Abstract
Brassinosteroids (Brs) are a newly recognized group of active steroidal hormones that occur at low concentrations in all plant parts and one of the active and stable forms is 24-epibrassinolide (EBR). We investigated the effect of EBR on tomato (Lycopersicon esculentum Mill.) and its mechanism when seedlings were exposed to low temperature and poor light stress conditions. Leaves of stress-tolerant ‘Zhongza9’ and stress-sensitive ‘Zhongshu4’ cultivars were pre-treated with spray solutions containing either 0.1 μM EBR or no EBR (control). The plants were then transferred to chambers where they were exposed to low temperatures of 12 °C/6 °C (day/night) under a low light (LL) level of 80 μmol · m−2 · s−1. Exogenous application of EBR significantly increased the antioxidant activity of superoxide dismutase, catalase and peroxidase, and decreased the rate of O2 · − formation and H2O2 and malondialdehyde contents. Additionally, the ATP synthase β subunit content was increased by exogenous hormone application. Based on these results, we conclude that exogenous EBR can elicit synergism between the antioxidant enzyme systems and the ATP synthase β subunit so that scavenging of reactive oxygen species becomes more efficient. These activities enable plants to cope better under combined low temperature and poor light stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brassinosteroids (BRs), first discovered in pollen from rapeseed (Brassica napus) (Grove et al. 1979), are a newly recognized group of highly active steroidal hormones that occur at low concentrations in all plant parts. Because their physiological activity is significantly higher than that of the five other types of hormones, BR was confirmed as the sixth plant hormone during the 16th International Conference on Plant Growth Substances (Abbas et al. 2013; Fujii and Saka 2001). As naturally occurring substances that promote growth (Vardhini et al. 2011), BRs regulate various aspects of plant development, including cell expansion and division, xylem differentiation, proton activity, and photosynthesis (Vardhini 2012). They are also possibly involved in protecting plants against various environmental stresses, e.g., drought (Bajguz and Hayat 2009), high temperature (Kumar et al. 2012), chilling (Singh et al. 2012), salinity (Hayat et al. 2010), and heavy metals (Arora et al. 2010). One of the most active and stable forms of this phytohormone is 24-epibrassinolide (EBR).
Tomato (Lycopersicon esculentum Mill.) is one of the most widely grown vegetables. Most cultivars are thermophilic and perform well under high light levels. Tomato plants are highly sensitive to low temperature and poor light at all stages of development, but especially during early seedling growth (Wang et al. 2010). These two stresses are the most significant factors restricting plant growth and crop productivity in Winter and Spring, when they can cause numerous metabolic changes by suppressing photosynthesis and respiration and inducing oxidative stress.
Under low temperature and poor light stress condition, crop plants with stronger resistance can improve the ability to resist stress by increasing the expression of specific genes and proteins. For examples, defensin and cold dehydrin genes were overexpressed in Oxytropis species and that enhanced the tolerance to low temperature (Archambault and Strömvik 2011). The increasing expressions of anthocyanidin synthase genes gave Brassica rapa strong tolerance to low temperature (Ahmed et al. 2015). Up-regulation of genes encoding chitinases and glucanases proteins enhanced the winter barley tolerance to low temperature (Janská et al. 2011). While, our experiment showed up-regulation of ATP synthase β subunit protein gave tomato a high capacity to cope with low temperature and poor light stresses.
In an effort to devise new strategies for protection against such stresses, we conducted experiments in which the activities of antioxidant enzymes, the rate of O2 · − formation and H2O2 and malondialdehyde contents were measured. Our objective was to determine whether exogenous EBR could improve the tolerance of tomato plants to low temperatures and poor light intensities. We also investigated the mechanism by means of which these changes might occur through analysis of the ATP synthase β subunit.
Materials and methods
Plant materials and treatments
Two tomato genotypes were used: ‘Zhongza9,’ which is tolerant of low temperature and poor light, and ‘Zhongshu4,’ which is sensitive. Seeds were placed on moistened filter paper for 7 days under darkness at 19 °C. The germinants were then sown in plastic pots containing 1:1 (v:v) peat moss and vermiculite. The seedlings were exposed to a 12-h photoperiod (light intensity of 350 μmol · m−2 · s−1) with a day/night temperature regime of 25 °C/18 °C in a greenhouse at the College of Horticulture, Northwest A&F University, Yangling (34° 20′ N, 108° 24 E′), Shaanxi Province, China.
