow rate at 500 µmol s−1, and the leaf temperature was maintained at 30 °C.
Chlorophyll fluorescence was measured using a pulse amplitude modulation fluorometer (PAM-2100, Walz, Effeltrich, Germany). All the chlorophyll fluorescence measurements and the various parameter calculations followed the procedures of Lichtenthaler and others (2005) and Maxwell and Johnson (2000). The minimal (F o) and maximal (F m) fluorescence emissions in the leaves were assessed after 30 min of dark adaptation. The maximum quantum efficiency of PSII (F v/F m) was calculated as [F v/F m = (F m − F o)/F m]. Photochemical quenching (q P) was computed as [q P = (F m′ − F s)/(F m′ − F o′)]. The quantum yield of PSII (ΦPSII) was calculated as (F m′ − F s)/F m′ and non-photochemical (q N) was calculated as [q N = (F m − F m′)/(F m − F o)].
Determination of Malondialdehyde, Hydrogen Peroxide, and Total Soluble Sugar Contents
Leaf samples were randomly selected from the control, EBR-treated and DHECD-treated plants to analyze the malondialdehyde (MDA), hydrogen peroxide (H2O2), and total soluble sugar contents on days 0, 1, 3, 5, and 7 of the heat stress conditions and on day 7 after recovery (re-7). The MDA content was measured by the thiobarbituric acid method according to Hodge and others (1999). The H2O2 content was evaluated according to Velikova and others (2000). The amount of H2O2 was calculated from the H2O2 standard curve. The total soluble sugar content was estimated by the anthrone method according to Fales (1951).
Determination of Seed Set
After the 7 days of recovery from the heat stress, all plants were grown in a greenhouse having average day/night temperatures of 30/25 °C until the final harvest. The panicles were harvested at 124 days after sowing. The percentage of seed set was calculated by counting the number of filled seeds per total seeds.
Statistical Analysis
All data were statistically analyzed by ANOVA and the different means between treatments were considered by applying Tukey–Kramer’s HSD (honestly significant difference) test at p ≤ 0.05. Each value was presented as the mean ± standard error (SE) with a minimum of five replicates.
Results
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) Concentration on Lethal Heat Temperature Tolerance
Rice plants treated with various concentrations of EBR or DHECD alleviated the lethal high temperature (47 °C for 2 h). EBR at 10−8, 10−7, and 10−6 M as well as DHECD at 10−7 and 10−6 M reduced leaf wilting by 50 %. The wilted leaves of all treatments did not recover after transfer of the plants to the normal temperature regime. The concentration of 10−9 M of EBR or DHECD did not reduce the percentage of wilted leaves when compared with plants that had not received EBR or DHECD (Fig. 1a). The changes in leaf wilting related to the changes in the RWC in the leaves. EBR and DHECD at concentrations of 10−8, 10−7, and 10−6 M significantly increased the RWC in leaves comparing with control (Fig. 1b). Moreover, application of EBR or DHECD produced a leaf greenness index that was higher than in the control. The EBR application of 10−8 and 10−7 M significantly increased the leaf greenness index by 11.23 and 10.51 % of the control treatment, respectively. DHECD applications of 10−9, 10−8, and 10−7 M significantly increased the leaf greenness index by 9.64, 9.63, and 7.19 % of the control treatment, respectively (Fig. 1c). Applications of EBR at 10−8 M and DHECD at 10−7 M were used as representative concentrations for further experimentation.
Effects of different concentrations of 24-epibrassinolide (EBR) and 7,8-dihydro-8α-20-hydroxyecdysone (DHECD) on percentage of wilted leaves (a), leaf relative water content; RWC (b), and leaf greenness index (c) of ‘Pathum Thani 1’ rice under lethal heat temperature. Data are means of ten replicates ± SE shown by vertical error bars. Means with the same letter are not significantly different at p ≤ 0.05 according to Tukey–Kramer’s honestly significant difference test
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) on the Growth of Rice Under Heat Stress
Rice plants treated with EBR or DHECD had significantly increased shoot fresh weight, root fresh weight, and leaf area before the plants were exposed to high temperature (day 0) (Table 1). On the first day after the rice plants were exposed to day/night temperatures of 40/30 °C, we found that EBR and DHECD produced similar effects to maintain high growth based on the shoot fresh weight, root fresh weight, and leaf area (Table 1). The shoot fresh weight and leaf area of the EBR and DHECD treatments were significantly higher than in the stress control treatment after 3, 5, and 7 days of heat stress. Furthermore, EBR and DHECD application increased the leaf area similar to the non-stress control plants from the third day of heat stress (Table 1). At 7 days after heat stress, high temperature decreased the shoot fresh weight and leaf area in the stressed control plants by 89.14 and 85.01 %, respectively, of the non-stress control treatment. However, the EBR treatment increased the shoot fresh weight and leaf area by 36.55 and 26.36 %, respectively, of the stress control treatment, whereas the DHECD treatment increased those growth parameters by 47.72 and 26.90 %, respectively, of the stress control treatment (Table 1). Moreover, EBR and DHECD treatments increased the leaf area in recovery plants after 7 days at normal temperature by 16.82 and 22.27 %, respectively, of the stress control treatment, and there were no significant differences between the non-stress control and BR-treated plants (Table 1).
