Abstract
Extracts of the brown seaweed Ascophyllum nodosum enhance plant tolerance against environmental stresses such as drought, salinity, and frost. However, the molecular mechanisms underlying this improved stress tolerance and the nature of the bioactive compounds present in the seaweed extracts that elicits stress tolerance remain largely unknown. We investigated the effect of A. nodosum extracts and its organic sub-fractions on freezing tolerance of Arabidopsis thaliana. Ascophyllum nodosum extracts and its lipophilic fraction significantly increased tolerance to freezing temperatures in in vitro and in vivo assays. Untreated plants exhibited severe chlorosis, tissue damage, and failed to recover from freezing treatments while the extract-treated plants recovered from freezing temperature of −7.5°C in in vitro and −5.5°C in in vivo assays. Electrolyte leakage measurements revealed that the LT50 value was lowered by 3°C while cell viability staining demonstrated a 30–40% reduction in area of damaged tissue in extract treated plants as compared to water controls. Moreover, histological observations of leaf sections revealed that extracts have a significant effect on maintaining membrane integrity during freezing stress. Treated plants exhibited 70% less chlorophyll damage during freezing recovery as compared to the controls, and this correlated with reduced expression of the chlorphyllase genes AtCHL1 and AtCHL2. Further, the A. nodosum extract treatment modulated the expression of the cold response genes, COR15A, RD29A, and CBF3, resulting in enhanced tolerance to freezing temperatures. More than 2.6-fold increase in expression of RD29A, 1.8-fold increase of CBF3 and two-fold increase in the transcript level of COR15A was observed in plants treated with lipophilic fraction of A. nodosum at −2°C. Taken together, the results suggest that chemical components in A. nodosum extracts protect membrane integrity and affect the expression of stress response genes leading to freezing stress tolerance in A. thaliana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses negatively affect plant growth and productivity accounting for more than 50% losses in productivity of major crops (Bray et al. 2000). Among abiotic stresses in the northern hemisphere freezing is important, resulting in millions of dollars in lost revenue and further limiting the extent of arable land in colder regions. Many economically important plants such as cotton, soybean, maize, rice, tomato; tropical fruits such as bananas, papayas, mangoes; and subtropical fruits such as grapes, guavas, and oranges are sensitive to low temperatures (Sharma et al. 2005). The freezing process, types of damages associated with freezing stress, tolerance responses in plants, and the current understanding of the genetic basis of freezing tolerance have been reviewed (Guy 2003; Sharma et al. 2005).
Seaweed and seaweed products have been used as fertilizers, biostimulants, and soil conditioners for centuries. Besides their use as farmyard manure (FYM), purified products of seaweed, commonly referred to as ‘seaweed extracts’, are sprayed as biostimulants on several crop plants. The brown alga, Ascophyllum nodosum (L.) Le Jol. is the most commonly used seaweed in commercial extracts and suspensions. A wide range of beneficial effects of products derived from Ascophyllum have been reported. Commercial formulations of A. nodosum extracts stimulate shoot growth and branching (Temple and Bomke 1989), increase root growth, promote lateral root development (Metting et al. 1990), improve nutrient uptake (Yan 1993), and enhance resistance to diseases (Featonby-Smith and van-Staden 1983) and tolerance to environmental stresses such as drought, salinity and frost (Nabati 1991; Nabati et al. 1994). It has been reported that some bioactive organic compounds, rather than mineral elements, account for the beneficial effects of these extracts (Blunden 1991; Crouch and van Staden 1993).
A number of seaweed extract products have been used to improve freezing tolerance in plants. In a field study, vineyard sprayed with extracts of giant Bull kelp Durvillea potatorum showed a significant reduction in shoot osmotic potential in response to freezing stress (Wilson 2001). Field studies on winter barley (Hordeum vulgare cv Igri) showed that the application of A. nodosum improved winter hardiness and increased frost resistance (Burchett et al. 1998). However, the physiological mechanisms responsible for the improvement in plant stress tolerance and the nature of the compound in A. nodosum responsible for this bioactivity remain unclear. Therefore, the present study aimed to investigate the effect of A. nodosum on enhancing freezing tolerance and to characterize the physiological and molecular basis of this freezing stress tolerance in Arabidopsis thaliana.
