Abstract
Seaweeds and their extracts have been used for centuries in agriculture to improve plant growth and impart stress tolerance. There has been historical evidence that phytohormones present in seaweeds lead to these effects, but questions of this mode of action have always been raised. By quantifying phytohormones in seaweed extracts coupled with the use of phytohormone biosynthetic and insensitive mutants, we conclude that the phytohormone levels present within the extracts are insufficient to cause significant effects in plants when extracts are applied at recommended rates. However, components within seaweed extracts may modulate innate pathways for the biosynthesis of phytohormones in plants. Phytohormone profiles of plant tissue extracts were analyzed following root application of a commercial seaweed extract produced from Ascophyllum nodosum (ANE) to in vitro-grown Arabidopsis plants. We found an increase in total concentration of cytokinins (CKs), in particular, of trans-zeatin-type CKs, 24 and 96 h after ANE application, with an increase in cis-zeatin-type CKs observed at 144 h. Concomitantly, increases in abscisic acid (ABA) and ABA catabolite levels were observed whereas auxin levels were reduced. Additionally, the profile of transcripts revealed that CK biosynthetic genes were upregulated, whereas the CK catabolic genes were repressed at 24 and 96 h following ANE application. Not surprisingly, the transcripts of ABA biosynthetic genes were increased whereas the auxin biosynthetic genes were repressed. These corroborated findings are the first to help explain the underlying physiological benefits derived from the application of ANE to plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascophyllum nodosum, a brown macroalga known as rockweed, is ubiquitous in cool coastal marine waters throughout the Northern Hemisphere. Unprocessed A. nodosum and alkaline extracts derived from the alga have been widely used as a biostimulant in agricultural production in a wide variety of crop species (Craigie 2011; MacKinnon and others 2010; Spann and Little 2011). Direct benefits from the application of A. nodosum and other seaweed extracts on crop performance include enhanced root vigor (Crouch and van Staden 1992), increased leaf chlorophyll content (Blunden and others 1996), an increase in the number of leaves (Rayirath and others 2008), improved fruit yield (Arthur and others 2003; Kumar and Sahoo 2011; Kumari and others 2011), heightened flavonoid content (Fan and others 2011), and enhanced vegetation propagation (Leclerc and others 2006). However, the more substantial improvements associated with application of seaweed extracts are improved tolerance toward abiotic stresses, including drought (Spann and Little 2011; Zhang and Ervin 2004), ion toxicity (Mancuso and others 2006), freezing (Rayirath and others 2009), and high temperature (Zhang and Ervin 2008).
The precise mechanisms by which seaweed extracts improve the growth and vigor of plants are not fully understood, but many of the extracts’ effects are attributed to a variety of constituents within the seaweed extracts, including betaines, plant nutrients (both micro and macro), and phytohormones (Khan and others 2009). Although the modes of action of some of the chemical components of seaweed extracts are known, the vast majority remain uncharacterized. Therefore, it is likely that many of these biochemical components within the extracts exhibit additive or synergistic activities (Fornes and others 2002). The chemical compositions of several seaweed extracts are known, and because they can maintain plant-promoting bioactivity at relatively low concentrations (<0.01 % w/v) (Crouch and van Staden 1993) or in the absence of ionic compounds (Rayirath and others 2008), it is unlikely that the growth-promoting ability is due to nutrient composition alone. Instead, the beneficial effects of seaweed extracts appear to be modulated through either plant growth regulators within the seaweed extracts or through a process of stimulation of the endogenous phytohormone biosynthesis in extract-treated plants.
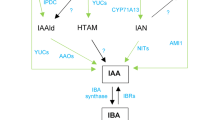
Phytohormones are low-molecular-weight natural products produced by plants. They essentially regulate all physiological and developmental processes from germination to senescence as well as plant responses to environmental stresses. These structurally diverse compounds include auxins, cytokinins (CK), abscisic acid (ABA), gibberellic acid (GA), ethylene, jasmonic acid, and salicylic acid. Several biosynthetic precursors and catabolic metabolites of these plant hormones can exhibit biological activity, giving rise to an intricate network of signalling molecules at the cellular level. To further complicate the picture, there is mounting evidence of considerable cross-talk among plant hormone-signaling pathways in regulating developmental and physiological processes (Peleg and Blumwald 2011; Su and others 2011; Werner and Schmulling 2009).
Based on phenotypic observations of plants treated with various seaweed extracts, CK and cytokinin-like activity is the most commonly reported phytohormone effect of seaweed extracts (Stirk and others 2003). CKs are among the most important plant growth regulators, controlling plant cell division, the release of axillary buds, and induction of photomorphogenic development, in addition to being the dominant signal that controls growth rates and development within the shoot apical meristem (Werner and Schmulling 2009). In Arabidopsis, trans-CKs are considered to be more active than cis-CKs, with trans-zeatin (tZ) and isopentyl adenine (2iP) types being dominant in controlling CK-dependent responses (Sakakibara 2006a, b). The initial step in the biosynthesis of trans-CKs in Arabidopsis is through the formation of iP-ribotides from adenosine subunits via the action of isopentenyl transferases (AtIPT1,3-8) (Miyawaki and others 2004, 2006). CK signalling follows a multi-component phosphorylation cascade. Initially, CKs are received by His protein kinases [Arabidopsis histidine kinases (AHK) 2, 3, and 4], which then phosphorylate His phosphotransfer proteins [Arabidopsis histidine phosphotransfer proteins (AHPs)], which are then transferred to the nucleus and phosphorylate Arabidopsis response regulators (ARRs) (Argueso and others 2010; D’Agostino and others 2000; Kieber and Schaller 2010; Perilli and others 2010; To and others 2007; Werner and Schmulling 2009). Phosphorylated ARRs act as transcription factors inducing the transcription of CK responsive genes (Perilli and others 2010). Degradation of CKs is controlled mainly through the action of cytokinin oxidase/dehydrogenase (CKX) which is encoded by a multigene family in Arabidopsis (AtCKX1-7) (Kudo and others 2010; Werner and others 2006).
Application of exogenous CKs at low levels resulted in increased leaf size, increased seed set, and delayed senescence while resulting in overall reduction in root mass (Nooden and others 1979; Singh and others 1992). Overexpression of various IPT genes under the control of induced or developmentally regulated genes resulted in plants that exhibited an increase in compound leaf numbers, leaf area, chlorophyll levels and seed set, while the plants exhibited decreased root mass and a delay in leaf senescence (Gan and Amasino 1995; Ghanem and others 2011; Rivero and others 2007, 2010; Shani and others 2010). In addition to improved above-ground plant growth, many of these enhanced CK-producing plants also had improved tolerance against salinity and drought stress (Havlova and others 2008; Merewitz and others 2011; Rivero and others 2007). In contrast, plants with mutations in IPT genes or that overexpressed CKX had reduced CK content leading to plants with reduced leaf area, decreased shoot formation, and an overall increase in root mass (Matsumoto-Kitano and others 2008; Shani and others 2010; Werner and others 2001, 2003, 2010).