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) on Chlorophyll and Carotenoid Contents Under Heat Stress
EBR-treated and DHECD-treated plants had significantly increased chlorophyll a, chlorophyll b, and chlorophyll (a + b) contents compared with stress control plants from 3 days after the plants received heat stress. Furthermore, the EBR and DHECD applications significantly increased total carotenoid contents from 5 days after heat stress (Table 2). On the final day of the heat stress application, the high temperature condition decreased chlorophyll a, chlorophyll b, chlorophyll (a + b), and total carotenoid contents by 43.84, 49.53, 45.03, and 61.84 %, respectively, of the non-stress control treatment (Table 2). The EBR application increased chlorophyll a, chlorophyll b, chlorophyll (a + b), and total carotenoid contents by 85.63, 73.58, 82.63, and 27.66 %, respectively, of the stress control treatment, whereas the DHECD application increased those pigments by 88.75, 77.36, 85.92, and 38.30 %, respectively, of the stress control treatment (Table 2). Moreover, the EBR-treated and DHECD-treated plants were able to increase the chlorophyll (a + b) and total carotenoid contents to comparable levels with the non-stress control after 7 days of recovery (Table 2).
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) on the Gas Exchange Rate Under Heat Stress
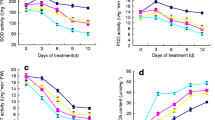
The EBR-treated and DHECD-treated plants had significantly increased net CO2 assimilation rates (A) before plant exposure to heat stress (day 0) (Fig. 2a). The BR-treated plants increased A, G s, and E and also decreased C i after high temperature stress (Fig. 2). EBR and DHECD had similar effects with regard to the increase in A and decrease in C i (Fig. 2a, d) but DHECD increased G s and E more than EBR (Fig. 2b, c). The DHECD-treated plants had G s and E levels significantly higher than in the non-stressed control plants after 7 days of heat stress (Fig. 2b, c). After 7 days of heat stress, the stress control plants significantly reduced A, G s, and E by 17.23, 27.05, and 31.11 %, respectively, of the non-stress control treatment. The EBR-treated plants increased A, G s, and E by 325.41, 240.25, and 235.04 %, respectively, of the stress control treatment, whereas the DHECD-treated plants increased A, G s, and E by 388.11, 494.75, and 290.16 %, respectively, of the stress control treatment (Fig. 2a–c). Moreover, heat stress increased C i by 7.96 % of the non-stress control treatment. EBR and DHECD decreased C i by 96.54 and 97.85 %, respectively, of the stress control treatment (Fig. 2d). The plants treated with EBR and DHECD recovered A and C i to a similar degree as in the non-stress control plants when the plants were transferred to the normal temperature regime (Fig. 2a, d). On the other hand, the EBR and DHECD application resulted in G s and E being higher than in the non-stress and stress control treatments at the day of recovery (Fig. 2b, c).