A 10-mM EBR stock solution was prepared by dissolving 0.048 g EBR in 10 mL absolute ethanol. A 0.1 μM EBR solution (containing 0.1 % Teepol) was prepared just before use, and 10 mL was sprayed on each plant (Singh et al. 2012). Uniformly sized seedlings were selected and when they had produced five true leaves, their foliage was pre-treated once a day for 7 days with spray solutions containing either no EBR (control) or 0.1 μM EBR (n = 60 plants per treatment). The seedlings in each treatment group (−EBR or +EBR) were then transferred to environmental chambers and exposed to combined-stress conditions of 12 °C/6 °C (day/night) and a low light intensity of 80 μmol · m−2 · s−1. This resulted in two treatment groups for each genotype: −EBR/stress and +EBR/stress. For each parameter, three tomato seedlings were randomly selected and sampled at a time. The plants were sampled after 0, 3, 6, 9, and 12 days of stress treatment, when they were immediately frozen in liquid N2 and stored at −80 °C.
Measurements of antioxidant enzyme activities
Leaf samples (0.2 g) from each treatment group were homogenized on ice in 5 mL of 50 mM sodium phosphate buffer (pH 7.8) containing 1 mM EDTA and 2 % (W/V) PVP. The homogenate was centrifuged at 12,000×g for 30 min at 4 °C. The supernatant was used for monitoring enzyme activities (Wu et al. 2014a).
Superoxide dismutase (SOD) activity was assayed according to the method of Vardhini et al. (2011), which is based on the measurement of inhibition in the photochemical reduction of nitro blue tetrazolium spectrophotometrically at 560 nm. Peroxidase (POD) activity was determined at 436 nm based on its ability to convert guaiacol to tetraguaiacol, per the method of Abedi and Pakniyat (2010). Catalase (CAT) activity was studied by monitoring the disappearance of hydrogen peroxide (H2O2) at 240 nm, according to the method of Farhad et al. (2011).
Measurements of O2 · − formation rate and H2O2 and malondialdehyde contents
For O2 · − formation rate and H2O2 content measurements, leaf samples (0.2 g) from each treatment group were homogenized on ice in 5 mL of 50 mM potassium phosphate buffer (pH 7.8). The homogenate was centrifuged at 12,000×g for 10 min. Next, 0.5 mL 50 mM potassium phosphate buffer (pH 7.8) and 1 mL 1 mM hydroxylamine hydrochloride were added to 0.5 mL supernatant. After incubation at 25 °C for 20 min, an equal volume of ethyl ether was added and the sample was centrifuged at 12,000×g for 20 min (Hu et al. 2010).
The rate of O2 · − formation and H2O2 content were assayed according to the method of Zhou et al. (2004). The rate of O2 · − formation is based on the measurement of nitrite formation from hydroxylamine in the presence of O2 · − and the absorbance was read at 530 nm. The H2O2 content was measured at 410 nm of the titanium-peroxidase complex.
For malondialdehyde (MDA) measurements, leaf samples (0.2 g) from each treatment group were homogenized on ice in 5 mL of 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA and 2 % (W/V) PVP. The homogenate was centrifuged at 12,000×g for 10 min. The supernatant was used to monitor the MDA content. MDA contents were determined by performing thiobarbituric acid (TBA) reactions, as described by Hu et al. (2010). Measurements were corrected for nonspecific absorbance by subtracting the values obtained at 440, 532, and 600 nm.
SDS-PAGE and target protein content measurement
Protein extracts were prepared from the leaves of seedlings exposed to low temperature and poor light for 1, 6, and 12 days. The samples were ground to powder in liquid nitrogen and melted in ice-cold extraction buffer [50 mM Tris-HCl (pH 8.0), 10 mM NaCl, 1 % sodium dodecyl sulfate (SDS), 5 % 2-mercaptoethanol, 0.1 mM PMSF, and 0.1 mM DTT]. This was followed by centrifugation at 10,000×g for 15 min at 4 °C.
Equal amounts of concentrated protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), using 15 % resolving gels and 4 % stacking gels. After 3 h of electrophoresis at 100 V/45 mA, the gels were stained with Coomassie Brilliant Blue R-250 (Khalifa 2012).