In addition to CK-like effects observed in plants treated with seaweed extracts, seaweeds themselves, their extracts, and seaweed-treated plants have been reported to contain high levels of auxins and auxin-like compounds (Crouch and van Staden 1993; Kumari and others 2011; Yokoya and others 2010). Auxins control the rate of cellular expansion through the action of auxin response factors (ARF) that perceive the auxin translocated through the action of Pin-formed (PIN) proteins and activate auxin responsive genes (Bishopp and others 2011; Moubayidin and others 2009). Auxin biosynthesis is complex, with five biosynthetic pathways described in Arabidopsis [4 tryptophan (trp)-dependent and one trp-independent (Zhao 2010)]. A commercial extract of A. nodosum had an estimated concentration as high as 50 mg g−1 dry weight (DW) of indole acetic acid (IAA) (Kingman and Moore 1982). Auxin-like responses have been observed through proliferation of adventitious roots in cut mung bean shoots immersed in a variety of aqueous seaweed extracts (Kumari and others 2011). In addition, the organic components of A. nodosum extracts were shown to activate the GUS expression driven by the synthetic auxin responsive promoter DR5 in transgenic Arabidopsis plants (Rayirath and others 2008).
In the present study, we attempted to identify the mechanisms by which application of the alkaline ANE alters phytohormone levels in plants by quantifying changes in phytohormone concentration and transcript abundance of phytohormone metabolic genes.
Materials and Methods
Plant Growth
Arabidopsis thaliana (L. Heyhn.) plants, ecotype Columbia (Col-0), were grown in in vitro culture. Seeds were first gas sterilized with NaClO (5 % v/v) and concentrated HCl (0.3 % v/v) for 3 h, suspended in 0.15 % agar (Bioshop Canada, Inc., Burlington, ON, Canada), and stored at 4 °C in the dark. After 2–3 days of stratification, seeds were sown on sterilized filter paper within a 100-ml glass jar containing 10 ml of ½ MS medium (Murashige and Skoog salt, Sigma, St. Louis, MO, USA) supplemented with 1 % (w/v) sucrose pH 5.7, which was sealed with a Magenta B cap (Sigma). The seeds were then grown in a growth chamber at 22 °C with a 16-h light/8-h dark cycle with a light intensity of 100 μmol m−2 s−1.
Chemical Treatment
Soluble seaweed extract powder (ANE), commercially produced from A. nodosum (Acadian Seaplants Limited, Dartmouth, NS, Canada), was used in the experiment. The control treatment was a modified Long Ashton Nutrient Solution (LANS) that contained inorganic ions that were present in the ANE [1.7 mM KOH, 1.65 mM KCl, 400 μM KNO3, 400 μM Ca(NO3)2, 150 μM MgSO4, 133 μM NaH2PO4, 10 μM NaCl, 5 μM C6H5FeO7, 3 μM H3BO3, 1 μM MnSO4, 500 nM CuSO4, 200 nM ZnSO4, 50 nM Na2MoO4, and 20 nM CoSO4]. Both test solutions were adjusted to pH 5.7 and filter sterilized before being added to 14-day-old plants at a final rate of 0.01 % w/v.
Phytohormone Analysis
Rosette leaves from 25 Arabidopsis plants were harvested [~ 1 g fresh weight (FW)] at 24, 96, and 144 h following ANE treatment. There were three biological replicates for each treatment and time point. The whole experiment was repeated twice over a period of 4 months. Samples were flash frozen and lyophilized, precisely weighed, and processed according to Chiwocha and others (2003). Seaweed extracts of powder and liquid origins were lyophilized, with 1 g used for analysis. Quantification of ABA, cytokinins, auxins, and GAs in control and ANE-treated plant tissue was conducted using ultraperformance liquid chromatography–electrospray tandem mass spectrometry (UPLC-ESI–MS/MS: http://www.nrc-cnrc.gc.ca/eng/facilities/pbi/plant-hormone.html). Particulate matter and other unwanted components were removed by solid-phase extraction (SPE) with C18 SepPak cartridges (Waters, Mississauga, ON, Canada). The procedure used for quantification of endogenous auxins, abscisic acid, and metabolites GA and cytokinins was performed as described in Chiwocha and others (2003). Internal standards used for analysis were synthesized or obtained as described previously (Abrams and others 2003; Chiwocha and others 2003, 2005; Zaharia and others 2005). Calibration curves were created for all compounds of interest. Quality control samples (QCs) were run along with the tissue samples. Detailed methods for phytohormone analysis are described in Supplementary Methods.
Root Growth Assay
In addition to wild-type seeds, the following Arabidopsis thaliana (Columbia ecotype Col-0) plants were obtained from the Arabidopsis Stock Center in Columbus, OH, USA: abi4-1 mutants, DR5:GUS, and the quadruple mutant ipt1,3,5,7 donated by Dr. Tatsuo Kakimoto (University of Osaka) (Matsumoto-Kitano and others 2008) and the ARR5::GUS in the Wassilewskija (WS-0) background. Gas-sterilized Arabidopsis seeds (wild-type Col-0; abi4-1, DR5:GUS, ARR5::GUS, and ipt1,3,5,7) were spread onto ½ MS media and stratified at 4 °C for 3 days before being oriented vertically in the growth chamber. Evenly germinated seedlings were transferred after 4 days to square ½ MS plates supplemented with the different concentrations of ANE. Root length was measured on 7-, 9-, and 11-day-old seedlings, and relative root growth rates were evaluated by comparison with the control using ImageJ software (Research Services Branch, NIH, Bethesda, MD, USA).
Lateral Root Quantification
The number of lateral roots were counted on transferred seedlings starting 7 days post germination and then grown on ½ MS medium containing ANE or control. The number of lateral roots on the primary root was determined with a dissecting microscope; all lateral roots that had emerged from the primary root were counted.
For root growth and GUS assays, gas-sterilized Arabidopsis seeds were plated onto ½ MS media solidified with 0.25 % (w/v) Phytagel (Sigma), stratified for 3 days at 4 °C, and then transferred vertically to growth chamber conditions. Four days after germination the seedlings were transferred to square plates containing solidified ½ MS media supplemented with different treatments.