Effects of 24-epibrassinolide (EBR) and 7,8-dihydro-8α-20-hydroxyecdysone (DHECD) on change of leaf net CO2 assimilation rate; A (a), stomatal conductance; G s (b), transpiration rate; E (c) and intracellular CO2 content C i; (d) in leaves of ‘Pathum Thani 1’ rice under heat stress. Vertical dashed line indicates transfer of plants to normal temperature (30/25 °C day/night) for recovery. Data are means of five replicates ± SE shown by vertical error bars. Means of each time after heat stress with the same letter are not significantly different at p ≤ 0.05 according to Tukey–Kramer’s honestly significant difference test. EBR and DHECD concentrations are 10−8 and 10−7 M, respectively
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) on the Chlorophyll Fluorescence Under Heat Stress
High temperature decreased F v/F m, q p, and ΦPSII whereas it increased F o and q N on all days that the control plants were exposed to heat stress (Fig. 3). EBR and DHECD utilized the same activity to alleviate heat stress by an increase in F v/F m (Fig. 3b) and a reduction in F o and q N on all 7 days of high temperature stress (Fig. 3a, e). In contrast, EBR tended to increase q p and ΦPSII more than the DHECD application (Fig. 3c, d). After plants had been subjected to a high temperature for 7 days, the stress control plants decreased F v/F m, q p, and ΦPSII by 92.14, 39.20, and 36.54 %, respectively, of the non-stress control treatment. The EBR treatment increased F v/F m, q p, and ΦPSII by 5.12, 102.56, and 111.50 %, respectively, of the stress control treatment, whereas DHECD treatment increased those parameters by 4.19, 64.15, and 68.50 %, respectively, of the stress control treatment (Fig. 3b–d). When the stressed plants were transferred to the normal temperature regime for 7 days (re-7), there was no significant difference in the chlorophyll fluorescence parameters between the BR-treated plants and the non-stress control plants. On the other hand, the stress control plants were unable to recover (Fig. 3).
Effects of 24-epibrassinolide (EBR) and 7,8-dihydro-8α-20-hydroxyecdysone (DHECD) on change of the minimal fluorescence; F o (a), the maximal quantum yield of PSII; F v/F m (b), photochemical quenching; q p (c), quantum efficiency of PSII; ΦPSII (d) and non-photochemical quenching; q N (e) in leaves of ‘Pathum Thani 1’ rice under heat stress. Vertical dashed line indicates transfer of plants to normal temperature (30/25 °C day/night) for recovery. Data are means of five replicates ± SE shown by vertical error bars. Means of each time after heat stress with the same letter are not significantly different at p ≤ 0.05 according to Tukey–Kramer’s honestly significant difference test. EBR and DHECD concentrations are 10−8 and 10−7 M, respectively
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) on the MDA Content, H2O2 Content, Total Soluble Content, and Relative Water Content in Leaves of Rice
The rice plants under heat stress had high levels of MDA and H2O2. The EBR and DHECD treatments significantly decreased the MDA content for all days that plants were under heat stress and for the recovery day (Fig. 4a). Moreover, the EBR and DHECD applications significantly decreased the H2O2 content after the third day of heat stress when compared with the stress control plants. The EBR-treated and DHECD-treated plants decreased the H2O2 content to approximately the same values as in the non-stress control at 5 and 7 days after heat stress (Fig. 4b). High temperature significantly decreased the total soluble sugar contents of all stress treatments at 1 and 3 days after heat stress. The application of EBR and DHECD increased the total soluble sugar contents at 5 days after heat stress by 23.12 and 26.98 %, respectively, of the stress control treatment and at 7 days after heat stress by 24.39 and 38.01 %, respectively, of the stress control treatment. Nevertheless, all treatments which were exposed to high temperature were unable to increase the total soluble sugar contents after recovery under the normal environment conditions (Fig. 4c). When compared with non-stress control plants, heat stress reduced the RWC in leaves after the third day of heat stress. The stress control treatment significantly decreased the RWC by 52.47 % of the non-stress control treatment at 7 days after high temperature. On the other hand, the EBR and DHECD treatments increased the RWC by 24.39 and 38.01 %, respectively, of the stress control treatment (Fig. 4d).
Effects of 24-epibrassinolide (EBR) and 7,8-dihydro-8α-20-hydroxyecdysone (DHECD) on change of malondialdehyde (MDA) content (a), H2O2 content (b), total soluble sugar content (c), and relative water content (d) in leaves of ‘Pathum Thani 1’ rice under heat stress. Vertical dashed line indicates transfer of plants to normal temperature (30/25 °C day/night) for recovery. Data are means of five replicates ± SE shown by vertical error bars. Means of each time after heat stress with the same letter are not significantly different at p ≤ 0.05 according to Tukey–Kramer’s honestly significant difference test. EBR and DHECD concentrations are 10−8 and 10−7 M, respectively
Effects of 24-Epibrassinolide (EBR) and 7,8-Dihydro-8α-20-Hydroxyecdysone (DHECD) Applications on Rice Seed Set
The percentages of filled seed were calculated after seed set. The high temperature in the vegetative phase of rice significantly reduced the filled seed in the stress control treatment to 51.07 %. The EBR and DHECD applications resulted in greater numbers of filled seed similar to the numbers for the non-stress control plants (Fig. 5).
