Abstract
This paper presents a study of the metabolic response (dark respiration intensity, photosystem II efficiency, metabolic activity) and the yield of barley treated with 24-epibrassinolide and subjected to high-temperature stress. Transport of exogenously applied 24-epibrassinolide in barley and changes in the profile of brassinosteroids that may occur in tissues after 24-epibrassinolide application were also studied. The water solution of 24-epibrassinolide (0.005 and 0.25 mg dm−3) was applied via infiltration of the first and second leaves of 12-day-old seedlings. Control plants were treated with water solution of hormone solvent (ethanol). Fifteen-day-old plants were subjected to high-temperature stress (42°C for 3 h). The influence of hormone treatment and stress conditions was investigated in the first and second leaves based on measurements of PSII efficiency. The aftereffect of plant treatment was investigated in the seventh leaf (measurements of PS II efficiency, dark respiration intensity, metabolic activity). The transport efficiency of 24-epibrassinolide exogenously applied to the first and second leaves, as well as the profile of other brassinosteroids, was also measured on the seventh leaf. Finally, yield formation was estimated. 24-epibrassinolide showed protective action, which manifested itself in the improved functioning of PSII, but this was observed in case of higher hormone concentration and only for the first, older leaf. The PSII efficiency of the seventh leaf was similar in plants treated with brassinosteroid and in the control plants, whereas the respiration intensity and metabolic activity decreased in plants previously treated with higher concentration of 24-epibrassinolide. The use of a higher hormone concentration at the seedling phase ultimately resulted also in lower crop yield. Brassinosteroids—brassinolide and castasterone—were detected in barley leaves. 24-epibrassinolide was found only in trace amounts in control plants. Its exogenous application directly to the apoplast of the first and second leaves resulted in an increase in the 24-epibrassinolide content in the seventh leaf, but did not depend on whether a high or low concentration had been applied to the plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brassinosteroids are plant growth regulator substances. The first brassinosteroid, a novel polyhydroxylated steroidal lactone with high growth-promoting activity, termed brassinolide, obtained from rape pollen (Brassica napus L.) was discovered in 1979 (Grove et al. 1979). This finding showed that steroids are generally signalling molecules in both animals and plants. While mammalian steroid hormones are recognised mainly by nuclear receptors, brassinosteroids in plants, according to current knowledge, are recognised by a cell surface receptor kinase, BRI1 (Wang et al. 2001). BRs include more than 70 compounds found in plant kingdom (Bajguz and Tretyn 2003). In addition to brassinolide, the following compounds belong to the brassinosteroid group: 24-epibrassinolide, castasterone, homobrassinolide, teasterone, dolicholide, typhasterol, etc. (Zullo and Adam 2002; Zullo and Kohout 2004). Brassinosteroid compounds are widely distributed throughout the reproductive and vegetative plant tissues. BRs generally occur in reproductive organs such as pollen (5–1,000 ng g−1 FW) and fruits (0.2–3.5 ng g−1 FW) in relatively high amounts (Symons et al. 2008). In seeds, BRs are present in the range 0.1–1,600 ng g−1 FW (Symons et al. 2008; Janeczko et al. 2010). BR content in shoot tissues is rather lower (0.12–2 ng g−1 FW) (Symons et al. 2008; Janeczko and Swaczynová 2010). In the lowest amounts (<0.05 ng g−1 FW), BRs are found in the roots (Symons et al. 2008).
As with their animal counterparts, BRs regulate the expression of numerous genes, influencing the activity of complex metabolic pathways. Investigations on brassinosteroid mutants confirm that brassinosteroids are important for vascular differentiation, senescence, fertility and photomorphogenesis. Brassinosteroids increase yield in plants of economic importance such as wheat, rice, groundnut, potato, cotton, mung bean and others (Ramraj et al. 1997; Zullo and Adam 2002; Fariduddin et al. 2008; Janeczko et al. 2010). BRs may also improve crop quality (Vardhini and Rao 1998, 2002; Janeczko et al. 2009). BRs have high activity in plant protection against different stressors, such as thermal and heavy metal stressors or pathogen attack (Janeczko et al. 2005; Janeczko et al. 2007a, b; Bajguz and Hayat 2009), and often have more effect when plants are under stress than when they grow under optimal conditions.
