Abstract
The effect of seaweed liquid extract (SLE) of Sargassum wightii on germination, growth and yield of Triticum aestivum var. Pusa Gold was studied. Application of a lower concentration (20%) of SLE enhanced the percentage of seed germination, growth and yield, as measured by kernel number and seed dry weight. All growth and yield parameters were found to be highest at the 20% concentration SLE treatment. Total (100%) seed germination was observed for the 20% concentration SLE treatment, an 11% increase over the control. The present study demonstrated that seaweed liquid extract could serve as an alternative biofertilizer as is eco-friendly, cheaper, deliver substantial economic and environmental benefits to farmers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
India is mainly an agricultural country with approximately 70% of the population located in rural areas and directly engaged in agriculture. The growing population is placing pressure on food production and to meet this increasing demand, farmers are using chemical fertilizers to enhance their crop production. Chemical fertilizers mixed with pesticides get accumulated in plants which lead to health problems in humans due to biomagnification (Hansra 1993). The adverse effect of inorganic fertilizers on soil and environment is leading science to examine alternative biofertilizers (Metting et al. 1990).
Seaweeds are important marine renewable resources. They are used as food, feed, fodder, fertilizer, agar, alginate, carrageenan and source of various fine chemicals (Sahoo 2000). In recent years, the use of natural seaweeds as fertilizer (Hong et al. 2007) has allowed for substitution in place of conventional synthetic fertilizer (Crouch and van Staden 1993). Seaweeds extracts are marketed as liquid fertilizers and biostimulants since they contain many growth regulators such as cytokinins (Durand et al. 2003; Stirk et al. 2003), auxins (Stirk et al. 2004), gibberellins (Wildgoose et al. 1978), betaines (Blunden et al. 1991; Wu et al. 1997), macronutrients such as Ca, K, P, and micronutrients like Fe, Cu, Zn, B, Mn, Co, and Mo (Khan et al. 2009), necessary for the development and growth of plants. Seaweeds and seaweed extracts also enhance soil health by improving moisture holding capacity (Moore 2004) and by promoting the growth of beneficial soil microbes (Khan et al. 2009).
Seaweed extracts are known to enhance the growth of vegetables, fruits, and other crops (Blunden 1991; Crouch and van Staden 1994; Washington et al. 1999). Seaweed extracts when applied to seeds, added to the soil or sprayed on crops at vegetative and flowering stages, can stimulate seed germination (Hong et al. 2007), growth (Moore 2004; Khan et al. 2009), and yield (Arthur et al. 2003; Norrie and Keathley 2006) of various crops (Masny et al. 2004; Ei-Zeiny 2007; Kumar 2008). Plants treated with seaweed extract have been reported to show increased nutrient uptake (Mancuso et al. 2006), better plant growth, deep root development by improving lateral root formation (Atzmon and van Staden 1994) and increased total volume of the root system (Slàvik 2005). Seaweed extract also enhances plant defense against pest and diseases (Allen et al. 2001; Cluzet et al. 2004) increase salt tolerance (Mancuso et al. 2006), drought tolerance (Zhang and Ervin 2004), and heat tolerance (Zhang and Ervin 2008). There is not much information available about the application of seaweed liquid extract (SLE) on growth and yield of wheat plants. Thus, the present study is an attempt to examine the effect of SLE on wheat plants.
Materials and methods
For this study, the seaweed Sargassum wightii Greville (Phaeophyceae, Sargassaceae) was collected from Thirumullavaram, Kerala, India in January, March, June and September, 2007. Plants collected in the field were cleaned with seawater to remove sand and epiphytes, shade-dried, and transported to laboratory.
The crop plant, used in the present study was wheat, i.e., Triticum aestivum var. Pusa Gold (Poaceae). Seeds of T. aestivum were obtained from National Seeds Corporation (IARI) Pusa, New Delhi, India.
Preparation of seaweed liquid extract
One kilogram of seaweed was finely chopped and boiled with 1 L of distilled water for 1 h in water bath and then extract was filtered through muslin cloth. The filtrate was allowed to cool at room temperature thereafter filtered through Whatman filter paper no. 41 (pore size 20–25 μm). The filtrate was a 100% seaweed extract (Bhosle et al. 1975). Different concentrations of SLE (5, 10, 20, 30, 40 and 50%) were prepared by diluting this extract with distilled water. The seaweed extract was stored at 4°C for further applications.