The grayscale percentages of the target protein in the crude protein were analyzed with the BrandScan software. Crude proteins were extracted from leaf samples and their contents (per gram) were calculated. Target protein contents were calculated using the formula: target protein content (mg/g) = crude protein content (mg/g) × grayscale percentage.
Mass spectrometry analysis
Target protein bands were excised from the gels, washed with a 100 mM NH4HCO3/30 % ACN solution, and dried in a vacuum concentrator. The proteins were digested overnight at 37 °C with 5 μL of 10 ng · μL−1 sequencing-grade modified trypsin (Promega, USA). Afterward, 100 μL of 60 % (v/v) ACN in 0.1 % aqueous trifluoroacetic acid (TFA) was added to the gel pieces, which were then sonicated for 15 min. The digested peptides were dissolved using 2 μL of 50 % acetonitrile/0.1 % TFA, and 0.5 μL aliquots were applied to the target disk. After air-drying, 0.5 μL of matrix solution [cyano-4-hydroxy cinnamic acid (CHCA) saturated in 50 % acetonitrile/0.1 % TFA] was added to the dried samples and again allowed to dry. The samples were then subjected to matrix-assisted laser desorption ionization time-of-flight/time-of-flight (MALDI-TOF/TOF) tandem mass spectrometry (MS/MS) analysis using a 4800 Plus MALDI TOF/TOF™ Analyzer instrument (Applied Biosystems, USA) with an Nd:YAG smart laser beam (335 nm wavelength). The acceleration voltage in the ion source was operated at 20 kV and a minimum signal-to-noise ratio of 50 was selected for MS/MS. The laser power was set to 4600 for MS and to 5200 for MS/MS with the CID off. The MS spectra were acquired across a mass range of 800 to 4000 Da (Kang et al. 2012).
The proteins were identified by searching the NCBI non-redundant database, using the MASCOT program (http://www.matrixscience.com, Matrix Science, UK). Search parameters included L. esculentum, set as taxonomy; trypsin cleavage, one missed cleavage allowed; carboxymethyl (C), set as a fixed modification; oxidation (Met) of methionines allowed as variable modification; peptide mass tolerance, within 100 ppm; and fragment mass tolerance of ±0.4 Da (Hu et al. 2012). According to the MASCOT probability analysis (P < 0.05), only significant hits were accepted for protein identification (Wen et al. 2013).
Statistical analysis
All physiological parameters were examined twice, with three replicates. Statistical analysis was performed with Microsoft Excel 2013 and SPSS16.0.
Results
Measurements of antioxidant enzyme activity
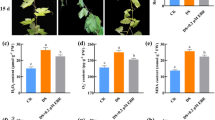
SOD activity followed similar trends in both cultivars (Fig. 1a), peaking on Day 3 before decreasing in the leaves of seedlings exposed to the combined low temperature and poor light stresses. However, SOD activity was stronger in the tolerant ‘Zhongza9’ than in the sensitive ‘Zhongshu4.’ Furthermore, SOD activity was higher in the +EBR seedlings. The subsequent decline in SOD activity was slower, and it remained at a relatively higher level, in the pre-treated seedlings. By Day 12, the SOD activities were 55.2 and 50.9 % greater in pre-treated ‘Zhongza9’ and ‘Zhongshu4,’ respectively, than in plants not exposed to EBR.
POD activity showed the same trend as SOD. By Day 12, POD activity had increased significantly by 73.9 and 35.9 % in +EBR ‘Zhongza9’ and +EBR ‘Zhongshu4,’ respectively, compared with the untreated samples (Fig. 1b).
CAT activity decreased over time for both +EBR genotypes, although the decline was more rapid in the sensitive cultivar. Compared with the non-pretreated seedlings, CAT activity on Day 12 was 69.4 % higher in +EBR ‘Zhongza9’ and 46.1 % higher in +EBR ‘Zhongshu4’ (Fig. 1c).
Measurements of O2 · − formation rate and H2O2 and malondialdehyde contents
When the seedlings were exposed to low temperature and poor light, the MDA contents significantly increased (Fig. 1d). This rise was more dramatic in the sensitive genotype. However, when compared with plants that did not receive hormone pretreatment, the MDA levels on Day 12 were 36.4 and 23.5 % lower for +EBR ‘Zhongza9’ and +EBR ‘Zhongshu4,’ respectively.