Effects of 24-epibrassinolide (EBR) and 7,8-dihydro-8α-20-hydroxyecdysone (DHECD) applications on percentage of filled seed of ‘Pathum Thani 1’ rice under heat stress. Data are means of five replicates ± SE shown by vertical error bars. Means with the same letter are not significantly different at p ≤ 0.05 according to Tukey–Kramer’s honestly significant difference test. EBR and DHECD concentrations are 10−8 and 10−7 M, respectively
Discussion
Brassinosteroids have been reported to counteract heat stress in several plants (Cao and Zhao 2008; Krishna 2003; Ogweno and others 2008; Singh and Shono 2005; Wu and others 2014). A temperature between 44 and 55 °C normally killed more than 50 % of the plants (Sutcliffe 1977). A temperature that causes more than 90 % plant death is known as a lethal heat temperature and in rice, the lethal heat temperature was 47 °C for 2 h (Katiyar-Agarwal and others 2003). Dhaubhadel and others (1999) and Singh and Shono (2005) reported that the foliar application of EBR induced a basic thermotolerance in rape and tomato. The current study observed that the application of either 10−8 M EBR or 10−7 M DHECD resulted in rice plants with decreased amounts of leaf wilting and increase in both the RWC and leaf greenness at the lethal heat temperature (Fig. 1). The RWC in leaves has been used to determine plant water status (Kaur and others 2011). A reduction in the RWC in the control plants indicated that these plants had high water loss which caused leaf wilting (Fig. 1a, b). Moreover, the EBR and DHECD applications inhibited the decrease in leaf greenness (Fig. 1c). Generally, the leaf greenness index is linearly correlated with the chlorophyll contents (Coste and others 2010; Uddling and others 2007). The results suggested that EBR and DHECD played a role in the maintenance of chlorophyll in the leaf under lethal heat stress conditions.
EBR and DHECD produced similar effects in maintaining high growth as indicated by the shoot fresh weight, root fresh weight, and leaf area under heat stress (Table 1). The increase in the shoot and root fresh weights under heat stress might be explained by the greater water uptake to those organs after EBR or DHECD application. Moreover, the increase in the plant biomass was associated with the increase in the photosynthetic rate (Dalio and others 2011). The EBR and DHECD treatments increased the leaf area before the exposure of plants to high temperature and maintained a higher leaf area under heat stress (Table 1). An increase in the leaf area after BR application has been reported in pigeon pea (Dalio and others 2011), tomato (Yu and others 2004), and wheat (Shahbaz and others 2008). The greater leaf area related to the high levels of photosynthetic pigments leading to an increase in photosynthesis (Dalio and others 2011).
High temperature significantly decreased the chlorophyll and carotenoid contents in the stress control plants. The EBR and DHECD treatments maintained the levels of chlorophyll a, chlorophyll b and the total carotenoids (Table 2). The application of BRs produced an increase in the chlorophyll contents which relieved stress effects in maize (Anjum and others 2011), Cajanus cajan (Dalio and others 2011), cucumber (Yuan and others 2012), and eggplant (Wu and others 2014). The chlorophyll contents in the leaf are one of the constituents used to investigate the biomass and the photosynthetic rate (Dalio and others 2011) because they provide a common reference system to qualify plant health (Wittmann and others 2001). The decrease in the levels of chlorophyll a and b due to heat stress indicated that high temperature eliminated the light absorbing capacity because chlorophyll a and b are the main pigments in the light harvesting complex (Calatayud and Barreno 2004; Zhu and others 2011). Heat stress also showed a diminution in the total carotenoid contents (Table 2). The loss of carotenoids affected the deterioration of the thermal dissipation capacity in plants under stress (Calatayud and Barreno 2004). The application of EBR or DHECD not only maintained higher chlorophyll and carotenoid contents, but also decreased the MDA and H2O2 production under heat stress (Fig. 4a, b). The enhancement of the chlorophyll and carotenoid contents was associated with a decrease in lipid peroxidation and oxidative stress (Calatayud and Barreno 2004; Kumar and others 2006).