Experiment
Seeds with uniform shape, size, color, and weight were selected for soaking in seaweed extract. One hundred seeds in ten replicates each were soaked for each concentration of seaweed liquid extract for 24 h. Control seeds were soaked in distilled water for 24 h. After an incubation period of 24 h at room temperature (24 ± 2°C), seeds were placed on Petri plates containing filter paper. Seed containing Petri plates were placed at temperature 21°C, light intensity of approximately 100 μmol photons m−2 s−1 for 12 h in a germinator. The filter paper was kept moist by regular addition of distilled water for control seeds and SLE for treatment seeds. Parameters such as seed germination, root length, shoot length, and number of lateral roots was regularly monitored every fifth day, till 25 days of growth. The soaked seeds were also transferred to the field and were regularly monitored with an interval of 5 days, for number of branches, number of kernels, kernel length, number of seeds per kernel, and dry weight of seeds. The experiment was terminated after a period of 4 months.
Statistical analysis
One-way analysis of variance was performed on each variable. Tukey’s Post hoc multiple mean comparison test was used for analysis of significant differences between treatments (at 5% level). All statistical analyses were performed with Statistical Package for Social Sciences (SPSS, version 10).
Results
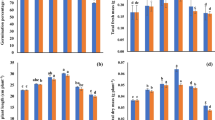
The effect of the 20% SLE concentration gave an 11% increase in seed germination over control (Fig. 1a). An increase in several growth parameters such as root length, number of lateral roots, shoot length and number of branches were also found to be highest for the 20% SLE treatment, and declining at higher concentrations. Root length was highest for the 20% treatment (Fig. 1b). Application of 20% SLE showed a 63.38% increase in the number of lateral root as compared to control (Fig. 1c). A 6.7% increase in shoot length over control was measured 25 days after germination (Fig. 2a) and a 46.15% increase in the number of branches plant−1 for the same 20% treatment (Fig. 2b). The number of kernels plant−1 and kernel length were observed to be highest that was 54.16% and 18.75%, respectively, increased over control at 20% and lowest at the 100% SLE treatment (Figs. 2c and 3a). Treatment of 20% also gave a 13.69% increase in number of seeds kernel−1 and 22.86% increase in dry weight of seeds over control (Fig. 3b and c). The effect of SLE on germination, growth and yield of T. aestivum is shown in Table 1.
Discussion
Seaweed extracts are known to enhance the growth of vegetables, fruits, and other crops as they are reported to contain growth regulators such as auxins (IAA and IBA), gibberellins, cytokinins, betaines, and major macro- and micronutrients (Blunden et al. 1991). Thus, seaweeds are now being used as biofertilizers (Bokil et al. 1974). This study showed that the application of seaweed liquid extract enhances the germination, growth, and yield of wheat plants.
This study showed that the application of S. wightii seaweed liquid extract increases seed germination percentage. Seed treatment with 20% seaweed extract gave highest percentage of seed germination while 100% concentration gave least. Hong et al. (2007) reported that the percentage of germination in various crops such as vegetables and fruits were increased by using seaweeds as a biofertilizer. Xavier and Jesudass (2007) also reported that 100% seed germination was found in lower concentrations of Caulerpa recemosa extract. The increased germination percentage at low concentrations may be due to the presence of some growth regulators, micro- and macronutrients (Challen and Hemingway 1965).
The application of SLE-enhanced root length, number of lateral roots, shoot length and number of branches increased maximum for the 20% treatment. Beyond this concentration, these variables decreased substantially. The lowest root length, number of lateral roots, shoot length, and number of branches were observed for the 100% SLE concentration. Rayorath et al. (2008) reported an increase in root length (up to 32%) with the application of extract from Ascophyllum nodosum. Seaweed concentrate prepared from Ecklonia maxima was found to enhance root growth of tomato at 0.4% concentration (Crouch and van Staden 1992).
The number of lateral roots in Vigna sinensis was observed to be highest at a 20% concentration and the minimum number of lateral roots was recorded for a 100% concentration of S. wightii extract (Sivasankari et al. 2006). Tomato plants treated with lower concentration (0.4%) of E. maxima extract showed an increase in plant height (Crouch and van Staden 1992). Anantharaj and Venkatesalu (2002) observed that the number of branching increases after the application of seaweed extracts on Dolichos biflorus. Significant differences at 5% level of significance (P ≤ 0.05) were observed for root length, number of lateral roots, and number of branches at 20% SLE treatment.