GUS Staining
Plant tissues were fixed in 90 % (v/v) acetone for GUS staining, which was performed as described previously (Weigel and Glazebrook 2002). Pictures were taken with a Canon digital camera (EOS Rebel) using a stereoscope microscope. Each treatment was performed using a minimum of three biological replicates.
qRT-PCR Analysis
Total RNA was isolated using the monophasic RNA extraction method (Chomczynski and Sacchi 1987). Two micrograms of RNA was treated with 2 units of RQ1 DNAse (Promega, Madison, WI, USA) according to the manufacturer’s instructions. cDNA was synthesized using an Applied Biosystems high-capacity cDNA synthesis kit (Applied Biosystems, Life Technologies, Carlsbad, CA, USA), using the manufacturer’s protocols. Relative transcript levels were assayed by real-time PCR analysis using gene-specific primers on a StepOne™ Plus Real-Time PCR System (Applied Biosystems), which used the Power SYBR® Green RT-PCR Reagents kit (Applied Biosystems). Primer sequences were taken from the Roche Universal Probe Library (Roche Diagnostics, Indianapolis, IN, USA). Wherever possible, the primers would flank an intron-spanning region. All amplicon-length ranges were between 68 and 120 bp. The reaction mixture contained 5 μL of Power SYBR® Green PCR Master Mix, 20 ng of cDNA, and 250 nM of each of the forward and reverse primers. The following default program was used: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min each. RNA relative quantification analyses were performed using Actin2 serving as an endogenous control, with efficiencies calculated by dilution series for individual primers (Pfaffl 2001). The list of primers used is shown in Supplementary Table 2. Each data point was determined in triplicate for each of the three biological replicates and presented as mean ± SE.
Statistical Analysis
For all analyses, ANE-treated plants were compared to control-treated plants, with the differences analyzed using analysis-of-variance Tukey’s honestly significant difference test (*p < 0.05, **p < 0.01, ***p < 0.001) with SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Phytohormone Analyses of A. nodosum Extracts
To determine if previously reported increases in “phytohormone-like”responses were due to phytohormones present within seaweed extracts themselves, we quantified the phytohormones in various seaweed extracts. Total phytohormone levels were quantified in A. nodosum alkaline extracts from Canadian Atlantic sources used in this study, in addition to various other commercially available extracts (Table 1). The phytohormone levels in all of the extracts were relatively low, with isopentenyl adenine (2iP) being the only CK detected in all of the samples at levels close to 10 ng g−1 DW of sample. Similarly, the concentrations of ABA and its catabolites and all of the GAs were less than 5 ng g−1 DW. The Norwegian ANE had levels of IAA in excess of 500 ng g−1 DW; however, the remaining samples had lower levels in the 20–25-ng g−1 DW range, which would be unlikely to induce any auxin-responsive phenotypes at field application because minimum concentrations required for phenotypic changes are reported to be approximately 100 nM.
Effect of Seaweed Extracts on Rooting
Addition of aqueous ANE (0.01 % w/v) to ½ Murashige and Skoog (MS) media decreased the length of the primary roots at 3 and 5 days following treatment, a reduction of over 20 % compared to unsupplemented ½ MS medium (Fig. 1, p < 0.01). There was also a reduction in the total number of secondary roots, with ANE-treated plants having approximately 35 % fewer secondary roots per root primordia. Additionally, the ratio of secondary roots was reduced from 2.7 to 2.2 branches cm−1 (p < 0.01, data not shown). The overall morphology of the roots was also altered, with the secondary roots supplemented with A. nodosum extracts exhibiting reduced horizontal growth and growing in a more vertical fashion (Fig. 1a).
Root growth and development was affected by ANE in agar plate system. a Twelve-day-old seedlings of wild-type Arabidopsis, 8 days after transfer to control (left) or ANE (0.01 % w/v) in ½ MS solid media. b Average length of primary roots in seedlings 3 and 5 days after transfer to indicated media. The data represent the mean ± SE (n = 3). c Number of lateral roots at all developmental stages (including lateral root primordial) in seedlings 3 and 5 days following transfer to media containing treatment
Both CKs and ABA are strong repressors of root growth in plants (Wasilewska and others 2008; Werner and Schmulling 2009). To determine if the observed reduction in root growth was due to the presence of either ABA or CKs within the ANE itself, we repeated the experiment with the ABA/CK-insensitive mutant abi4-1 (Shkolnik-Inbar and Bar-Zvi 2010) and the CK biosynthesis quadruple mutant ipt1,3,5,7 (Matsumoto-Kitano and others 2008). The abi4-1 mutants exhibited decreased length of primary roots and increased lateral rooting ratios compared to Col-0 plants in both control and ANE treatment. However, the increased lateral root ratio was greater following ANE treatment with the abi4-1 plants, which displayed a 50 % increase in lateral root number compared to only a 35 % increase with the control media (Supplementary Fig. 1). Additionally, there was no significant difference in primary root length in abi4-1 plants grown on either control or ANE-supplemented media compared to the nearly 20 % reduction in primary root length in control Col-0 plants. When ipt1,3,5,7 plants were grown on control medium, they produced thinner primary roots and a greater number of both secondary and adventitious roots, with the primary root similar in length to that of Col-0 plants (Supplementary Figs. 1 and 2). Similar to Col-0 plants, the mean length of primary roots for ipt1,3,5,7 plants was also reduced. However, the length was reduced to a lesser extent than for Col-0 (Supplementary Figs. 1 and 2). The phenotype observed is possibly due to increased biosynthesis of endogenous phytohormones (that is, CKs and ABA) within the plant rather than an effect of the extract itself.
Effect on DR5 and ARR5 Reporters
Since we observed a decrease in root growth in the agar plate system following ANE treatment, we proceeded to determine if auxin and CK responses were altered using the synthetic auxin-responsive promoter DR5 driving GUS genes in Arabidopsis Col-0, and the CK-sensitive promoter ARR5 driving GUS expression in the WS-0 background of Arabidopsis. Forty-eight hours after transfer to media containing 0.01 % w/v ANE, GUS activity was greatly reduced throughout the DR5::GUS seedlings (Fig. 2). The activity in the controls was found throughout the vasculature of the cotyledons, with increasing intensity found at the root tips. The expression pattern was similar in plants transferred to media containing ANE, but with an overall lower intensity (Fig. 2). Five days after transfer the differences persisted. The GUS activity dissipated from the cotyledons of the control plants and was present within the vascular tissues of the fully formed leaves and relatively more intense within the newly emerging leaves (not shown). In contrast, leaves of the ANE-treated plants exhibited much lower levels of GUS activity, with nearly undetectable levels within the vasculature of fully formed leaves and relatively weak levels in the expanding leaves, with the bulk of the activity found in the tips of both the leaves and the cotyledons. The differences in the root tissues were more pronounced. After 48 h the control exhibited GUS activity throughout the primary root, with increased activity at the points of initiation of the secondary roots and within the elongating secondary roots. Heightened activity was found near the root meristems compared to ANE-treated plants, which exhibited much weaker GUS activity in the primary root with fewer and more weakly staining secondary root structures. The differences persisted in the root tissues 5 days following ANE treatment, with reduced GUS activity most prominent in the expanding root section of both the primary and the lateral roots (data not shown).