The aim of the present work was to study the influence of 24-epibrassinolide on photosynthesis (energy flows in PS II), metabolic activity, dark respiration and the yield formation of barley. Since brassinosteroids very often exhibit their activity in plants growing under stress conditions (Janeczko et al. 2005), barley was exposed to heat shock (42°C for 3 h). As a part of the study, the content of brassinosteroids in barley leaves and efficiency of transport of 24-epibrassinolide after its exogenous application were also analysed with HPLC–MS technique. To estimate the efficiency of energy flow in PSII during the light stage of photosynthesis, measurements of chlorophyll a fast fluorescence were used (Strasser et al. 2000). Unfavourable plant growth conditions alter the transients of fluorescence curves, which are connected with changes in the positions of “F” points and have an effect on the calculated values of phenomenological fluxes that describe PS II efficiency (Strasser et al. 2000). Plant metabolic activity was measured based on the amount of heat released from plant tissues. Measurements were performed using the calorimetric method. Heat is an inevitable by-product of all metabolic processes, but the amount of heat emitted by plant tissues depends on plant age, growth intensity or external factors influencing the plant, such as hormonal treatment or exposure to stress conditions—salinity, drought, pathogen infections, etc. (Criddle et al. 1989; Rapacz and Żur 1996; Criddle and Hansen 1999; Płażek and Rapacz 2000; Smith et al. 2000; Hansen et al. 2004; Stokłosa et al. 2006; Janeczko et al. 2007b). An important part of plant metabolic activity is the respiration process. Analysis of dark respiration provides information about catabolic processes, which also depend on plant age and growth conditions (Brown and Thomas 1980; Lambers and Ribas-Carbó 2005). Dark respiration measurements may also be useful for predicting plant yield (Smith et al. 1995).
Materials and methods
Plant culture and treatments
Seedlings of spring barley (Hordeum vulgarum L. cv. Sezam) were grown in pots, in a greenhouse at day/night temperatures of 20/17°C and under natural light conditions (April, latitude: 50°03′ north; longitude: 19°55′ east). Twelve-day-old plants, with two leaves, were treated with 24-epibrassinolide (0.005 and 0.25 mg dm−3) with the use of the leaf infiltration technique. The solutions were injected into the first (older) and second (younger) leaf using a plastic syringe without needle. This method allows applying of the solution directly to the leaf apoplast space and thus results in better hormone penetration within the plant tissue. Three days after hormonal treatment, the fast fluorescence kinetics of chlorophyll a (PS II efficiency) was measured in the first and second leaves of 15-day-old plants. The plants were then immediately exposed to a high temperature (42°C, 3 h in darkness), and the PS II efficiency was measured 30 min after heat stress. For the third time, PS II efficiency was measured in the first and second leaves of 22-day-old plants (7 days after heat shock). The plants continued to grow in a greenhouse for the next 20 days until the seventh leaf was fully expanded and then the following physiological measurements were performed: PS II efficiency, dark respiration, metabolic activity and brassinosteroid content. Dry weight and the surface area of the seventh leaf were also measured. The plants were then transferred to the soil in the field and finally yield parameters were counted: the fresh weight of the plant (aboveground part) after harvesting, the number of spikes and seeds per plant, the weight of 1,000 seeds (g) and seed yield per plant (g).
Preparation of hormonal and control solutions
The 4.1 mM stock solution of 24-epibrassinolide (Sigma-Aldrich, Poznań, Poland) was prepared in 50% ethanol and this stock was diluted with distilled water to obtain a final concentration of 0.005 and 0.25 mg dm−3 BR27 used for treatments. Control plants were treated with water containing traces of hormone solvent (ethanol). Ethanol concentration in all working solutions was adjusted to 0.00625% (v/v) to match the amount of ethanol present in the solution with the highest concentration of BR27. Additionally, the absolute control (plants without treatment) was also cultured, but since the results of physiological measurements (dark respiration, PS II efficiency) obtained for them were similar to that in ethanol-treated control, these data will not be presented.
Estimation of water capacity of the apoplast and calculation of 24-epibrassinolide content after application to the first and second leaves
To calculate the amount of 24-epibrassinolide injected into the apoplast of one leaf, the water capacity of the apoplast of the first and second leaves was estimated. A leaf of a 12-day-old seedling was cut off, weighed (Mass 1), injected with water and weighed again (Mass 2). Water capacity of the apoplast = (Mass 2 − Mass 1)/0.998 (water density at 20°C). In the first leaf, the average water apoplast capacity was 0.05 cm3 and for the second 0.04 cm3 (calculated based on 7 replicates, SE ±0.01 for both leaves). Based on this, the approximate amount of 24-epibrassinolide injected into leaf apoplast was mathematically calculated and expressed in ng per fresh weight (Mass 1) of leaf (Table 1).
Extraction, purification and determination of brassinosteroids
Endogenous brassinosteroids were extracted and quantified using HPLC–MS technique as described previously in Swaczynová et al. (2007) and Janeczko and Swaczynová (2010). Samples of every fresh seventh leaf of barley (0.5 g) were taken for analysis. The following brassinosteroids were analysed: 24-epibrassinolide, brassinolide, castasterone, 24-epicastasterone, 28-homobrassinolide and 28-homocastasterone. The analysis was done in three replicates (1 replicate = sample from 2 leaves).