The study clearly demonstrated that high temperature significantly decreased the net CO2 assimilation rate (Fig. 2a). EBR and DHECD ameliorated heat stress by maintaining a high net CO2 assimilation rate (A) and plants treated with these recovered A when they were transferred to the normal temperature condition (Fig. 2a). Singh and Shono (2005) reported that tomato seedlings treated with EBR had a photosynthetic rate higher than in untreated tomato under a high day/night temperature regime of 35/27 °C and EBR application increased the photosynthetic rate when the plants were exposed to 25/20 °C day/night for 24 h. In this study, the increase in net photosynthesis caused by EBR or DHECD application resulted in an increase in G s (Fig. 2b) and was related to the increase in the transpiration rate (Fig. 2c). Furthermore, it indicated that BR-treated plants had a greater number of open stomata than in the stress control treatment (Serna and others 2012). When plants had a greater number of open stomata, they had a greater chance to allow more CO2 into the leaves (Serna and others 2012). The EBR and DHECD applications produced a lower intracellular CO2 content than in the stress control treatment (Fig. 2d) indicating that plants could utilize CO2 to increase photosynthesis (Serna and others 2012; Singh and Shono 2005). In the current study, the treatments involving EBR or DHECD under heat stress produced similar effects of A increasing and C i decreasing (Fig. 2a, d). DHECD tended to increase G s and E more than EBR (Fig. 2b, c), which implied that DHECD could alleviate heat stress by mainly increasing G s which is one of the stomatal factors. Moreover, we found that DHECD-treated plants had G s and E levels higher than in the non-stressed control plants at 7 days after heat stress and also found higher levels of these parameters in plants subjected to EBR or DHECD treatment at the recovery day (Fig. 2b, c). The results suggested that BRs directly enhance stomatal opening in rice leaves. This phenomenon indirectly led to alleviated heat stress because the regulation of stomatal conductance is the main process that plants use to control the temperature in their leaves (Pospíšilová 2003). Therefore, the increase in the levels of G s and E by treatment with BRs initiated a reduction in the leaf temperature (Janeczko and others 2011). The declination of A in higher plants is not only caused by stomatal limitations—which include a decrease in stomatal conductance (G s), transpiration rate (E), and intracellular CO2 content (C i)—but also by non-stomatal limitations indicated by the decrease in PSII efficiency (Farquhar and Sharkey 1982; Neves and others 2008).
Chlorophyll fluorescence is an important characteristic used to study plant responses to environmental stress (Rascher and others 2000). Heat stress decreased chlorophyll fluorescence parameters including F v/F m, q p, and ΦPSII whereas it increased F o and q N (Fig. 3). Normally, non-stressed plants have F v/F m values around 0.83 and this value declines when plants are subjected to biotic or abiotic stress (Björkman and Demmig 1987). High temperature significantly decreased F v/F m in the stress control plants. However, EBR-treated and DHECD-treated plants maintained F v/F m values around 0.80 on all days under heat stress (Fig. 3b). The value of F v/F m was used as an indicator of photoinhibition; the reduction in F v/F m related to photodamage of the PSII reaction centers which resulted in a decreased photosynthetic rate (Calatayud and Barreno 2004). EBR and DHECD application demonstrated the high level of F v/F m under heat stress and indicated that BRs could protect against PSII damage. PSII is the most sensitive photosynthetic apparatus under high temperature conditions (Wu and others 2014). EBR and DHECD utilized the same activity to alleviate heat stress by an increase in F v/F m (Fig. 3b). On the other hand, EBR tended to increase q p and ΦPSII more than the DHECD application (Fig. 3c, d). Photochemical quenching (q p) indicates the ratio of opened to closed PSII reaction centers (Maxwell and Johnson 2000). A high q p value showed enhancement of the reduced consumption rate and ATP production by non-cyclic electron transport that is associated with an increase in photosynthesis (Nogués and Baker 2000; Xia and others 2009). The greater value of q p in EBR-treated plants than in DHECD-treated plants under heat stress (Fig. 3c) was related to the higher quantum efficiency of PSII (ΦPSII) in the EBR treatment compared to the DHECD treatment (Fig. 3d). A high q p value has the benefit of separation of the electron charge in the reaction center which causes the high ΦPSII and electron transport rate (Guo and others 2006). Furthermore, heat stress significantly increased the non-photochemical quenching (q N) (Fig. 3e). The increase in q N was closely associated with the plant’s ability to get rid of excess energy to protect the photosynthetic apparatus (Calatayud and Barreno 2004; Vasil’ev and others 1998). A high q N value in a plant indicates that the plant has a declination of photosynthetic rate because q N represents the dissipation of energy that cannot be utilized for electron transportation in the photochemical process (Vasil’ev and others 1998). Therefore, the decrease in q N leads to a reduction in chlorophyll fluorescence yield (Vasil’ev and others 1998). Using EBR or DHECD had the same effect of inhibiting the increase of q N under heat stress (Fig. 3e). Ogweno and others (2008) suggested that BR-treated plants protected the PSII from over-excitation and damage of the thylakoid membrane from high temperature.