Additionally, transgenic WS-0 Arabidopsis harboring the CK-sensitive promoter ARR5, which controls GUS expression, was visualized on ANE-treated or control plants. Forty-eight hours after transferring the seedlings on media containing ANE, the histochemical GUS activity was present in nearly all of the tissues of both ANE and control-treated seedlings. There was activity throughout the cotyledons, hypocotyls, and roots, with particularly strong activity in the vascular and meristematic tissues. ANE-treated plants exhibited relatively more intense GUS staining 48 h after treatment relative to the controls, particularly in the cotyledon mesophyll cells, the hypocotyls, and the roots (Fig. 3). Five days after treatment the control plants displayed ARR5-driven GUS activity, primarily in the vascular tissue, with the activity moving away from the aging tissues toward the newly expanding leaves and at the root meristems (both primary and secondary). Relative to the controls, the ANE-treated seedlings had more intense GUS activity within the newly expanding leaves (notably in the mesophyll cells in addition to the vascular tissues), and intense staining near the shoot apical meristems and the newly formed immature leaves. When roots were examined 5 and 7 days after transfer to the ANE treatment, the heightened ARR5 expression was maintained, with the primary root staining intensely and increased activity maintained around both primary and secondary root tips relative to the controls (Fig. 3b–d).
Effect of ANE on Phytohormone Levels
Since we determined that the levels of phytohormones present in the various commercial seaweed extracts are insufficient to alter plant phenotypes or induce promoter expression in the GUS transgenic Arabidopsis plants, we hypothesized that the extracts were altering endogenous, biosynthetic pathways of plants. To this end, we quantified the levels of a complete spectrum of phytohormones in Arabidopsis plants grown under highly controlled conditions to minimize biological variations.
Endogenous levels of auxins, including free IAA, indole-3-butyric acid (IBA), and IAA conjugated forms [that is, IAA-aspartic acid (IAA-asp), IAA-glutamic acid (IAA-glu), IAA-leucine, and IAA-alanine], were quantified by UPLC-ESI–MS/MS. Of the auxins detected, only IAA, IAA-asp, and IAA-glu were present in all of the samples, of which IAA was the most dominant auxin representing nearly 90 % of the total detected. There was a high level of variation in the absolute IAA levels between experimental repeats and therefore the data could not be pooled (Supplementary Fig. 3). Despite high levels of variation in auxin levels, similar trends were observed and differences were apparent when treated as ratios of the controls 24 h after treatments (Fig. 4). The overall level of IAA increased in the control plants by approximately 20 % between 24 and 96 h before falling rather sharply by nearly 50 % between 96 and 144 h. The ratios of IAA were reduced in the rosette leaves of ANE-treated Arabidopsis by 33 % and 42 % at 24 and 96 h following treatment, respectively (p < 0.05), with the ratio falling by 144 h after treatment to a level that was similar to the control-treated plants.
Levels of various CKs, including bioactive forms as well as conjugated ones, were analyzed in the rosette leaves of control and ANE-treated Arabidopsis plants. Results show that the bioactive free-base cytokinins, that is, Z (both cis- and trans-CKs), dhZ, and 2iP, were not detected; however, their precursors ZR and iPA were present in good amounts (except for dhZR which was not detected). Moreover, ZOG (zeatin-O-glucoside), which is another catabolism product of Z, was also found in considerable amounts. Of the CKs detected, the trans-CKs were the most abundant at 24 h after ANE treatment, whereas t-ZR and iPA were the most abundant, representing nearly 65 % of the total CKs detected. Ninety-six and 144 h after treatment, the cis-CKs corresponded to a much greater ratio of the total CK pool, with c-ZOG becoming the dominant isomer. Treatment with ANE increased the overall levels of CK in Arabidopsis rosette leaves nearly 50 %; moreover, whereas the levels of cis-CK isoforms were unaffected, increases of 100, 54, and 76 % were found for t-ZR, iPA, and total-trans isomers, respectively (p < 0.05). Overall, the levels of CK decreased at 96 h in both control and ANE-treated plants, whereas ANE treatments maintained a 40 % increase in total CK relative to 96-h control treatment, corresponding to increases in trans-CKs (Fig. 5, p < 0.05). Conversely, at 144 h following treatment, there was no significant difference in the total CK levels; however, the levels of trans-CKs were slightly higher in the control plants (p < 0.1), whereas the cis-CKs, in particular, c-ZOG, were found to be increased by 40 % in ANE-treated plants (p < 0.05). The ANE treatments resulted in an early increase in the overall levels that returned to control levels by 144 h after the treatments.
Effect of ANE treatment on cytokinin concentration in rosette leaves of Arabidopsis. Quantification of individual CK metabolites (top) and total CK metabolites in addition to total cis and trans metabolites (bottom) from 24, 96, and 144 h following ANE (0.01 % w/v) treatment or control (bar represents ± SE)
Because seaweed extracts have been implicated in ABA-mediated responses, we quantified the concentration of ABA and its catabolites in Arabidopsis leaves following ANE treatment (Fig. 6). The ABA levels increased with time in both control and ANE-treated plants, increasing by 100 % from 24 to 144 h post treatment, possibly due to age and size of the plants. There was also an increase in the ABA levels in plants that had ANE treatment, showing 40, 23, and 25 % increases at 24, 96, and 144 h, respectively (p < 0.1).
Metabolites of ABA were examined to gain insight into ABA homeostasis following ANE treatment. Catabolic products of ABA metabolism through 7′-, 8′-, and 9′-hydroxylation as well as conjugation were examined. Phaseic acid (PA), dihydrophaseic acid (DPA), and ABA glucose-ester (ABA-GE) were the only catabolites detected in the samples analyzed. Similar to ABA, the levels of these catabolites were found to increase with time by more than 100 % from 24 to 144 h. There were relatively large increases of 75 and 86 % at 144 h for PA and DPA in ANE-treated plants, respectively (p < 0.05). These increases indicated that the ANE-treated plants were converting the enhanced ABA produced to less active forms to alleviate growth-reducing effects of ABA. The application of ANE increased the ABA-GE levels relative to controls only at 144 h following treatment.
Bioactive gibberellins control a range of functions in plants that include several phenotypes observed in plants treated with seaweeds extracts. A complete suite of bioactive GAs, GA precursor, and GA degradation products was examined in Arabidopsis rosette leaves (Supplementary Table 1). Data suggest that representatives of both the nonhydroxylation GA biosynthetic pathway (GA12 → GA15 → GA24 → GA9 → GA4 → GA34) as well as the early 13-hydroxylation pathway (GA53 → GA44 → GA19 → GA20 → GA1 → GA8) are present in small amounts. The presence of larger amounts of GA24 and its further catabolism product GA34 suggests that bioactive forms GA9 and GA4 must have been produced. Smaller amounts of GA53 and GA19 are also present. Results of the analysis show that ANE treatment slightly increased the overall levels of GAs, although not significantly.