Materials and methods
Seeds of A. thaliana (col-0) were purchased from Lehle Seeds (Round Rock, TX). Murashige and Skoog basal salt mixture (Cat No: M5524), sucrose and agar, were purchased from Sigma Aldrich (Oakville, ON). Powdered alkaline extract of A. nodosum (Acadian®) was provided by Acadian Seaplants Limited, Dartmouth, Nova Scotia, Canada. The total organic and inorganic composition and elemental analysis of this alkaline extract is presented in Supplementary Table 1 (Acadian Seaplants Limited, technical information).
Preparation of seaweed extracts and its organic sub-fractions
An aqueous solution of the commercial formulation (Acadian®) of A. nodosum extract (hereafter termed as ANE) was prepared by dissolving 1 g of the extract powder in 20 ml of sterile distilled water by constant stirring with a magnetic stirrer for 15 min. The solution was then filter sterilized using a 0.22 μm SFCA syringe filter (Corning Inc., NY, USA) and stored in sterile glass centrifuge tubes at 4°C until further use.
A methanol organic fraction of ANE was prepared by extracting 10 g in 50 ml methanol for 15 min. The extract was centrifuged at 4,000×g for 10 min, and the supernatant transferred to a clean centrifuge tube. The solvent was then evaporated to dryness under a stream of nitrogen and stored in sterile glass centrifuge tubes at −20°C until further use. The dried methanol organic fraction was re-suspended in a minimal quantity of methanol (100 μl g−1 equivalent) and the volume made up with sterile distilled water for use in experiments.
For sub-fractionation, the methanol organic fraction was prepared as described above and the pellet was re-suspended in 50 ml of water. This aqueous solution was further sub-fractionated by sequential extraction with three volumes (75 ml each) of hexane, chloroform, and ethyl acetate. The sub-fractions were dried under a stream of nitrogen and stored in sterile glass centrifuge tubes at −20°C until further use. The dried sub-fractions were re-suspended in minimal quantity of methanol (100 μl g−1 equivalent) and the volume made up with sterile distilled water for use in experiments.
NMR spectroscopy analysis of ANE and the ethyl acetate fraction of ANE
Proton nuclear magnetic resonance (1H NMR) of these fractions was measured on a Bruker Advance DRX-500 spectrometer (Bruker Canada Ltd.) at 500.13 MHz. The ANE and its ethyl acetate fraction were dissolved in D2O (99.9%, Sigma Aldrich, Oakville, ON) and CD3OD (98.8% D, Sigma Aldrich, Oakville, ON), respectively and then placed in a 5-mm NMR tube containing 0.03% of the internal reference trimethylsilyl propionic- 2,2,3,3-d4 acid (sodium salt). The spectrum in CD3OD was referenced to the signal at 3.30 ppm and the D2O run was referenced to 4.80 ppm.
Fatty acid analysis
Fatty acid analysis of the ethyl acetate fraction of ANE was conducted by Maxxam Analytics Inc. (Mississauga, ON) using a gas chromatograph equipped with a flame ionization detector using AOAC method 996.06.
Sterol analysis
The sterol content of the ethyl acetate fraction of ANE was obtained by gas chromatography–mass spectrometry analysis of the trimethylsilyl ether (TMS) derivatives of the sterols (Kamal-Eldin et al. 1998). Briefly, TMS derivatives were prepared by addition of 100 μl of N,O-bis(trimethylsilyl)trifluoroacetamide (Fluka cat no. 15238, Sigma-Aldrich Canada Ltd, Oakville, ON) to glass screw-top vials containing fucosterol standards or the ethyl acetate subfraction. The vials were flushed with nitrogen, sealed and heated at 60°C for 1 h. Excess solvent was evaporated from the samples which were then resuspended in dichloromethane. Analysis of the TMS derivatives was performed on a SP-2340-60 m capillary column (Supelco, PA) fitted on an Agilent 6890 N gas chromatograph coupled to an Agilent 5975 Inert XL mass selective detector. Separation was performed using the following temperature program: 100°C, 25°C min−1, 200°C, 5°C min−1, 250°C (16 min). Quantitation of the fucosterol content was afforded using a calibration curve constructed from derivatized fucosterol standards.