The present investigation clearly demonstrated that SLE enhanced the reproductive parameters. The number of kernels plant−1, kernel length, number of seeds kernel−1 and dry weight of seeds increased to highest at 20% concentration and lowest in 100% concentration of SLE. Plants sprayed with 0.4% concentration of seaweed concentrate of Ecklonia showed 10% increase in fruit number (Crouch and van Staden 1992). The pod yield for beans increased after foliar spray with crude extracts of Macrocystis integrifolia and E. maxima (Temple and Bomke 1989). Similarly, a substantial increase in yield was achieved in Thompson seedless’ grape Vitis vinifera and peppers (Norrie and Keathley 2006; Arthur et al. 2003), respectively. The effects of the application of seaweed fertilizers in improving total fruit production may be related to the effect of cytokinins (Featonby-Smith 1984). Kumar et al. (2007) also reported highest fruit length of Abelmoscus esculantus following treatment with a 5% SLE fertilizer.
Foliar applications of an aqueous extract of Hypnea musciformis increased the yield of tea by 14% over control (Thevanathan et al. 2005). Kelpak seaweed concentrate increased the number of grains in wheat (Beckett and van Staden 1989). The highest increase in dry weight was observed at 20% SLE and the lowest for the 100% treatment with S. wightii extract (Sivasankari et al. 2006). Kelpak at lower concentrations increased the grain weight of wheat (Beckett and van Staden 1989). Significant differences at 5% level of significance (P ≤ 0.05) were observed for number of kernels plant−1, kernel length, number of seeds kernel−1, and dry weight of seed. Treatment of 20% SLE produced positive response over others. Liquid extract prepared from S. wightii was found to have promising fertilizer activity. Finally, the application of low SLE concentrations may increase plant growth and deliver substantial economic and environmental benefits to farmers. Thus, the present study demonstrated that seaweed liquid extract could serve as an alternative biofertilizer as they are eco-friendly, cheaper and can overcome the ill-effect of chemical fertilizers along with pesticides.
References
Allen VG, Pond KR, Saker KE, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Schmidt RE, Fike JH, Zhang X, Ayad JY, Brown CP, Miller MF, Montgomery JL, Mahan J, Wester DB, Melton C (2001) Tasco: influence of a brown seaweed on antioxidants in forages and livestock—a review. J Anim Sci 79:E21–E31
Anantharaj M, Venkatesalu V (2002) Studies on the effect of seaweed extracts on Dolichos biflorus. Seaweed Res Util 24:129–137
Arthur GD, Stirk WA, van Staden J (2003) Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S Afr J Bot 69:207–211
Atzmon N, van Staden J (1994) The effect of seaweed concentrate on the growth of Pinus pinea seedlings. New For 8:279–288
Beckett RP, van Staden J (1989) The effect of seaweed concentrate on the growth and yield of potassium stressed wheat. Plant Soil 116:29–36
Bhosle NB, Untawale AG, Dhargalkar VK (1975) Effect of seaweed extract on the growth of Phaseolus vulgaris L. Indian J Mar Sci 4:208–210
Blunden G (1991) Agricultural uses of seaweeds and seaweed extracts. In: Guiry MD, Blunden G (eds) Seaweed resources in Europe: uses and potential. Wiley, Chichester, pp 65–81
Blunden G, Smith BE, Mason TG, Ying Xu, Yvin JC, Chabot R (1991) Betaines in seaweed extracts. J Eur Cosmet 13:113–118
Bokil KK, Mehta VC, Datar DS (1974) Seaweeds as manure: II pot culture manorial experiments on wheat. Phykos 13:1–5
Challen SB, Hemingway JC (1965) Growth of higher plants in response to feeding with seaweed extracts. Proc Int Seaweed Symp 5:359–367
Cluzet S, Torregrosa C, Jacquet C, Lafitte C, Fournier J, Mercier L, Salamagne S, Briand X, Esquerré-Tugayé MT, Dumas B (2004) Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from the green alga Ulva spp. Plant Cell Environ 27:917–928
Crouch IJ, van Staden J (1992) Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J Appl Phycol 4:291–296
Crouch IJ, van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Crouch IJ, van Staden J (1994) Commercial seaweed products as biostimulants in horticulture. J Home Consum Hortic 1:19–76
Durand N, Briand X, Meyer C (2003) The effect of marine bioactive substances (NPRO) and exogenous cytokinins on nitrate reductase activity in Arabidopsis thaliana. Physiol Plant 119:489–493
Ei-Zeiny OAH (2007) Effect of bio-fertilizers and root exudates of two weeds as a source of natural growth regulators on growth and productivity of bean plants (Phaseolus vulgaris L.). Res J Agric Biol Sci 3:440–446
Featonby-Smith BC (1984) Cytokinins in Ecklonia maxima and the effect of seaweed concentrate on plant growth. Ph.D Thesis, University of Natal, Pietermaritzburg, pp 153.