The MDA content reflects oxidative damage which causes membrane lipid peroxidation (Balestrasse and others 2010). An increase in lipid peroxidation resulting from heat stress might damage the scavenging process in the reactive oxygen species. The high MDA content in the stress control plants (Fig. 4a) implied that the cell membrane was severely injured by stress (Genisel and others 2013). The EBR and DHECD treatments significantly decreased the MDA and H2O2 contents under heat stress (Fig. 4a, b). It was assumed that BRs induced the antioxidant defensive system in heat stress (Khripach and others 2000). Moreover, the reduction of H2O2 was one of the reactive oxygen species related to the increase in photosynthesis because many enzymes in chloroplasts are extremely sensitive to high levels of H2O2. The inhibition of photosynthetic enzymes by H2O2 causes a decline in CO2 fixation (Ogweno and others 2008; Zhou and others 2004). The current study showed that heat stress decreased the total soluble sugar content. Treatment with EBR or DHECD promoted the accumulation of the soluble sugar content which was one of the osmoprotectants under heat stress (Fig. 4c) and indicated that BRs improve the heat resistance system in plants (Wu and others 2014). Generally, stressed plants accumulate compatible osmolytes, including sugar, in the cytosol to maintain intracellular osmotic homeostasis (Elsheery and Cao 2008). The EBR-treated and DHECD-treated plants increased the relative water content in leaves more than in the stress control plants (Fig. 4d), and this might be related to the effect of BRs on water uptake (Dalio and others 2011).
BRs were reported to increase the yield in many plants including chickpeas (Ali and others 2007), lettuce (Serna and others 2012), rice (Cao and Zhao 2008), tomato (Singh and Shono 2005), and yellow passion fruit (Gomes and others 2006). We found that the application of EBR and DHECD produced the same number of filled seed as in the non-stress control plants whereas the stress control plants had lower numbers of filled seed (Fig. 5). Singh and Shono (2005) reported that the high photosynthetic rate related to the increase in tomato yield. In this study, BR treatment resulted in a higher net CO2 assimilation rate than in the heat stress control treatment (Fig. 2a). Therefore, EBR and DHECD might increase the seed set because of the high level of photosynthesis and biomass accumulation after the plants were exposed to heat stress.
The results of this study clearly demonstrated that EBR and DHECD were effective in increasing the photosynthetic rate under heat stress by reducing stomatal and non-stomatal limitations. Moreover, EBR and DHECD ameliorated high temperature stress by decreasing lipid peroxidation and increasing the total soluble sugar contents, biomass, and rice seed set. DHECD—a BR mimic compound—influenced activities that alleviated heat stress as did EBR. Therefore, both DHECD and EBR were a good candidate for application in agriculture.