Petri dish freezing tolerance assay
The in vitro Petri dish freezing tolerance assay used to investigate the plants’ responses in ANE-induced freezing tolerance was conducted as described by Xin and Browse (1998). Briefly, sterilized Arabidopsis seeds were grown on solidified Murashige and Skoog basal salt medium (Murashige and Skoog 1962) containing 1% sucrose and supplemented with different concentrations of ANE or its organic subfractions. Required concentrations of filter sterilized ANE (0.5 g L−1) or organic fractions (1.0 g L−1 equivalent of ANE) were added to molten (50°C) media, and plated in partitioned petri dishes (Fisher Scientific, Ottawa, ON). Two sets of controls, one set of plates with distilled water used to dissolve ANE and a second set with equal amount of methanol used to dissolve the ethyl acetate fraction (100 μl L−1 medium) were maintained. Seeds were evenly distributed in the Petri dishes by placing individual seeds with a 100 μL micro pipette at the rate of 10–15 seeds per partition. The Petri dishes were incubated at 22/18°C day/night temperatures, 16:8 photoperiod and light intensity 100 μmol photons m−1 s−1 for 10 days.
Ten days after germination, Petri dishes were transferred to a temperature-controlled incubator set to −2 ± 0.1°C. To achieve uniform freezing, the Petri dishes were equilibrated at −2°C for 24 h before further lowering of the temperature. After equilibration, the temperature of the chamber was progressively lowered at the rate of 1°C per day until the desired sub-zero temperatures were attained. The temperature was monitored by wireless temperature sensor modules (Traceable® Remote sensor module, Fisher Scientific, Ottawa, ON, Canada) placed inside the petri plates. At each temperature, five Petri plates per treatment were withdrawn from chamber, thawed at 4°C for 12 h in the dark, and returned to the original growth conditions (16:8 photoperiod, 22/18°C day/night temperatures). Survival of plants (by degree of chlorosis and shoot damage) was recorded visually, 48 h after returning to the original growth conditions.
Peat pellet freezing assay
An in vivo assay, peat pellet freezing assay, was carried out to study the effect of ANE and its organic subfractions in imparting freezing tolerance at the whole plant level in green house conditions. Arabidopsis plants were grown in peat pellets (4 cm dia pellets—Jiffy-7®, Jiffy Products Ltd, NB, Canada) in greenhouse conditions set at 22/18°C day/night temperatures and 16:8 photoperiod and light intensity 100 μmol photons m−1 s−1. Plants were irrigated once every 2 days and fertilized weekly [20:20:20 NPK (1 g L−1) @ 10 ml per plant] for 2 weeks. Three-week-old seedlings were used for the experiment. Extract treatments were given 48 h prior to freezing treatment by irrigating with desired concentrations of ANE (1.0 g L−1) or organic fractions (1.0 g L−1 equivalent made up with sterile distilled water) at the rate of 20 ml per plant.
Forty-eight hours after treatment with extracts, plants were transferred to a low-temperature incubator set to 0°C. To achieve uniform freezing, plants were equilibrated at 0°C for 24 h. Freezing was initiated by spraying ice-cold water and the temperature of the chamber was progressively lowered at the rate of 1°C every 24 h until the desired sub-zero temperature was attained. The temperature was monitored by wireless temperature sensor modules placed at different points inside the freezing cabinet. At desired temperatures, ten randomly selected plants from each treatment were withdrawn from the chamber, thawed at 4°C for 12 h in dark, and then returned to the original growth conditions of the greenhouse. Two days later, survival of plants was recorded visually according to the degree of chlorosis and leaf damage. The plants which were completely damaged or those which were chlorotic and failed to show signs of regrowth were considered as dead. The experiment was repeated three times with ten plants per treatment.