Measurements of photosystem II efficiency (chlorophyll a fast fluorescence)
Chlorophyll a fast fluorescence giving information about PS II efficiency was measured using a Plant Efficiency Analyzer (PEA; Hansatech Ltd., King’s Lynn, Norfolk, England) with an excitation light intensity of 3 mmol m−2 s−1 (peak 650 nm). The leaves were measured after 30 min of adaptation to darkness (clips with a 4-mm diameter hole) at a temperature of 20°C. Fluorescence intensity was measured with a PIN-photodiode, and changes in fast fluorescence were registered during illumination for a time from 10 μs to 1 s. During the initial 300 μs, data were collected every 10 μs and, after this period, the frequency of measurements was reduced automatically. Based on the fluorescence curves obtained, technical fluorescence parameters were extracted using a software (Handy PEA Software Ver: v1.30, Hansatech Instruments Ltd.) and calculated according to Strasser et al. (2000). Based on data extracted from the fast fluorescence transient, the following parameters of PS II efficiency (phenomenological fluxes or phenomenological activities) were calculated based on Strasser et al. (2000): energy absorption by antennas ABS/CSm; energy flux for trapping (energy transferred to reaction centre) TRo/CSm; energy flux for electron transport ETo/CSm; energy dissipation (energy lost as a heat) DIo/CSm, where CSm is the cross section of the sample. The analysis was done in 20 replicates (1 replicate = 1 leaf).
Measurements of metabolic activity (heat production)
Metabolic activity was measured as heat production from barley leaves using an isothermal calorimeter (TAM III; Thermometric, Järfälla, Sweden) at 20°C; 20 cm3 ampoules equipped with lids, which enable natural air exchange, were used. The seventh leaf was cut off from the plant and placed in a glass vessel with 0.1 cm3 of water and then into a calorimetric ampoule. The reference ampoule contained a vessel with only water. Data on heat production were collected for 20 min. An example of heat curve production is shown in Fig. 1. After measurement, leaf area and DW were estimated. Based on data recorded by TAM Assistant software (SciTech Software AB, Thermometric AB), the following parameters were defined (see Fig. 1):
- HPmax :
-
the highest value of heat production recorded during 20 min (mW)
- HPAREA, T=20 min :
-
the area (integral) of heat production under the curve recorded during 20 min (mJ)
- HPAREA max :
-
the area (integral) of heat production under the curve recorded to the moment of reaching maximal value of heat production (mJ).
HPmax is most often used to express plant metabolic activity (Criddle et al. 1989; Smith et al. 2000; Stokłosa et al. 2006). Parameters: HPAREA, T=20 min and HPAREA max are proposed and calculated in this study to interpret heat emission curves (Fig. 1). Values of heat production were expressed per 0.1 g of leaf DW (Table 3), as well as per leaf area (50 cm2). The analysis was done in five replicates (1 replicate = 1 leaf).
Measurements of dark respiration intensity
The dark respiration of the seventh leaf was measured with an infrared gas analyser for CO2 (LCA-2, Analytical Development Corp., UK) with a Parkinson PLC(N) assimilation chamber. The airflow through the assimilation chamber [350 μmol CO2 mol−1 (of the air)] amounted to 350 cm3 min−1. Measurements were performed at a temperature of 25°C in darkness. Values of dark respiration intensity were expressed per 0.1 g of leaf DW (Table 3), as well as leaf area (50 cm2). The analysis was done in five replicates (1 replicate = 1 leaf).
Measurements of leaf dry weight and area
The seventh leaf area was measured with the use of a CI-202 Area Meter (CiD Inc., USA); then the leaves were dried at 60°C for 72 h and weighted. The measurements were done in five replicates (1 replicate = 1 leaf).
Yield components
Plants were harvested and the following yield parameters were counted: the fresh weight of the plant (aboveground part), the number of spikes and seeds per plant, the weight of 1,000 seeds (g) and seed yield per plant (g). Estimation of yield was done for 20 plants per treatment.
Results
Brassinosteroid content in barley
After infiltration of the first and second leaves of barley with 24-epibrassinolide at concentrations of 0.005 and 0.25 mg dm−3, the content of this hormone in the apoplast of the treated leaf tissue amounted to 2 and 100 ng g−1 FW, respectively (Table 1). In the seventh leaf of control barley, two important brassinosteroids—brassinolide and castasterone—were detected (Table 1). 24-Epibrassinolide was found only in trace amounts. The content of 24-epibrassinolide in the seventh leaf increased to an easily detectable level in BR27-treated plants (Table 1). The content of 24-epibrassinolide in the seventh leaf did not differ between plants treated with BR27 at concentrations of 0.005 and 0.25 mg dm−3. The content of brassinolide and castasterone in BR27-treated plants was similar to that of the control. 24-Epicastasterone, 28-homobrassinolide and 28-homocastasterone were not detected in barley.