References
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ Exp Bot 59:217–223. doi:10.1016/j.envexpbot.2005.12.002
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185. doi:10.1111/j.1439-037X.2010.00459.x
Balestrasse KB, Tomaro ML, Batlle A, Noriega GO (2010) The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71:2038–2045. doi:10.1016/j.phytochem.2010.07.012
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plant of diverse origins. Planta 170:489–504. doi:10.1007/bf00402983
Calatayud A, Barreno E (2004) Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments and lipid peroxidation. Plant Physiol Biochem 42:549–555. doi:10.1016/j.plaphy.2004.05.002
Cao Y, Zhao H (2008) Protective roles of brassinolide on rice seedlings under high temperature stress. Rice Sci 15:63–68. doi:10.1016/s1672-6308(08)60021-9
Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26:573–582. doi:10.1046/j.1365-313x.2001.01055.x
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Phys 49:427–451. doi:10.1146/annurev.arplant.49.1.427
Coste S, Baraloto C, Leroy C, Marcon É, Renaud A, Richardson AD, Roggy J, Schimann H, Uddling J, Hérault B (2010) Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann For Sci 67:607p1–607p5. doi:10.1051/forest/2010020
Dalio RJD, Pinheiro HP, Sodek L, Haddad CRB (2011) The effect of 24-epibrassinolide and clotrimazole on the adaptation of Cajanus cajan (L.) Millsp. to salinity. Acta Physiol Plant 33:1887–1896. doi:10.1007/s11738-011-0732-x
Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342. doi:10.1023/a:1006283015582
Elsheery NI, Cao KF (2008) Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol Plant 30:769–777. doi:10.1007/s11738-008-0179-x
Fales FW (1951) The assimilation and degradation of carbohydrate by yeast cells. J Biol Chem 193:113–124
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345. doi:10.1146/annurev.pp.33.060182.001533
Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54:137–164. doi:10.1146/annurev.arplant.54.031902.134921
Genisel M, Turk H, Erdal S (2013) Exogenous progesterone application protects chickpea seedlings against chilling-induced oxidative stress. Acta Physiol Plant 35:241–251. doi:10.1007/s11738-012-1070-3
Gomes MMA, Compostrini E, Leal NR, Viana AP, Ferraz TM, Siqueira LN, Rosa RCC, Netto AT, Nuñez-Vázquez M, Zullo MAT (2006) Brassinosteroid analogue effects on the yield of yellow passion fruit plants (Passiflora edulis f. flavicarpa). Sci Hortic (Amsterdam) 110:235–240. doi:10.1016/j.scienta.2006.06.030
Guo HX, Liu WQ, Shi YC (2006) Effects of different nitrogen forms on photosynthetic rate and the chlorophyll fluorescence induction kinetics of flue-cured tobacco. Photosynthetica 44:140–142. doi:10.1007/s11099-005-0170-3
Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M (2006) Global temperature change. Proc Natl Acad Sci USA 103:14288–14293. doi:10.1073/pnas.0606291103
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112. doi:10.1016/j.envexpbot.2010.03.004
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. doi:10.1007/s004250050524
Homvisasevongsa S (2006) Synthesis and structure–activity relationship studies of ecdysteroid and brassinosteroid analogues. Dissertation, Ramkhamhaeng University, Thailand
Janeczko A, Okleštková J, Pociecha E, Koscielniak J, Mirek M (2011) Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiol Plant 33:1249–1259. doi:10.1007/s11738-010-0655-y
Katiyar-Agarwal S, Agarwal M, Grover A (2003) Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol Biol 51:677–686. doi:10.1023/A:1022561926676
Kaur G, Kumar S, Thakur P, Malik JA, Bhandhari K, Sharma KD, Nayyar H (2011) Involvement of proline in response of chickpea (Cicer arietinum L.) to chilling stress at reproductive stage. Sci Hortic (Amsterdam) 128:174–181. doi:10.1016/j.scienta.2011.01.037
Khripach V, Zhabinskii V, Groot AD (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447. doi:10.1006/anbo.2000.1227
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297. doi:10.1007/s00344-003-0058-z
Kumar N, Gupta S, Tripathi AN (2006) Gender-specific responses of Piper betle L. to low temperature stress: changes in chlorophyllase activity. Biol Plant 50:705–708. doi:10.1007/s10535-006-0111-4
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem F4.3.1–F4.3.8. doi: 10.1002/0471142913.faf0403s01
Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 43:379–393. doi:10.1007/s11099-005-0062-6
Lobell DB, Asner GP (2003) Climate and management contributions to recent trends in U.S. agricultural yields. Science 299:1032. doi:10.1126/science.1078475
Lobell DB, Field CB (2007) Global scale climate–crop yield relationships and the impacts of recent warming. Environ Res Lett 2:014002 (7 pp). doi:10.1088/1748-9326/2/1/014002
Matsui T, Omasa K, Horie T (2001) The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Prod Sci 4:90–93. doi:10.1626/pps.4.90