Similarly, low temperature and poor light stresses increased the rate of O2 · − formation and H2O2 content in tomato seedling leaves, while EBR pretreatment significantly alleviated this increase (Fig. 2). The O2 · − formation rates on Day 12 were 59.0 and 42.8 % lower for +EBR ‘Zhongza9’ and +EBR ‘Zhongshu4,’ respectively. The H2O2 contents on Day 12 were 32.7 and 27.3 % lower for +EBR ‘Zhongza9’ and +EBR ‘Zhongshu4,’ respectively.
SDS-PAGE and target protein content measurement
After 1, 6, and 12 days of induced stress, total leaf proteins were extracted and separated by SDS-PAGE. We noted that one protein band expression was different in the two treatment groups for each genotype. Using the BandScan software, we calculated the content of this target protein. As shown in Fig. 3, with prolonged stress, the expression of the protein gradually decreased, albeit more slowly in leaves from the tolerant genotype ‘Zhongza9.’ Furthermore, under stress conditions, the protein levels were much higher in +EBR seedlings than in those that had not been pretreated.
Mass spectrometry analysis
We selected this band for analysis via MALDI-TOF/TOF MS. The protein was identified following a search of the NCBI non-redundant database. Most of the stronger mass spectra peaks were matched with y1, y2, y4–10, y13–16, y18–22, y*14, y*23, b3, and immonium ions (Fig. 4). Therefore, we deduced that the partial peptide sequence was IFNVLGEPVDNLGPVDTSTTSPIHR by aligning the ions with amino acids (Fig. 5). Through a BLAST alignment of this peptide, we confirmed that the protein was the ATP synthase β subunit of L. esculentum Mill (gi/6688527) with 17 % protein sequence coverage (Fig. 6).
Discussion
Low temperature and poor light stresses not only affect plant growth and development, but also lead to oxidative damage and even death of plant tissues (Ogweno et al. 2009). This is mainly caused by the excessive accumulation of reactive oxygen species (ROS) from plant membrane damage. These ROS can also react with nucleic acids, proteins and lipids, destroying cellular structure and function, and even causing cell death (Wu et al. 2014b). O2 · − and H2O2 are the main ROS and can be efficiently scavenged in vivo by three key antioxidant enzymes—SOD, CAT, and POD (Xi et al. 2013). Among them, SOD is the first line of defense against ROS-associated damage, catalyzing the dismutation of O2 · − to H2O2 and molecular oxygen. Subsequently, H2O2 is further metabolized to simple water molecules through the action of POD and CAT. Therefore, the activities of antioxidant enzymes can serve as an important index of plant stress tolerance.
In our experiments, both SOD and POD activities increased in the early phase of induced stress in both tomato cultivars. This was a general adaptive strategy that plants used to overcome such challenges. After 3 days, the activities of SOD and POD decreased. However, the O2 · − formation rate and H2O2 and MDA contents increased, showing that the balance of ROS production and removal was disturbed. Cytoplasmic membranes under ROS attack produced peroxides. EBR pretreatment significantly increased the SOD, POD, and CAT activities and reduced the rate of O2 · − formation and H2O2 content in both tomato cultivars. Excess ROS were efficiently scavenged in vivo, and membrane lipid peroxidation was alleviated. Our observations were consistent with previous reports that EBR could improve plants’ capacity to cope with the stresses of low temperature and poor light, especially during early seedling development (Sasse 2003; Divi and Krishna 2010). This was mainly because EBR induced stress tolerance by triggering the accumulation of H2O2 and O2 · − which subsequently up-regulated the antioxidant system (Jiang et al. 2012; Fariduddin et al. 2014).
As the main biological energy source, ATP is indispensable for many metabolic pathways in higher plants, while ATP synthase plays a key role in the synthesis of ATP in all living organisms (Cheng et al. 2010). ATP synthase comprises an integral membrane CF0 portion and an extrinsic CF1 portion. The enzyme complex CF1 has five subunits. Of those, the β subunit, composed of a catalytic and ADP-binding unit, catalyzes ATP formation from ADP and Pi in the presence of a transmembrane proton gradient (Ye et al. 2013).