PS II efficiency of barley seedlings before and after heat shock
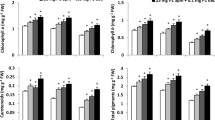
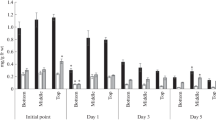
In 15-day-old barley, infiltration of the first and second leaves with BR27 generally had no impact on PSII efficiency (Table 2). Only the amount of energy dissipated as heat in the second leaf increased. Heat shock in control plants caused damage to the PS II efficiency of leaves (Table 2). The absorption of energy (ABS/CSm), energy flux for trapping (TRo/CSm) and energy flux for electron transport (ETo/CSm) significantly decreased (Table 2). For example, ETo/CSm decreased by about 89% for the first leaf and 85% for the second in comparison to the values noted before heat stress. Seven days after stress in the first leaf of control, the values of ABS/CSm, TRo/CSm and ETo/CSm increased in comparison to the values noted just after heat shock by about 76, 161 and 296%, respectively, remaining however still lower than for control before stress. In contrast, in the second leaf, these values continued to decrease. ETo/CSm just after heat shock decreased by about 85% as compared to the control before stress, whereas after 7 days the decrease was by about 89%.
The protective effect of BR27 on PSII efficiency was observed for a concentration of 0.25 mg dm−3 in the first leaf, but not in the second (Table 2). For the first leaf, the values of energy flux for electron transport just after heat shock decreased by about 80% as compared to the values before stress, while after 7 days it was only 33% lower than values noted before stress. A similar tendency was noticed for lower concentrations of BR27, though statistically insignificant.
PSII efficiency, metabolic activity and dark respiration in the seventh leaf of barley plants
After approximately 4 weeks from the moment of heat shock, PSII efficiency measured on the seventh leaf in 42-day-old plants did not differ in control and BR27-treated plants (Table 2). In addition to PS II efficiency, metabolic activity and dark respiration of the seventh leaf were measured. Calorimetric studies on the seventh leaf showed that metabolic activity, calculated per of 0.1 g DW, decreased in 24-epibrassinolide-treated barley (Table 3). In plants treated with a higher concentration of BR27, the values of the heat production parameters such as HPmax and HPAREA, T=20 min, which represent cell metabolic activity, significantly decreased by about 17 and 20% (Table 3). HPAREA max also showed a tendency to decrease, though statistically insignificant. A decrease in the values of metabolic activity was also observed in plants treated with 0.005 mg dm−3 BR27, but statistical significance was found only for HPAREA, T=20 min (value lower then control by about 12%). When all the aforementioned parameters were expressed per leaf area, they showed no differences between the BR27-treated plants and the control (data not shown).
In plants treated with BR27 at a concentration of 0.25 mg dm−3, dark respiration of the seventh leaf (calculated per 0.1 g DW) was 41% lower then in the control (Table 3). The dark respiration of plants treated with 0.005 mg dm−3 BR27 also decreased, but the difference was statistically insignificant (Table 3). In contrast to the parameters of metabolic activity, the same tendency was observed and statistically proved if the data were calculated per leaf area (data not shown).
There were no statistically significant differences in DW and leaf area of the seventh leaf of 42-day-old plants between the control and BR27-treated barley (data not shown). Only a slight tendency toward decrease in the leaf area and mass was observed for barley treated previously with a higher BR concentration.
Barley yield components
Infiltration of leaves with 24-epibrassinolide at a concentration of 0.005 mg dm−3 had no statistically significant impact on yield; however, some tendency to decrease was observed (Table 4). Leaf infiltration with 50 times higher concentration of BR27 negatively affected barley yield. The plants had lower fresh mass and produced less number of tillers. The number of seeds per spike, the number of seeds per plant and the final yield were lower by about 16, 43 and 39%, respectively, in comparison to the control (Table 4).
Discussion
According to data in the literature available, this is the first time that an analysis of brassinosteroid content in barley has been performed. Brassinosteroids—brassinolide and castasterone—were detected in barley leaves. 24-epibrassinolide was found only in trace amounts in control plants. Among the Poaceae family, brassinosteroids are found in Lolium perenne L., Oryza sativa L., Phalaris canariensis L., Secale cereale L., Triticum aestivum L. and Zea mays L. (Bajguz and Tretyn 2003; Janeczko and Swaczynová 2010).
In biological experiments BRs move only slowly, if at all, after their application to leaves by plant spraying (Nishikawa et al. 1994, 1995; Symons et al. 2008; Janeczko and Swaczynová 2010). Significant in this case may be the presence of the cuticle barrier and problems in the penetration of hormone solution into the leaf. In the present study, we bypassed the barrier of the leaf surface and injected BR27 directly into the apoplast of the first and second leaves of barley seedlings using the leaf infiltration method. This way of application resulted in an increase in the 24-epibrassinolide content in the newly developed leaves (the seventh leaf was analysed). Apparently then, 24-epibrassinolide was transported from leaf to leaf. Interestingly, however, its content in the seventh leaves was similar after applying low and high BR27 concentration to two-leaf seedlings. A similar phenomenon was noticed in our earlier study, where no significant difference in 24-epibrassinolide content in wheat leaves was observed after BR27 application (0.1 or 2.0 μM) to the roots (Janeczko and Swaczynová 2010). Apparently, plants regulate the uptake or transport of applied brassinosteroids. 24-Epibrassinolide might then be transported from leaf to leaf under the control of the internal mechanism of BR homeostasis. Exogenously applied BRs undergo various chemical modifications, i.e. hydroxylation and glucosylation in the side chain or conjugation with fatty acids (Sembdner et al. 1994; Asakawa et al. 1996; Srivastava 2002). These metabolites probably play a role in BR homeostasis and some of them may represent a way to store brassinosteroids (Srivastava 2002). Cell cultures of Ornithopus sativus Brot. transformed the exogenously applied 24-epibrassinolide to produce fatty acids (lauric, myristic and palmitic) conjugates with 3,24-bisepibrassinolide, esterified at the 3β-position (Kolbe et al. 1995). The non-conjugated compound, 3,24-bisepibrassinolide, was isolated from the culture medium, whereas the fatty acyl esters were present within the cells (Kolbe et al. 1995). It is possible that in our experiment the mechanisms controlling BR homeostasis allowed transport of only part of the applied 24-epibrassinolide. The rest of it could be immobilised at the place of application. It is also conceivable that BR27 was conjugated to fatty acid or glycoside forms that are impossible to detect using our protocol.