Effect of ANE on Gene Expression
Since we observed that ANE treatment altered the endogenous concentrations of auxins, CKs, and ABA in Arabidopsis, we proceeded to investigate the effect of ANE on the expression of key genes involved in the metabolism of auxins, CKs, and ABA. In addition, we measured the expression of ABA- and CK-responsive genes to validate findings of phytohormone quantification.
For CK metabolism, we examined the expression of Arabidopsis IPT genes involved in the synthesis of bioactive CKs (that is, tZ and iP-type CKs) primarily from the methylerythritol phosphate (MEP) pathway, through ATP/ADP IPTs (IPT3, 4, and 5) (Miyawaki and others 2004, 2006), CK hydroxylase CYP735As (Takei and others 2004), in addition to the activation genes CK nucleoside 5′- monophosphate phosphorohydrolase [LONELY GUY (LOG) (Kuroha and others 2009)]. The expression levels of IPT3, 4, and 5 were quite low, indicating very low transcript levels in the leaf and root tissues examined. However, there were increases in transcript levels of between 1.6- and 3-fold of the control levels following ANE treatment at 24 h which dropped to between 1.4- and 2-fold by 96 h (Fig. 7). Expression of CK hydroxylases was subsequently examined; due to the overall low transcript abundance of ATP/ADP IPTs, there was extensive variation within our CYP735A1 measurements, which were not included. However, the expression of CYP735A2 followed a similar pattern to that of IPT3, 4, and 5 in ANE-treated plants (Fig. 7). Because there was an increase in some cis-CKs in ANE treatment at 96 and 144 h, we examined whether their formation was due to isomerization of the existing trans-CKs or whether there was delayed activation of the mevalonate (MVA) pathway using tRNA IPTs. Transcript profiles of both IPT2 and IPT9 were examined, with no significant alteration in expression levels for either gene following ANE application, indicating that the shift towards cis-CKs at later time points was not due to MVA pathway activation (Fig. 7).
Expression of the key CK IPT biosynthetic genes acting as part of the methylerythritol phosphate (MEP) pathway (IPT3, 4, and 5) and CK hydroxylase CYP735A2 involved in production of trans-CKs in Arabidopsis with the tRNA IPTs (IPT2, 9) involved in the formation of cis-CKs following ANE application. Data represent the mean ± SE
Final conversion of riboside CK precursors to their highly active free-base CKs is through either a direct conversion through LOG genes or a multiple-enzyme process that is not completely understood. Quantification of the transcripts of the most abundant LOG genes (LOG1, LOG7, and LOG8) in Arabidopsis leaves from control or ANE-treated plants revealed no significant increase in the transcript levels (Supplementary Fig. 4).
Potential increases in total CKs are possible through reduced expression of CKX genes. Key genes involved in CK catabolism, CKX2 and CKX4, were examined to determine if CK breakdown was affected due to ANE treatment. Expression of CKX2 was relatively low and no significant difference was observed between ANE-treated and control plants. The CKX4 transcript level was more abundant compared to that of CKX2, with the expression of both genes increasing with the age of the plant. However, ANE treatment of seedlings reduced the expression of CKX4 dramatically from 75 % of the control level 24 h following application to less than 25 % of the control levels at 96 and 144 h following application.
Increased ARR5 expression was observed in response to ANE treatment (Fig. 3). In this experiment we used Wassilewskija (WS-0) backgrounds of Arabidopsis, which are impaired in root-based perception of beneficial growth-promoting microorganisms (Ton and others 2001) and may respond differently to the bioactive compounds found in ANE. To determine if similar CK responses were occurring within the Col-0 background, we quantified the ARR5 transcript following ANE application in Arabidopsis Col-0. We observed a greater than twofold increase in ARR5 transcripts at 24 and 96 h post application before returning to levels similar to that of control at 144 h. In addition, one of the main implications of exogenous CK application or increased endogenous CK levels is the delay of the onset of senescence. There was a strong inhibition in the transcription of the Senescence Associate Gene 13 (SAG13) at 96 and 144 h following ANE application relative to the control (Fig. 8), indicating a delay in overall leaf senescence due to ANE treatment.
We next examined the effect of ANE on key auxin biosynthetic genes. Transcript levels of the genes P450 cytochrome oxidases CYP79B2 and B3, which are key rate-controlling enzymes for auxin biosynthesis, were examined. In a pattern consistent with the total auxin levels, the expression of CYP79B2 was repressed by 0.7-fold that of the control at 24 and 96 h before returning back to levels similar to that of control at 144 h (Fig. 9a). However, the expression of CYP79B3 was nearly identical but there was greater variation between biological replicates with no statistically significant differences beyond 24 h (data not shown). As the maximum suppression of the auxin level was observed 24 h following ANE treatment, the expression of a number of auxin biosynthetic genes was examined, including indole-3-glycerol phosphate synthase (IGPS1), phosphoribosylanthranilate transferase 1 (PAT1), tryptophan synthase alpha chain (TSA1), and nitrilase 1,2 (NIT1,2). All of these biosynthetic genes were found to be reduced to below 0.4-fold of control levels except PAT1, which was only weakly suppressed by ANE treatment (Fig. 9b).
As a result of a slight increase in the accumulation of the active GA precursor GA24, we examined the expression of the key GA biosynthetic genes GA3ox1 and GA2ox2. The expression of GA3ox1 and GA2ox2 was decreased by 40 and 75 %, respectively, in Arabidopsis 24 h after application of ANE. The expression of GA2ox2 returned to control levels at 96 and 144 h, whereas GA2ox1 transcript abundance was higher than that of the control 96 and 144 h after ANE treatment (Supplementary Fig. 5).
We investigated the expression of key ABA biosynthetic genes to study the mode of action of ANE. These genes included ABA2 (xanthoxin dehydrogenase) and 9-cis-epoxycarotenoid dioxygenase 3 (NCED3), which regulate the key steps of ABA biosynthesis. Quantification of the transcript levels of ABA biosynthetic genes followed a pattern similar to that of ABA concentration in ANE-treated plants. ANE application resulted in an increased expression of ABA biosynthetic genes. Furthermore, the increase in ABA concentration in ANE-treated plants was confirmed by quantifying the transcript abundance of the ABA-responsive gene RD29a. There was a significant increase in the transcription of RD29a (up to 4-fold) (Fig. 10).