Our investigation was the first to show a link between EBR and the ATP synthase β subunit. In our experiment, the expression of the ATP synthase β subunit decreased in both tomato cultivars under low temperature and poor light stress conditions. This may be because H2O2 and O2 · − are signal molecules and can directly interact with ATP synthase β subunit (target or sensor). Accumulation of H2O2 and O2 · − under low temperature and poor light stresses inhibited ATP synthase β subunit synthesis. A decrease in ATP formation limited photosynthesis and respiration, ultimately inhibiting the growth and development (Rott et al. 2011). After pretreatment with exogenous EBR, the tomato seedlings showed increased antioxidant activity, and the excess H2O2 and O2 · − were effectively scavenged. Thus, inhibition of the ATP synthase β subunit was removed. The increase in ATP synthase β subunits activated biological energy metabolism and improved ATP levels, with most of that energy being used for growth and development. This additional energy enabled the plants to cope with the combined low temperature and poor light stresses. This demonstrated that exogenous EBR could make the antioxidant enzyme system and ATP synthase β subunit act synergistically, so that ROS were scavenged efficiently in vivo, and plant growth and development could gradually return to normal under low temperature and poor light stress conditions.
In this study, exogenous EBR induced higher antioxidant activities and ATP synthase β subunit content in the tolerant genotype ‘Zhongza9’ than the sensitive genotype ‘Zhongshu4.’ This gave ‘Zhongza9’ a higher capacity to removing H2O2 and O2 · −. These results indicated that EBR pretreatment potentially gave ‘Zhongza9’ better protection against the oxidative damage caused by low temperature and poor light stresses.
Abbreviations
- BRs:
-
Brassinosteroids
- CAT:
-
Catalase
- EBR:
-
24-Epibrassinolide
- H2O2 :
-
Hydrogen peroxide
- MALDI-TOF/TOF MS:
-
Matrix-assisted laser desorption ionization time-of-flight/time-of-flight mass spectrometry
- MDA:
-
Malondialdehyde
- MS/MS:
-
Tandem mass spectrometry
- O2 · − :
-
Superoxide radical
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SOD:
-
Superoxide dismutase
References
Abbas S, Latif HH, Elsherbiny EA (2013) Effect of 24-epibrassinolide on the physiological and genetic changes on two varieties of pepper under salt stress conditions. Pak J Bot 45:1273–1284
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46:27–34
Ahmed NU, Park JI, Jung HJ, Hur Y, Nou IS (2015) Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct Integr Genomics 15:383–394
Archambault A, Strömvik MV (2011) PR-10, defensin and cold dehydrin genes are among those over expressed in Oxytropis (Fabaceae) species adapted to the arctic. Funct Integr Genomics 11:497–505
Arora P, Bhardwaj R, Kanwar MK (2010) 24-epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plants 16:285–293
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Cheng J, Zhang XA, Shu YG, Yue JC (2010) F0F1-ATPase activity regulated by external links on β subunits. Biochem Biophys Res Commun 391:182–186
Divi UK, Krishna P (2010) Overexpression of the brassinosteroid biosynthetic gene AtDWF4 in Arabidopsis seeds overcomes abscisic acid-induced inhibition of germination and increases cold tolerance in transgenic seedlings. J Plant Growth Regul 29:385–393
Farhad MS, Babak AM, Reza ZM, Mir Hassan RS, Afshin T (2011) Response of proline, soluble sugars, photosynthetic pigments and antioxidant enzymes in potato (Solanum tuberosum L.) to different irrigation regimes in greenhouse condition. Aust J Crop Sci 5:55–60
Fariduddin Q, Yusuf M, Ahmad I, Ahmad A (2014) Brassinosteroids and their role in response of plants to abiotic stresses. Biol Plant 58:9–17
Fujii S, Saka H (2001) The promotive effect of brassinolide on lamina joint-cell elongation, germination and seedling growth under low-temperature stress in rice (Oryza sativa L.). Plant Prod Sci 4:210–214
Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Cook JC (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112