In no-stress conditions, brassinosteroids can stimulate net photosynthesis and ribulose bisphosphate activity of plants (Braun and Wild 1984; Fariduddin et al. 2003; Hayat et al. 2000). In cucumber, BR27 increases the quantum yield of PS II electron transport, which is due to a significant increase in photochemical quenching (Yu et al. 2004). In the present experiment, no influence of BR27 on the studied parameters of photosystem II was found in barley seedlings before stress. Similarly, no influence of 24-epibrassinolide on the improvement of energy utilisation in PS II in oilseed rape seedlings grown in vitro had been noticed earlier (Janeczko et al. 2005). However, under stress conditions (heavy metal application, pathogen infection), BR increased the values of energy flux for trapping and for electron transport (Janeczko et al. 2005; Skoczowski et al. 2010). In the present study, 24-epibrassinolide favoured PS II recovery in barley damaged by high temperature. It is possible that this is a non-specific mode of BR action on PSII under various stresses. Interestingly, the BR effect was dependent on which barley leaf was being treated. BR protection was observed for the older (first) leaf, while the younger leaf was insensitive to BR27 action and simultaneously less capable of post-stress regeneration. The mechanism of this phenomenon remains unexplained, but one of the hypothetical possibilities is that in younger tissue an insufficient number of receptors developed to accept steroid ligand and activate the signal transduction pathways responsible for brassinosteroid-induced counter-stress changes in cells.
The mechanisms of plant adaptation and defence against thermal stress have been well known (Berry and Bjorkman 1980; Maestri et al. 2002). However, the participation of BR in the onset of resistance (especially, for the protection of photosynthesis) to these kinds of stressors is only partially explained. Higher values of net photosynthesis rate, better survival and finally higher yield were found in heat-stressed tomato plants treated with BR (Singh and Shono 2005). Transpiration rate and stomata conductance, increased by BR27, probably reduce the temperature of leaves. Brassinosteroids at high temperature can regulate the production of heat shock proteins in tomato and oilseed rape (Dhaubhadel et al. 1999, 2002; Singh and Shono 2005). Heat shock proteins synthesised to protect cellular protein structures against destruction play a crucial role in the adaptation to stress (Sarhan and Perras 1987; Vierling 1991, Ortiz and Cardemil 2001; Korotaeva et al. 2001). Small chloroplast HSP protect electron transport pathways against heat stress (Heckathorn et al. 1998). Dhaubhadel et al. (1999, 2002) and Singh and Shono (2005) did not study the synthesis of HSP in chloroplasts separately, but it is likely that this HSP contributes to the reduction of damage and better regeneration of photosynthetic pathways. These mechanisms appear most likely to explain the protective effect of BR on photosynthetic pathways under heat stress in our experiment on barley. On the other hand, BR also has an impact on the activity of antioxidative enzymes (Mazorra and Núñez 2000; Mazorra et al. 2002). The high temperature contributes to hyper-reduction in the electron transportation chain, which leads to the production of free radicals (Dash and Mohanty 2002). For example, reactive oxygen species inactivate cellular enzymes and disorganise membrane functions (lipid peroxidation and fatty acid de-esterification). The antioxidant defence system of the plant comprises a variety of antioxidant molecules and enzymes (superoxide dismutase, ascorbate peroxidase, catalase) (Chaitanya et al. 2002). Antioxidative enzymes are an important element of protective systems in various types of stresses, including thermal stress (Miller-Morey and Van Dolah 2004; Almeselmani et al. 2006). As our preliminary experiment shows, spraying barley with BR27 increases catalase activity after heat shock (Pociecha and Janeczko 2008). Similar data concerning antioxidative enzymes were obtained for tomato subjected to heat stress (Mazorra and Núñez 2000; Mazorra et al. 2002). Reduction in chlorophyll content and membrane damage, with an increase in ion leakage, is also a result of high-temperature stress (El-Shintinawy et al. 2004). Brassinosteroids reduce cell membrane permeability and prevent chlorophyll degradation in response to low temperature stresses (Krishna 2003; Janeczko et al. 2007a, b). It is very likely that BR action may be similar also in the case of plants exposed to heat shock.