Hansra BS (1993) Transfer of agricultural technology on irrigated agriculture. Fer News 38(4):31–33
Hong DD, Hien HM, Son PN (2007) Seaweeds from Vietnam used for functional food, medicine and fertilizer. J Appl Phycol 19:817–826
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. Plant Growth Regul 28:386–399
Kumar G (2008) Effect of seaweed extract on growth and development of wheat. M.Phil. Thesis, University of Delhi, Delhi, pp 66.
Kumar RAS, Effie A, Saravanababu S (2007) Studies on the effect of seaweed and seagrass liquid fertilizers on the fruit length and weight of Abelmoschus esculentus L. Var. Hybrid-10. Seaweed Res Util 29:101–103
Mancuso S, Azzarello E, Mugnai S, Briand X (2006) Marine bioactive substances (IPA extract) improve ion fluxes and water stress tolerance in potted Vitis vinifera plants. Adv Hortic Sci 20:156–161
Masny A, Basak A, Żurawicz E (2004) Effects of foliar applications of KELPAK SL and GOËMAR BM 86® preparation on yield and fruit quality in two strawberry. J Fruit Ornam Plant Res 12:23–27
Metting B, Zimmerman WJ, Crouch IJ, van Staden J (1990) Agronomic uses of seaweed and microalgae. In: Akatuska I (ed) Introduction to applied phycology. SPB, The Hague, pp 267–307
Moore KK (2004) Using seaweed compost to grow bedding plants. BioCycle 45:43–44
Norrie J, Keathley JP (2006) Benefits of Ascophyllum nodosum marine-plant extract applications to ‘Thompson seedless’ grape production. Acta Hortic 727:243–247
Rayorath P, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008) Rapid bioassay to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. Using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429
Sahoo D (2000) Farming the ocean: seaweeds cultivation and utilization. Aravali, New Delhi
Sivasankari S, Venkatesalu V, Anatharaj M, Chandrasekaran M (2006) Effect of seaweed extract on the growth and biochemical constitutes of Vigna sinensis. Bioresour Technol 97:1745–1751
Slàvik M (2005) Production of Norway spruce (Picea abies) seedlings on substrate mixes using growth stimulants. J For Sci 51:15–23
Stirk WA, Novak O, Strnad M, van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41:13–24
Stirk WA, Arthur GD, Lourens AF, Novak O, Strnad M, van Staden J (2004) Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J Appl Phycol 16:31–39
Temple WD, Bomke AA (1989) Effects of kelp (Macrocystis integrifolia and Ecklonia maxima) foliar application on bean crop growth. Plant Soil 117:85–92
Thevanathan R, Rajarajan R, Bhavan ILG (2005) Liquid fertilizer preparation of marine microalgae to enhance the yield and quality of tea. Seaweed Res Util 27:117–123
Washington WS, Engleitner S, Boontjes G, Shanmuganathan N (1999) Effect of fungicides, seaweed extracts, tea tree oil and fungal agents on fruit rot and yield in strawberry. Aust J Exp Agric 39:487–494
Wildgoose PB, Blunden G, Jewers K (1978) Seasonal variation in gibberellin activity of some species of Fucaceae and Laminariaceae. Bot Mar 21:63–65
Wu Y, Jenkins T, Blunden G, Whaphan C, Hankin SD (1997) The role of betaines in alkaline extracts of Ascophyllum nodosum in the reduction of Meloidogyne javanica and M. incognita infestations of tomato plants. Fundam Appl Nematol 20:99–102
Xavier GSA, Jesudass LL (2007) Effect of seaweed extracts on cluster bean. Seaweed Res Util 29:85–87
Zhang X, Ervin EH (2004) Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci 44:1737–1745
Zhang X, Ervin EH (2008) Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci 48:364–370
Acknowledgments
The authors would like to thank the University Grants Commission, New Delhi for granting fellowship for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented at the 7th Asia Pacific Congress on Algal Biotechnology, New Delhi, 2009
Rights and permissions
About this article
Cite this article
Kumar, G., Sahoo, D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J Appl Phycol 23, 251–255 (2011). https://doi.org/10.1007/s10811-011-9660-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-011-9660-9