Estimation of chlorophyll
Chlorophyll degradation during the post-freezing recovery period is one of the morphological markers of freezing-induced tissue damage in plants (Huner et al. 1993). The effect of ANE in maintaining the stability of chlorophyll content and reduced degradation during post-freezing recovery was analyzed by comparing the total chlorophyll, chlorophyll a, and chlorophyll b content of freeze-damaged leaf tissues of the treated and control plants from the “Peat pellet freezing assay”. Leaf tissue (250 mg each) was collected (second whorl of leaves) from 15 plants per treatment and chlorophyll was estimated using the method described by Arnon (1949). Two independent experiments were carried out with 15 plants per treatment.
Electrolyte leakage assay
Plant materials, growth conditions, extract, and low-temperature treatments were the same as described in the “Peat pellet freezing assay”. Ten random plants from each treatment were removed from the incubator and thawed at 4°C for 12 h in dark. After thawing, two leaves from the second whorl were excised from ten plants and placed in screw-capped glass tubes containing 10 ml of deionized water. The electrolyte leakage was quantified according to the standard protocol described by Gilmour et al. 1988. A plot of temperature versus percent electrolyte leakage was used to determine the LT50 values. The experiment was repeated three times with equal number of replicates.
Quantification of freezing damage by cell viability staining
Trypan blue stain (3,3-[(3,3-dimethyl-4,4-biphenylylene) bis (azo)] bis(5-amino-4-hydroxy-2,7-naphthalenedisulfonic acid) tetra sodium salt) was used to visualize the extent of freezing-induced tissue damage in extract-treated and control plants. Damaged tissues stain dark blue, whereas the viable cells do not stain due to an intact cell membrane barrier (Rate et al. 1999).
The plant growth conditions, extract treatments, and freezing temperatures were as described in the “Peat pellet freezing assay”. At each treatment/temperature combination, five plants per treatment were removed from the incubator, thawed at 4°C for 12 h in the dark, and then returned to the normal growth conditions (16:8 h day:night cycle; 22/18°C day/night temperatures and light intensity 100 μmol photons m−1 s−1). Two days later, uniform sized leaves from the second or third whorl were sampled from plants (by excising at the base of the petiole) and the tissue damage was assessed as described previously by Rate et al. (1999). The area of the damaged tissue was measured using the image processing and analysis software Image-j® (Research Services Branch, NIH). For each treatment at least 15 individual leaves, collected from five random plants were stained and analyzed. The experiment was repeated twice with similar results.
Histological studies
Light microscopy (brightfield transmitted light)
Uniform sized leaves from the second/third whorl were sampled from treated plants by excising at the base of the petiole. The leaves were washed with sterile distilled water to remove the soil and other extraneous matter and blotted dry on filter paper pads. Segments (approximately 5 mm × 3 mm) parallel to the leaf midrib were excised 5 mm inside the leaf margin using a sterile scalpel blade (Fig. 5a). Care was taken to select the section from same regions of the leaves across the temperature/extract treatment combination. The leaf segments were submerged in Karnovsky’s fixative (paraformaldehyde–glutaraldehyde solution, 2.5% glutaraldehyde + 2% formaldehyde in 0.1 M Sorensen’s phosphate buffer; Electron Microscopy Sciences, Hatfield, PA) and fixed for 12 h at 22 ± 0.5°C. Samples were stored in fixative solution at 4°C until further use. The samples were embedded in spur resin (longer pot life recipe) following the protocol described by Spurr (1969). The resin was allowed to polymerize at 60°C for 24 h and sectioned using glass knives with a Reichert Ultra CutE ® ultramicrotome [Leica Microsystems (Canada) Inc., Richmond Hill, ON] set to a thickness of 2 μm.