We examined the transcript levels of CYP707A3, which encodes a key ABA 8′-hydroxylase, to determine if the heightened level of observed ABA catabolic end-products PA and DPA following ANE treatments was due to altered expression of ABA catabolic genes. ANE application initially repressed CYP707A3 (Fig. 10), consistent with an early increase in ABA, with relatively little change in the levels of PA and DPA (Fig. 6). From 96 to 144 h following ANE treatment, CYP707A3 transcript levels increased, coinciding with an increase in the ABA catabolite levels at these times (Fig. 6). The strong increase in PA and DPA potentially allows the plants to deal with the growth inhibitory effects of excess ABA under optimal or near-optimal growth conditions.
Discussion
Extracts of Ascophyllum nodosum have been used extensively as plant biostimulants in a variety of forms to improve crop performance and yield. Apart from containing naturally occurring plant growth stimulatory compounds such as vitamins, oligosaccharides, and micronutrients, seaweed extracts also have been reported to contain phytohormones (Craigie 2011), with numerous reports indicating that seaweed species (both brown and red seaweeds) contain the same hormones as terrestrial plants (Boyer and Dougherty 1988; Stirk and others 2003, 2004; Yokoya and others 2010). Rayirath and others (2008) and Khan and others (2011) supported this argument, showing that extracts of A. nodosum, both whole and organic fractions, induce auxin and cytokinin-like effects using transgenic Arabidopsis plants harboring GUS reporters. Here we demonstrated that alteration in plant phenotype following seaweed extract application results from a modulation of biosynthesis, quantity, and ratios of the endogenously produced cytokinins, auxins, and abscisic acid metabolites, rather than from the exogenous phytohormones present within the extracts themselves. There was an overall increase in the concentration of both CK and ABA and their metabolites in Arabidopsis leaf tissue and a complimentary decrease in auxin levels following treatment with ANE. This overall increase in endogenous CKs, coupled with a reduction in IAA levels, likely explains previous reports of increased vegetative plant growth and resistance toward drought and salinity stress (Craigie 2011; Khan and others 2009).
Auxins are key hormones involved in promoting elongation of primary roots, initiation of lateral root primordia, and development of formed lateral roots (Aloni and others 2006; Nibau and others 2008; Osmont and others 2007). In addition, ABA antagonizes auxin activity, which can be observed through inhibition of root elongation and lateral root formation, whereas CKs can induce auxin activity in developing tissues or inhibit auxin in established tissues (De Smet and others 2003, 2006; Fukaki and Tasaka 2009; Wasilewska and others 2008). CKs have been shown to inhibit the formation and establishment of an auxin gradient that is required for root elongation and initiation through the reduction in expression of PIN proteins (Ruzicka and others 2009). The inhibitory effects of ABAs on root formation are not fully understood; however, recent evidence suggests that elevated ABA levels inhibit polar auxin transport to the roots under optimal growing conditions, resulting in decreased root formation (Shkolnik-Inbar and Bar-Zvi 2010). Application of ANE resulted in an overall reduction in primary root growth and reduced lateral root formation of Arabidopsis plants grown in vitro on a nutrient enriched agar plate system (Fig. 1). This lateral root reduction, typical when CK or ABA are increased, would be further decreased by suppression of auxin biosynthesis and may help to explain the rooting phenotypes observed (Fukaki and Tasaka 2009; Werner and others 2001). Experiments conducted with the ABA- and CK-insensitive mutant abi4-1 allowed us to conclude that the inhibition of root branching was due in part to the reduced perception of CK because there was no reduction in primary root length of abi4-1 plants grown on ANE-containing medium and the reduction in number of lateral roots was similar to the reduction observed with abi4-1 plants grown on the surface of media containing low concentrations of CKs (Shkolnik-Inbar and Bar-Zvi 2010). To determine if the phytohormone effects were due to levels within the ANE itself, we grew the quadruple mutant ipt1,3,5,7, which is deficient in CK biosynthesis but can respond to exogenously applied CKs (Matsumoto-Kitano and others 2008), on media containing ANE and monitored the shoot and root growth. There was less reduction in primary root growth in ipt1,3,5,7 plants grown on media supplemented with ANE than in the wild-type Col-0 plants, which is consistent with our hypothesis that the observed CK-enhanced phenotypes are not due to hormones coming from the ANE itself.
Previous reports indicated an increase in activation of the synthetic auxin-responsive promoter DR5 and an increase in root formation in Arabidopsis following application of organic subfractions of alkaline ANE (Rayirath and others 2008), whereas the use of the whole aqueous ANE resulted in activation of the CK-responsive promoter ARR5 (Khan and others 2011). Strong antagonistic relationships exist between CK and auxin biosynthesis (Bishopp and others 2011; Moubayidin and others 2009; Ruzicka and others 2009; Werner and others 2010), indicating that it would be unlikely that supplementing plants with a single beneficial compound would lead to accumulation of both CKs and auxins. As the seaweed extracts contain a variety of different organic components that could potentially stimulate plant growth through a number of distinct pathways, the individual effects found within ANE could be enhanced via removal or augmentation of the different organic components through organic purification and separation to provide distinct bioactive compounds, whereas the whole ANE exhibits a dominant CK response. Consistent with the decreased levels of IAA within the treated tissues, there was also a strong decrease in auxin biosynthetic genes, with many of the key genes being downregulated by as much as 50 % 24 h after application of ANE.
Biologically significant levels of CKs and cytokinin-like components have been reported to be the most dominant plant growth regulators within seaweed extracts produced from different sources (Stirk and van Staden 1996; Stirk and others 2003, 2004; Yokoya and others 2010). However, when the CK levels were directly quantified by UPLC-ESI–MS/MS, we found the levels of CKs to be biologically insignificant at levels recommended for application (Table 1). These apparent contradictions may be due to the method by which CK levels were detected, results often relying on callus growth studies using known quantities of CKs to generate a standard curve (Stirk and van Staden 1996). Callus growth assessment does not actually measure the levels of CKs present in the samples; rather it measures the inducible “cytokinin-like” activities that could be induced by responses within the plant tissues themselves. Consistent with the findings presented here, the levels of CKs increased strongly in Arabidopsis rosettes within 24 h of ANE application. Highly active, free-base CKs exist in relatively low abundance in all tissues examined, presumably due to their high level of activity and detrimental effects at high concentrations. The dominant forms detected were those of the riboside forms tZR and iPA (precursors of the biologically active forms Z and 2iP) at 24 and 96 h following ANE treatment, whereas the catabolite of cis-Z, the cis O-glucoside c-ZOG, appeared at much higher concentrations after 144 h. This increase was likely due to heightened induction of the MVA CK biosynthetic genes IPT3, 4, and 5 in concert with the down regulation of the CKX genes, in particular CKX4. Alteration in the total expression levels of IPTs or CKXs has previously been demonstrated to alter the total accumulation of CKs in a variety of plants (Bartrina and others 2011; Ghanem and others 2011; Guo and others 2010; Merewitz and others 2011; Rivero and others 2010), and therefore likely to explain the metabolic alteration in CK profiles observed in ANE-treated Arabidopsis.