The effect of BR on PSII efficiency discussed above pertained to the first and second leaves of barley seedlings subjected to high-temperature stress. The same energy flow measurements in PSII conducted on newly expanded seventh leaf no longer showed any effect of 24-epibrassinolide. The PSII efficiency of the seventh leaf was similar in plants treated with brassinosteroid and in the control plants, whereas, for example, metabolic activity decreased in plants after treatment with higher concentration of 24-epibrassinolide.
Calorimetric measurements of heat production, which reflect the intensity of plant metabolic activity, showed decreased values of all calculated HP parameters and it was particularly noticeable in plants treated with higher BR concentration. According to other studies, metabolic activity is increased in plants as a result of pathogen infection (Płażek and Rapacz 2000; Janeczko et al. 2007b). Cold-hardened callus demonstrate higher frost resistance, which is accompanied by the highest heat emission and dry matter accumulation, as well as the best morphology characteristics (Rapacz and Żur 1996). Salinity stress lowers the metabolic activity of barley (Baltruschat et al. 2008). Calorimetric methods are useful tests for the detection of resistance to fenoxaprop and diclofop in wild oat biotypes (Stokłosa et al. 2006). Resistant biotypes treated with these herbicides shows higher metabolic activity than susceptible ones. In all the studies mentioned above, calorimetric measurements were useful as a method complementary to other physiological measurements for examining metabolic activity and the viability of plants. In our study, plants showed lower values of metabolic activity parameters, which correlated with the low intensity of dark respiration and with a lower yield of barley.
The value of all parameters, both HPmax, universally used in the literature, as well as the additionally proposed parameters (HPAREA, T=20 min and HPAREA max), which provide an interpretation of the heat effect curve, showed a downward trend in barley treated with BR. Note that the statistical significance of the difference from the control was obtained only for HPmax and HPAREA, T=20 min. Due to the correlation between HP and the crop yield as mentioned above, these parameters could be useful in the future to predict barley yield, especially in experiments where the plants are subjected to stress factors or to the actions of various regulators.
An important part of plant metabolic activity is the process of dark respiration. The relationship between plant yield and dark respiration measured on leaves may be different for various species (or even cultivars) and may depend on plant growth conditions. Wheat cultivars with higher yield potential are characterised by low intensity of dark respiration in comparison to cultivars with lower yield (Jiang et al. 2000). Two cultivars of barley, ‘Graphic’ and ‘Kym’, with different yield potential have similar leaf dark respiration intensity; however, the respiration intensity in cultivar with a higher yield potential (‘Graphic’) showed a tendency to decrease (Tambussi et al. 2005). In our experiment, hormonal treatment caused a decrease in dark respiration, which theoretically could result in higher crop yield, but surely respiration is not the only process modified by biologically active brassinosteroid that is capable of influencing plant yield.
Generally, leaf infiltration with a higher hormone concentration at the seedling phase ultimately resulted in lower crop yield. The same or even higher concentration of brassinosteroids applied via a less invasive method with a limited possibility of hormone penetration inside tissues (like spraying) may have a beneficial effect on the yield of other Poaceae plants such as wheat and rice (Ramraj et al. 1997; Janeczko et al. 2010). For example, in case of drought, homobrassinolide stimulates wheat yield, which is manifested by an increase in the number of grains per spike, spikes per m2 and weight of 1,000 grains (Sairam 1994). In case of salt-stress, 24-epibrassinolide applied via spraying in a greenhouse experiment stimulates plant biomass production and increases leaf area, but without any effect on grain yield in wheat cv. S-24 (salt resistant) and MH-97 (salt sensitive) (Shahbaz et al. 2008). Root application of this hormone results in an enhanced total grain yield and 100-grain weight of both salt-stressed cultivars referred to above (Ali et al. 2008).
To conclude, in spite of the initially positive effect of BR27 (at higher concentration) on PSII efficiency of barley, especially under high-temperature stress, its aftereffect on leaf metabolism and yield was negative. The amount of hormone that moved to newly expanded leaves after exogenous application was the same in plants treated with higher and lower BR27 concentration. Notwithstanding that, some physiological processes, as well as yield, differed much more from those of the control in plants treated with BR27 at higher concentration. Apparently, BR27 in this case modified metabolic cell pathways in a manner, which resulted in an unfavourable aftereffect on the efficiency of some physiological processes and plant yield.