Hu WH, Wu Y, Zeng JZ, He L, Zeng QM (2010) Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica 48:537–544
Hu M, Qiu ZH, Zhou P, Xu LF, Zhang JK (2012) Proteomic analysis of ‘Zaosu’ pear (Pyrus bretschneideri Rehd.) and its red skin bud mutation. Proteome Sci 10:51
Janská A, Aprile A, Zámečník J, Cattivelli L, Ovesná J (2011) Transcriptional responses of winter barley to cold indicate nucleosome remodelling as a specific feature of crown tissues. Funct Integr Genomics 11:307–325
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen ZX, Yu JQ (2012) Cellular glutathione redox homeostasis plays an important role in the brassinosteroid induced increase in CO2 assimilation in Cucumis sativus. New Phytol 194:932–943
Kang GZ, Li GZ, Zheng BB, Han QX, Wang CY, Zhu YJ, Guo TC (2012) Proteomic analysis on salicylic acid-induced salt tolerance in common wheat seedlings (Triticum aestivum L). Biochim Biophys Acta 1824:1324–1333
Khalifa NS (2012) Protein expression after NaCl treatment in two tomato cultivars differing in salt tolerance. Acta Biol Cracov 54:79–86
Kumar S, Sirhindi G, Bhardwaj R, Kumar M, Arora P (2012) Role of 24-epibrassinolide in amelioration of high temperature stress through antioxidant defense system in Brassica juncea L. Plant Stress 6:55–58
Ogweno JO, Song XS, Hu WH, Shi K, Zhou YH, Yu JQ (2009) Detached leaves of tomato differ in their photosynthetic physiological response to moderate high and low temperature stress. Sci Hortic 123:17–22
Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM (2011) ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23:304–321
Sasse JM (2003) Physiological actions of brassinosteroids: an update. J Plant Growth Regul 22:276–288
Singh I, Kumar U, Singh SK, Gupta C, Singh M, Kushwaha SR (2012) Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol Mol Biol Plants 18:229–236
Vardhini BV (2012) Effect of brassinolide on certain enzymes of sorghum grown in saline soils of Karaikal. J Phytol 4:30–33
Vardhini BV, Sujatha E, Rao SSR (2011) Brassinosteroids: alleviation of water stress in certain enzymes of sorghum seedlings. J Phytol 3:38–43
Wang M, Jiang WJ, Yu HJ (2010) Effects of exogenous epibrassinolide on photosynthetic characteristics in tomato (Lycopersicon esculentum Mill) seedlings under weak light stress. J Agric Food Chem 58:3642–3645
Wen L, Tan TL, Shu JB, Chen Y, Liu Y, Yang ZF, Zhang QP, Yin MZ, Tao J, Guan CY (2013) Using proteomic analysis to find the proteins involved in resistance against Sclerotinia sclerotiorum in adult Brassica napus. Eur J Plant Pathol 137:505–523
Wu XX, Yao XF, Chen JL (2014a) Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol Plant 36:251–261
Wu XX, He J, Zhu ZW, Yang SJ, Zha DS (2014b) Protection of photosynthesis and antioxidative system by 24-epibrassinolide in Solanum melongena under cold stress. Biol Plant 58:185–188
Xi ZM, Wang ZZ, Fang YL, Hu ZY, Hu Y, Deng MM, Zhang ZW (2013) Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul 71:57–65
Ye JX, Wang SP, Zhang FJ, Xie DQ, Yao YH (2013) Proteomic analysis of leaves of different wheat genotypes subjected to PEG 6000 stress and rewatering. Plant Omics J 6:286–294
Zhou YH, Yu JQ, Huang LH, Nogues S (2004) The relationship between CO2 assimilation, photosynthetic electron transport and water-water cycle in chill-exposed cucumber leaves under low light and subsequent recovery. Plant Cell Environ 27:1503–1514
Acknowledgments
This research was supported by the Key Laboratory of Protected Horticultural Engineering in Northwest, Ministry of Agriculture, PR China; and by the State Key Laboratory of Crop Stress Biology for Arid Areas, NWAFU, P R China, and by the Agriculture Research System in China (No. CARS-25-D-02). We also thank Dr. Fengwang Ma (Northwest A&F University, China) and Priscilla Licht for their assistance in revising this composition.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, L., Zou, Z., Zhang, J. et al. 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). Funct Integr Genomics 16, 29–35 (2016). https://doi.org/10.1007/s10142-015-0464-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-015-0464-x