We observed an increase in the levels of ABA and ABA catabolic metabolites in rosettes following application of ANE (Fig. 6). The differences in levels between the treatments increased with time, with larger differences observed in the tissues collected at 96 and 144 h post treatment. Increased ABA metabolites coincide with a concurrent increase in ABA biosynthetic genes ABA2 and NCED3 (Fig. 10). Since ABA is known as the key phytohormone involved in abiotic stress tolerance (Nambara and Marion-Poll 2005), the increased levels found in ANE-treated plants could potentially explain many of the reports on plants with improved tolerance to freezing, salinity, heat, and drought following treatment with A. nodosum extracts (Mancuso and others 2006; Rayirath and others 2009; Spann and Little 2011; Zhang and Ervin 2004, 2008). In contrast to ABA’s benefits to plants under stress conditions, ABA is a potent inhibitor of plant growth through regulating stomatal closure and inhibiting root growth (Nambara and Marion-Poll 2005). Although the increase in ABA was higher following ANE treatment, we observed only moderate inhibition of root growth. The lack of growth inhibition may be explained by accumulation of relatively high levels of the catabolic products of ABA degradation following ANE treatment (Fig. 6), mainly PA and DPA, which exhibit relatively low levels of biological activity (Sharkey and Raschke 1980). The accumulation of both PA and DPA is coupled with an increase in the transcript levels of the key ABA 8′-hydroxylase CYP707A3 that irreversibly deactivates ABA to PA (Okamoto and others 2006, 2011). CYP707As are strongly induced in tissues with excess free ABA present (Kushiro and others 2004) and potentially allow the plants to deal with the growth inhibitory effects of increased ABA under optimal or near-optimal growth conditions, including conditions following treatment with ANE.
We can conclude that root application of ANE resulted in alteration in the endogenous phytohormone biosynthesis and their relative ratios within the plant, which resulted in the beneficial phenotypes that have been reported previously in ANE-treated plants. These findings do not directly contradict previous reports of phytohormones present within the extracts themselves, with many of the previous bioassays for phytohormones examining growth parameters that would also be sensitive to upregulation of the endogenous pathways (Stirk and van Staden 1996; Stirk and others 2003). Further research is required to determine the different bioactive compounds within the ANE that lead to activation of the different biosynthetic pathways, and to determine if the effects are consistent between extracts of different seaweeds and across different plant species.
References
Abrams SR, Nelson K, Ambrose SJ (2003) Deuterated abscisic acid analogs for mass spectrometry and metabolism studies. J Label Compd Radiopharm 46:273–283
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 97:883–893
Argueso CT, Raines T, Kieber JJ (2010) Cytokinin signaling and transcriptional networks. Curr Opin Plant Biol 13:533–539
Arthur GD, Stirk WA, van Staden J (2003) Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S Afr J Bot 69:207–211
Bartrina I, Otto E, Strnad M, Werner T, Schmulling T (2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23:69–80
Bishopp A, Benkova E, Helariutta Y (2011) Sending mixed messages: auxin-cytokinin crosstalk in roots. Curr Opin Plant Biol 14:10–16
Blunden G, Jenkins T, Liu YW (1996) Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol 8:535–543
Boyer GL, Dougherty SS (1988) Identification of abscisic acid in the seaweed Ascophyllum nodosum. Phytochemistry 27:1521–1522
Chiwocha SD, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross AR, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35:405–417
Chiwocha SD, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross AR, Kermode AR (2005) The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42:35–48
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Craigie J (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Crouch IJ, van Staden J (1992) Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J Appl Phycol 4:291–296
Crouch IJ, van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
D’Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis Response Regulator gene family to cytokinin. Plant Physiol 124:1706–1717
De Smet I, Signora L, Beeckman T, Inze D, Foyer CH, Zhang HM (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33:543–555
De Smet I, Zhang HM, Inze D, Beeckman T (2006) A novel role for abscisic acid emerges from underground. Trends Plant Sci 11:434–439
Fan D, Hodges DM, Zhang JZ, Kirby CW, Ji XH, Locke SJ, Critchley AT, Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Fornes F, Sãnchez-Perales M, Guardiola JL (2002) Effect of a seaweed extract on the productivity of ‘de Nules’ Clementine Mandarin and Navelina Orange. Bot Mar 45:486–489
Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69:437–449
Gan SS, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270:1986–1988
Ghanem ME, Albacete A, Smigocki AC, Frebort I, Pospisilova H, Martinez-Andujar C, Acosta M, Sanchez-Bravo J, Lutts S, Dodd IC, Perez-Alfocea F (2011) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62:125–140
Guo JC, Duan RJ, Hu XW, Li KM, Fu SP (2010) Isopentenyl transferase gene (ipt) downstream transcriptionally fused with gene expression improves the growth of transgenic plants. Transgenic Res 19:197–209
Havlova M, Dobrev PI, Motyka V, Storchova H, Libus J, Dobra J, Malbeck J, Gaudinova A, Vankova R (2008) The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant, Cell Environ 31:341–353
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Khan W, Hiltz D, Critchley AT, Prithiviraj B (2011) Bioassay to detect Ascophyllum nodosum; extract-induced cytokinin-like activity in Arabidopsis thaliana. J Appl Phycol 23:409–414
Kieber JJ, Schaller GE (2010) The perception of cytokinin: a story 50 years in the making. Plant Physiol 154:487–492
Kingman AR, Moore J (1982) Isolation, purification and quantitation of several growth-regulating substances in Ascophyllum nodosum. Bot Mar 25:149–153
Kudo T, Kiba T, Sakakibara H (2010) Metabolism and long-distance translocation of cytokinins. J Integr Plant Biol 52:53–60
Kumar G, Sahoo D (2011) Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J Appl Phycol 23:251–255
Kumari R, Kaur I, Bhatnagar A (2011) Effect of aqueous extract of Sargassum johnstonii Setchell on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633
Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H (2009) Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21:3152–3169
Leclerc M, Caldwell CD, Lada RR, Norrie J (2006) Effect of plant growth regulators on propagule formation in Hemerocallis spp. and Hosta spp. HortScience 41:651–653
MacKinnon SL, Hiltz D, Ugarte R, Craft CA (2010) Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Mancuso S, Azzarello E, Mugnai S, Briand X (2006) Marine bioactive substances (IPA extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv Hort Sci 20:156–161
Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Vaclavikova K, Miyawaki K, Kakimoto T (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105:20027–20031
Merewitz EB, Gianfagna T, Huang BR (2011) Photosynthesis, water use, and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera. J Exp Bot 62:383–395