References
Ali Q, Athar H, Ashraf M (2008) Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56:107–116
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Sci 171:382–388
Asakawa S, Abe H, Nishikawa N, Natsume M, Koshioka M (1996) Purification and identification of new acyl-conjugated teasterons in lily pollen. Biosci Biotechnol Biochem 60:1416–1420
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bajguz A, Tretyn A (2003) The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62:1027–1046
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel K-H, Schäfer P, Schwarczinger I, Zuccaro A, Skoczowski A (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180:501–510
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Braun P, Wild A (1984) The influence of brassinosteroid on growth and parameters of photosynthesis of wheat and mustard plants. J Plant Physiol 116:189–196
Brown KW, Thomas JC (1980) The influence of water stress preconditioning on dark respiration. Physiol Plant 49:205–209
Chaitanya KV, Sundar D, Masilamani S, Reddy AR (2002) Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul 36:175–180
Criddle R, Hansen L (1999) Calorimetric methods for analysis of plant metabolism. In: Kemp RB (ed) Handbook of thermal analysis and calorimetry. Elsevier, Amsterdam, pp 711–763
Criddle RS, Hansen LD, Breidenbach RW, Ward MR, Huffake RC (1989) Effects of NaCl on metabolic heat evolution rates by barley roots. Plant Physiol 90:53–58
Dash S, Mohanty N (2002) Response of seedlings to heat-stress in cultivars of wheat: growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity source. J Plant Physiol 159:49–59
Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29:681–691
El-Shintinawy F, Ebrahim MKH, Sewelam N, El-Shourbagy MN (2004) Activity of photosystem 2, lipid peroxidation, and the enzymatic antioxidant protective system in heat shocked barley seedlings. Photosynthetica 42:15–21
Fariduddin Q, Ahmad A, Hayat S (2003) Photosynthetic response of Vigna radiata to pre-sowing seed treatment with 28-homobrassinolide. Photosynthetica 41:307–310
Fariduddin Q, Hasan SA, Ali B, Hayat S, Ahmad A (2008) Effect of modes of application of 28-homobrassinolide on mung bean. Turk J Biol 32:17–21
Grove MD, Spencer GF, Rohwedder WK, Mandawa N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Cook JC (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Hansen LD, Macfarlane C, McKinnon N, Smith BN, Criddle RS (2004) Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochim Acta 422:55–61
Hayat S, Ahmad A, Mobin M, Hussain A, Fariduddin Q (2000) Photosynthetic rate, growth, and yield of mustard plants sprayed with 28-homobrassinolide. Photosynthetica 38:469–471
Heckathorn SA, Downs CA, Sharkey TD, Coleman JS (1998) The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol 116:439–444
Janeczko A, Swaczynová J (2010) Endogenous brassinosteroids in wheat treated with 24-epibrassinolide. Biol Plant 54:477–482
Janeczko A, Kościelniak J, Pilipowicz M, Szarek-Łukaszewska G, Skoczowski A (2005) Protection of winter rape photosystem II by 24-epibrassinolide under cadmium stress. Photosynthetica 43:293–298
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007a) Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358
Janeczko A, Tóbias I, Skoczowski A, Dubert F, Gullner G, Barna B (2007b) Bacterial infection and pretreatment with 24-epibrassinolide markedly affect the heat emission and membrane permeability of rape cotyledons. Thermochim Acta 458:89–91
Janeczko A, Biesaga-Kościelniak J, Dziurka M (2009) 24-Epibrassinolide modifies seed composition in soybean, oilseed rape and wheat. Seed Sci Technol 37:625–637
Janeczko A, Biesaga-Kościelniak J, Oklešťková J, Filek M, Dziurka M, Szarek-Łukaszewska G, Kościelniak J (2010) Role of 24-epibrassinolide in wheat production: physiological effects and uptake. J Agron Crop Sci 196:311–321
Jiang GM, Hao NB, Bai KZ, Zhang QD, Sun JZ, Guo RJ, Ge QY, Kuang TY (2000) Correlation between variables of gas exchange and yield potential in different winter wheat cultivars. Photosynthetica 38:227–232
Kolbe A, Schneider B, Porzel A, Schmidt J, Adam G (1995) Acyl-conjugated metabolites of brassinosteroids in cell suspension cultures of Ornithopus sativus. Phytochemistry 38:633–636
Korotaeva NE, Antipina AI, Grabelnykh OI, Varakina NN, Borovskii GB, Voinikov VK (2001) Mitochondrial low-molecular weight heat-shock proteins and the tolerance of cereal mitochondria to hyperthermia. Russ J Plant Physiol 48:798–803
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297
Lambers H, Ribas-Carbó M (2005) Advances in photosynthesis and respiration—plant respiration: from cell to ecosystem. Springer, Dordrecht
Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol Biol 48:667–681
Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol 39:23–52
Mazorra LM, Núñez M (2000) Brassinosteroid analogues differentially modify peroxidase activity, superoxide dismutase activity and protein content in tomato seedlings. Cultiv Trop 21:29–33
Mazorra LM, Núñez M, Hechavarria M, Coll F, Sanchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol Plant 45:593–596