Epoxy Tissue Stain, a double stain comprised of Toluidine blue and Basic fuchsin (Electron Microscopy Sciences, Hatfield, PA), was used for staining the sections to impart general contrast (Spurlock et al. 1966). Sections were viewed using an Olympus BX50 light microscope (Olympus Canada, Markham, ON) under standard brightfield conditions and photographed using a Nikon Coolpix® 995 digital camera (Nikon Canada, Mississauga, ON) attached to the microscope.
Fluorescence microscopy
A fluorescent dye, Nile Red (5H-benzo[α]phenoxazine-5-one, 9-diethylamino) was used to visualize the damage to the lipid bilayer of the plasma membrane. The staining protocol was adopted from Ashrafi et al. (2003), originally used for staining fat deposition in animal tissue (Ashrafi et al. 2003 and references therein). Staining solution was prepared by dissolving Nile Red (Sigma Aldrich, Oakville, ON) in acetone (5% w/v) and further diluting with water to a final concentration of 0.05 μg mL−1. The leaf sections were mounted on microscope slides and were stained by flooding with staining solution at room temperature for 30 min in dark and subsequently washed with several changes of sterile distilled water. Stained sections were viewed using Leica DM 1000 fluorescence microscope (Leica Microsystems (Canada) Inc., Richmond Hill, ON) under a fluorescent filter attached with a Leica DFC camera [Leica Microsystems (Canada) Inc., Richmond Hill, ON] and digital images were acquired using Leica Application Suite®-image processing and analysis application software [Leica Microsystems (Canada) Inc., Richmond Hill, ON].
Quantification of the pectin methyl esterase (PME) activity
In order to explore the possible role of pectin methyl esterases (PMEs) in affecting the cell-wall responses during abiotic stresses, a gel diffusion assay to compare the PME activity between treated and control plants was performed as described by Downie et al. (1998). Total protein was extracted from leaf tissue using PME extraction buffer (0.1 M citrate/0.2 M Na2HPO4 buffer containing 1.0 M NaCl, pH 5.0). The quantity of protein was normalized over the samples and used as crude enzyme extract for quantification of PME activity following the method described by Downie et al. (1998). The experiment was repeated two times with five plates for each treatment.
Molecular analysis of A. nodosum induced freezing tolerance
The molecular basis of ANE-induced freezing tolerance was analyzed by studying the expression of key genes known to be involved in freezing tolerance in Arabidopsis. A two-step RT-PCR method was adopted for quantifying transcript abundance. Plant growth conditions, extract, and freezing temperatures treatments were as described in "Peat pellet freezing assay". Leaf samples were collected 0°C, −2°C, 24 h thawing at 4°C and 24 h after returning to the normal growth conditions (16:8 h day:night cycle and 22/18°C day/night temperatures). For each temperature/treatment combination, 15 plants were randomly withdrawn, leaves harvested (leaves harvested from 5 plants each were pooled and constituted a replicate, n = 3) and flash-frozen in liquid nitrogen. The frozen leaf samples were ground in a pre-chilled mortar and pestle and stored at −80°C until further use.
Total RNA was isolated using the RNAqueous® Plant RNA isolation Kit (Ambion Inc., Austin, TX) following the manufacturer’s instruction. A ‘Two-step RT-PCR’ was used for quantifying the transcript yield and comparing differential gene expression. Ten micrograms of total RNA was treated with DNase using the TURBO DNA-free® kit (Ambion Inc., Austin, TX), and first-strand cDNA was synthesized using the Retroscript® Reverse Transcription Kit (Ambion Inc., Austin, TX). The cDNA samples were purified with QIAquick® PCR purification Kit (Qiagen Inc., Mississauga, Ontario) and normalized with the QuantumRNA® Universal 18S internal standard (Ambion Inc., Austin, TX). A standard PCR reaction protocol was used to amplify the transcripts and the differential gene expression was compared on an agarose gel.