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37:128–138
Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103:16598–16603
Moubayidin L, Di Mambro R, Sabatini S (2009) Cytokinin-auxin crosstalk. Trends Plant Sci 14:557–562
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Nibau C, Gibbs DJ, Coates JC (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179:595–614
Nooden LD, Kahanak GM, Okatan Y (1979) Prevention of monocarpic senescence in soybeans with auxin and cytokinin - antidote for self-destruction. Science 206:841–843
Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E (2006) CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol 141:97–107
Okamoto M, Kushiro T, Jikumaru Y, Abrams SR, Kamiya Y, Seki M, Nambara E (2011) ABA 9′-hydroxylation is catalyzed by CYP707A in Arabidopsis. Phytochemistry 72:717–722
Osmont KS, Sibout R, Hardtke CS (2007) Hidden branches: developments in root system architecture. Annu Rev Plant Biol 57:93–113
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Perilli S, Moubayidin L, Sabatini S (2010) The molecular basis of cytokinin function. Curr Opin Plant Biol 13:21–26
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Rayirath P, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429
Rayirath P, Benkel B, Hodges DM, Allan-Wojtas P, MacKinnon S, Critchley AT, Prithiviraj B (2009) Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 230:135–147
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104:19631–19636
Rivero RM, Gimeno J, Van Deynze A, Walia H, Blumwald E (2010) Enhanced cytokinin synthesis in tobacco plants expressing P-SARK:iPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol 51:1929–1941
Ruzicka K, Simaskova M, Duclercq J, Petrasek J, Zazimalova E, Simon S, Friml J, Van Montagu MCE, Benkova E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA 106:4284–4289
Sakakibara H (2006a) Cytokinin biosynthesis and regulation. In: G.Litwack (ed) Plant hormones. Elsevier, San Diego, pp 271–287
Sakakibara H (2006b) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449
Shani E, Ben-Gera H, Shleizer-Burko S, Burko Y, Weiss D, Ori N (2010) Cytokinin regulates compound leaf development in tomato. Plant Cell 22:3206–3217
Sharkey TD, Raschke K (1980) Effects of phaseic acid and dihydrophaseic acid on stomata and the photosynthetic apparatus. Plant Physiol 65:291–297
Shkolnik-Inbar D, Bar-Zvi D (2010) ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22:3560–3573
Singh S, Letham DS, Palni LMS (1992) Cytokinin biochemistry in relation to leaf senescence. Endogenous cytokinin levels and exogenous applications of cytokinins in relation to sequential leaf senescence of tobacco. Physiol Plant 86:388–397
Spann TM, Little HA (2011) Applications of a commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown ‘Hamlin’ Sweet Orange nursery trees. Hort Sci 46:577–582
Stirk WA, van Staden J (1996) Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J Appl Phycol 8:503–508
Stirk WA, Novák O, Strnad M, van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41:13–24
Stirk W, Arthur G, Lourens A, Novák O, Strnad M, van Staden J (2004) Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J Appl Phycol 16:31–39
Su YH, Liu YB, Zhang XS (2011) Auxin-cytokinin interaction regulates meristem development. Mol Plant 4:616–625
To JPC, Deruere J, Maxwell BB, Morris VF, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ (2007) Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell 19:3901–3914
Ton J, Davison S, van Wees SCM, van Loon LC, Pieterse CMJ (2001) The Arabidopsis ISR1 locus controlling rhizobacteria-mediated induced systemic resistance is involved in ethylene signaling. Plant Physiol 125:652–661
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frey NFD, Leung J (2008) An update on abscisic acid signaling in plants and more. Mol Plant 1:198–217
Weigel D, Glazebrook J (2002) Arabidopsis: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Werner T, Schmulling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12:527–538
Werner T, Motyka V, Strnad M, Schmulling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98:10487–10492
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15:2532–2550
Werner T, Kollmer I, Bartrina I, Holst K, Schmulling T (2006) New insights into the biology of cytokinin degradation. Plant Biol 8:371–381
Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22:3905–3920
Yokoya NS, Stirk WA, van Staden J, Novak O, Tureckova V, Pencik A, Strnad M (2010) Endogenous cytokinins, auxins, and abscisic acid in red algae from Brazil. J Phycol 46:1198–1205
Zaharia LI, Galka MM, Ambrose SJ, Abrams SR (2005) Preparation of deuterated abscisic acid metabolites for use in mass spectrometry and feeding studies. J Label Compd Radiopharm 48:435–445
Zhang XZ, Ervin EH (2004) Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci 44:1737–1745
Zhang XZ, Ervin EH (2008) Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci 48:364–370
Zhao YD (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Acknowledgments
The research team graciously acknowledges funding received from the National Research Council (NRC), Industrial Research Assistance Program (IRAP), and particularly Dr. D. Douglas without whom this collaborative work would not have been possible. We also thank V. Cekic and M. Lafond (Plant Biotechnology Institute, National Research Council) for hormone-profiling sample preparation and Chaminda deSilva (Nova Scotia Agriculture College) for collection of mutant seeds. BP’s lab is supported by grants from the Natural Sciences and Engineering Research Council of Canada, Nova Scotia Department of Agriculture, and Acadian Seaplants Limited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
344_2012_9301_MOESM1_ESM.tif
Supplementary Fig. 1: Root growth and development of abi4-1 and ipt1,3,5,7 mutants affected by ANE treatment. A Average length of primary roots in Col-0, abi4-1, and ipt1,3,5,7 seedlings 5 days after transfer to indicated media. The data represent the mean ± SE. B Number of lateral roots per centimeter of newly grown roots at all developmental stages (including lateral root primordial) in seedlings 5 days following transfer
344_2012_9301_MOESM2_ESM.tif
Supplementary Fig. 2: Effect of ANE on root growth of ABA and CK mutants. Representative photos of 12-day-old seedlings of abi4-1, wild-type Col-0, or quadruple ipt1,3,5,7 mutant Arabidopsis 8 days after transfer to control (A) or ANE (B) (0.01 % w/v) and ½ MS solid media
344_2012_9301_MOESM3_ESM.tif
Supplementary Fig. 3: Absolute IAA levels in ANE-treated plants. Endogenous IAA levels from two independent trials. Error bars represent mean ± SE
344_2012_9301_MOESM4_ESM.tif
Supplementary Fig. 4: Expression of the key CK one-step activating genes LOG1,7 and LOG8 at indicated time points following ANE application. Data represent the mean ± SE
344_2012_9301_MOESM5_ESM.tif
Supplementary Fig. 5: Expression of the key GA biosynthetic genes GA2ox2 and GA3ox1 at indicated time points following ANE application. Data represent the mean ± SE
Rights and permissions
About this article
Cite this article
Wally, O.S.D., Critchley, A.T., Hiltz, D. et al. Regulation of Phytohormone Biosynthesis and Accumulation in Arabidopsis Following Treatment with Commercial Extract from the Marine Macroalga Ascophyllum nodosum . J Plant Growth Regul 32, 324–339 (2013). https://doi.org/10.1007/s00344-012-9301-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9301-9