Miller-Morey JS, Van Dolah FM (2004) Differential expression of stress proteins, antioxidant enzymes, and photosynthetic efficiency to physiological stresses in the Florida red tide dinoflagellate, Karenia brevis. Comp Biochem Physiol 138:493–505
Nishikawa N, Toyama S, Shida A, Futatsuya F (1994) The uptake and the transport of 14C-labeled epibrassinolide in intact seedlings of cucumber and wheat. J Plant Res 107:125–130
Nishikawa N, Shida A, Toyama S (1995) Metabolism of 14C-labeled epibrassinolide in intact seedlings of cucumber and wheat. J Plant Res 108:65–69
Ortiz C, Cardemil L (2001) Heat-shock responses in two leguminous plants: a comparative study. J Exp Bot 52:1711–1719
Płażek A, Rapacz M (2000) The intensity of respiration and heat emission from seedlings of Festuca pratensis (Hud.) and Hordeum vulgare L. during pathogenesis caused by Bipolaris sorokiniana (Sacc.) Shoem. Acta Physiol Plant 22:25–30
Pociecha E, Janeczko A (2008) Antioxidant enzymes activity and development of barley after high temperature stress—impact of 24-epibrassinolide. Zesz Probl Post Nauk Rol 524:83–93
Ramraj VM, Vyas BN, Godrej NB, Mistry KB, Swami BN, Singh N (1997) Effects of 28-homobrassinolide on yields of wheat, rice, groundnut, mustard, potato and cotton. J Agric Sci 128:405–413
Rapacz M, Żur I (1996) The use of microcalorimetric measurements in the investigations on the effect of various cold-acclimation methods on winter rape (Brassica napus var. oleifera) callus growth and frost resistance. In: Grzesiak S, Miszalski Z (eds) Ecophysiological aspects of plant responses to stress factors. ZFR PAN, Krakow, pp 483–495 (in Polish with abstract in English)
Sairam RK (1994) Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul 14:173–181
Sarhan F, Perras M (1987) Accumulation of a high molecular weight protein during cold hardening of wheat (Triticum aestivum L.). Plant Cell Physiol 28:1173–1179
Sembdner G, Atzorn R, Schneider G (1994) Plant hormone conjugation. Plant Mol Biol 26:1459–1481
Shahbaz M, Ashraf M, Athar H (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119
Skoczowski A, Janeczko A, Gullner G, Tóbias I, Kornaś A, Barna B (2010) Response of brassinosteroid treated oilseed rape cotyledons to infection with wild type and HR-mutant of Pseudomonas syringe or with P. florescence. J Therm Anal Cal (in press) doi:10.1007/s10973-010-1204-z
Smith BN, Lytle CM, Hansen LD (1995) Predicting plant growth rates from dark respiration rates: an experimental approach. In: Roundy BA, McArthur ED, Haley JS, Mann DK (eds) Proceedings: wildland shrub and arid land restoration symposium 1993 October 19–21; Las Vegas, NV. Gen. Tech. Rep. INT-GTR-315. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, pp 243–245
Smith BN, Criddle RS, Hansen LD (2000) Plant growth, respiration and environmental stress. J Plant Biol 27:89–97
Srivastava LM (2002) Brassinosteroids, chap 9. In: Srivastava LM (ed) Plant growth and development. Hormones and environment. Academic Press/Elsevier, USA, pp 212–213
Stokłosa A, Janeczko A, Skoczowski A, Kieć J (2006) Isothermal calorimetry as a tool for estimating resistance of wild oat (Avena fatua L.) to aryloxyphenoxypropionate herbicides. Thermochim Acta 441:203–206
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterise and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor & Francis, London, pp 445–483
Swaczynová J, Novák O, Hauserová E, Funksová K, Šíša M, Strnad M (2007) New techniques for estimation of naturally occurring brassinosteroids. J Plant Growth Regul 26:1–14
Symons GM, Ross JJ, Jager CE, Reid JB (2008) Brassinosteroid transport. J Exp Bot 59:17–24
Tambussi EA, Nogues S, Ferrio P, Voltas J, Araus JL (2005) Does higher yield potential improve barley performance in Mediterranean conditions? A case study. Field Crop Res 91:149–160
Vardhini BV, Rao SSR (1998) Effect of brassinosteroids on growth, metabolite content and yield of Arachis hypogaea. Phytochemistry 48:927–930
Vardhini BV, Rao SSR (2002) Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61:843–847
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42:579–620
Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410:380–383
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 5:1135–1143
Zullo MAT, Adam G (2002) Brassinosteroid phytohormones: structure, bioactivity and applications. Braz J Plant Physiol 14:83–121
Zullo M, Kohout L (2004) Semisystematic nomenclature of brassinosteroids. Plant Growth Regul 42:15–28
Acknowledgments
This study was supported by the European Union CROPSTRESS Program, Project QLK5-CT-2002-30424, by GAAS CR (IAA 400550801) and Grant MSM 6198959216. The authors would like to thank Dr Balázs Barna (Plant Protection Institute, Hungarian Academy of Sciences) for the method of leaf infiltration and Dr Ondrej Novak for help with HPLC–MS/MS measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Janeczko, A., Oklešťková, J., Pociecha, E. et al. Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiol Plant 33, 1249–1259 (2011). https://doi.org/10.1007/s11738-010-0655-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0655-y