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence–a practical guide. J Exp Bot 51:659–668. doi:10.1093/jexbot/51.345.659
Neves JPC, Ferreira LFP, Vaz MM, Gazarini LC (2008) Gas exchange in the salt marsh species Atriplex portulacoides L. and Limoniastrum monopetalum L. in Southern Portugal. Acta Physiol Plant 30:91–97. doi:10.1007/s11738-007-0094-6
Nogués S, Baker NR (2000) Effect of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317. doi:10.1093/jexbot/51.348.1309
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57. doi:10.1007/s00344-007-9030-7
Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno GS, Khush GS, Cassman KG (2004) Rice yields decline with higher night temperature from global warming. Proc Natl Acad Sci USA 101:9971–9975. doi:10.1073/pnas.0403720101
Pospíšilová J (2003) Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol Plant 46:491–506. doi:10.1023/A:1024894923865
Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant, Cell Environ 23:1397–1405. doi:10.1046/j.1365-3040.2000.00650.x
Serna M, Hernández F, Coll F, Amorós A (2012) Brassinosteroid analogues effect on yield and quality parameters of field-grown lettuce (Lactuca sativa L.). Sci Hortic (Amsterdam) 143:29–37. doi:10.1016/j.scienta.2012.05.019
Shahbaz M, Ashraf M, Athar H (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64. doi:10.1007/s10725-008-9262-y
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119. doi:10.1007/s10725-005-3252-0
Suksamrarn A, Tanachatchairatana T, Sirigarn C (2002) Stereoselective catalytic hydrogenation of Δ7-6-ketosteroids in the presence of sodium nitrite. Tetrahedron 58:6033–6037. doi:10.1016/S0040-4020(02)00580-X
Sutcliffe J (1977) Plants and temperature. Edward Arnold Publishers Limited, London 57 pp
Thussagunpanit J, Jutamanee K, Kaveeta L, Chai-arree W, Pankean P, Suksamrarn A (2013) Effects of brassinosteroid and ecdysone analogue on pollen germination of rice under heat stress. J Pestic Sci 38:105–111. doi:10.1584/jpestics.D13-029
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007) Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth Res 91:37–46. doi:10.1007/s11120-006-9077-5
Vasil’ev S, Wiebe S, Bruce D (1998) Non-photochemical quenching of chlorophyll fluorescence in photosynthesis. 5-hydroxy-1,4-naphthoquinone in spinach thylakoids as a model for antenna based quenching mechanisms. Biochim Biophys Acta 1363:147–156. doi:10.1016/s0005-2728(97)00096-0
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective roles of exogenous polyamines. Plant Sci 151:59–66. doi:10.1016/S0168-9452(99)00197-1
Werawattanametin K, Podimuang V, Suksamrarn A (1986) Ecdysteroids from Vitex glabrata. J Nat Prod 49:365–366. doi:10.1021/np50044a041
Wittmann C, Aschan G, Pfanz H (2001) Leaf and twig photosynthesis of young beech (Fagus sylvatica) and aspen (Populus tremula) trees grown under different light regime. Basic Appl Ecol 2:145–154. doi:10.1078/1439-1791-00047
Wu X, Yao X, Chen J, Zhu Z, Zhang H, Zha D (2014) Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol Plant 36:251–261. doi:10.1007/s11738-013-1406-7
Xia X, Huang L, Zhou Y, Mao W, Shi K, Wu J, Asami T, Chen Z, Yu J (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230:1185–1196. doi:10.1007/s00425-009-1016-1
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143. doi:10.1093/jxb/erh124
Yuan L, Shu S, Sun J, Guo S, Tezuka T (2012) Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth Res 112:205–214. doi:10.1007/s11120-012-9774-1
Zhang YP, Zhu XH, Ding HD, Yang SJ, Chen YY (2013) Foliar application of 24-epibrassinolide alleviates high-temperature induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthetica 51:341–349. doi:10.1007/s11099-013-0031-4
Zhou YH, Yu JQ, Huang LF, Nogués S (2004) The relationship between CO2 assimilation, photosynthetic electron transport and water–water cycle in chill-exposed cucumber leaves under low light and subsequent recovery. Plant, Cell Environ 27:1503–1514. doi:10.1111/j.1365-3040.2004.01255.x
Zhu X, Song F, Liu S, Liu T (2011) Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 346:189–199. doi:10.1007/s11104-011-0809-8
Zullo MAT, Adam G (2002) Brassinosteroid phytohormones—structure, bioactivity and applications. Braz J Plant Physiol 14:143–181. doi:10.1590/s1677-04202002000300001
Acknowledgments
This research was supported by the Thailand Research Fund (RDG5490011) and by partial support from the Center of Excellence for Innovation in Chemistry, Office of the Higher Education Commission, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thussagunpanit, J., Jutamanee, K., Kaveeta, L. et al. Comparative Effects of Brassinosteroid and Brassinosteroid Mimic on Improving Photosynthesis, Lipid Peroxidation, and Rice Seed Set under Heat Stress. J Plant Growth Regul 34, 320–331 (2015). https://doi.org/10.1007/s00344-014-9467-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-014-9467-4