Quantitative Real-Time PCR was performed to assess the fold change in transcript abundance of selected marker genes. Quantitative Real-Time PCR was carried out in a ‘Step One Plus® Real-Time PCR System’ (Applied Biosystems) using ‘Fast Start Universal SYBR Green Master®’ (Roche Diagnostics, Indianapolis, IN) adopting the manufacturer’s instructions. Data were analyzed using ‘Step One software V2.0’ with a ‘Relative Standard Curve’ mode. The primer sequences of the selected genes and key PCR parameters are given in Supplementary Table 2.
Statistical analysis and experimental controls
For the in vitro experiments, each Petri dish (containing about 50 plants) constituted a replicate. For in vivo experiments, each plant grown in peat pellet was a replicate, unless otherwise mentioned. The data were analyzed using Tukey’s HSD (Honestly Significantly Different) Test with P ≤ 0.05 using CoStat statistical software (CoHort Software, Monterey, CA).
The control plants were grown under identical growth conditions to plants used for extract treatment except that they received an equal volume of distilled water instead of ANE or organic fractions of ANE. During preliminary studies, a separate set of plants treated with equal volume of methanol used for re-suspending the sub-fractions was maintained. There was no difference between the aqueous methanol (100 μl L−1) treated and water controls, therefore only distilled water controls were used for further experiments.
Results
NMR spectroscopy analysis of ANE and the ethyl acetate fraction of ANE
The 1H NMR spectra of ANE and its ethyl acetate sub-fraction were obtained using different deuterated NMR solvents (Fig. 1). Despite this difference, the 1H NMR spectrum of the ethyl acetate fraction of ANE showed loss of resonances between 3.3 and 4.5 ppm compared to that of the ANE. The spectrum of the ANE was dominated by resonances between 3.5 and 4.5 ppm while the 1H NMR spectrum of the ethyl acetate fraction of the ANE was dominated by resonances between 0.8 and 2.5 ppm. (Fig. 1a, b). In general, 1H NMR spectra of the ethyl acetate fraction of ANE shows that this fraction is dominated by the resonances corresponding to the lipophilic components such as fatty acids and sterols (Fig. 1a, b).
Fatty acid and sterol analysis
Fatty acid analysis revealed that the ethyl acetate sub-fraction of ANE contained 0.8% (w/w) butyric acid, 2.3% (w/w) palmitic acid, 9.3% (w/w) oleic acid, 1.6% (w/w) linoleic acid and 1.6% (w/w) arachidonic acid for a total fatty acid content of 15.6%. Analysis of the sterol content showed that fucosterol was the only sterol present in significant quantities. The fraction was determined to contain 50.0% fucosterol.
Petri plate freezing tolerance assay
Plants treated with ANE and/or its organic fractions (hexane, chloroform or ethyl acetate) showed a higher tolerance against freezing stress as compared to water controls. At −5.5°C, the control plants showed severe chlorosis and exhibited a significant amount of tissue damage during recovery, whereas the treated plants were green and showed very little tissue damage (Fig. 2). At −6.5°C, the control plants showed 70% mortality, whereas the plants treated with the organic sub-fractions of ANE were healthy and recovered from stress-induced damage. Slight chlorosis was evident in the older leaves of the treated plants, and the plants treated with the ethyl acetate sub-fraction showed the highest tolerance. There was 100% mortality in control plants at −7.5°C, whereas the treated plants displayed considerably reduced damage.
Ascophyllum nodosum induced freezing tolerance in Arabidopsis thaliana in vitro. Effect of (a) Ascophyllum nodosum extract (ANE) and (b) its organic sub-fractions on freezing tolerance in Petri dish assay. (c) Comparison of the survival rate of plants treated with ANE and its organic sub-fractions over untreated controls in Petri dish assay at different freezing temperatures
Peat pellet freezing assay
Plants treated with ANE and its ethyl acetate subfraction showed significant tolerance against freezing and subsequent thawing over untreated controls (Fig. 3a). At −2.5°C, the control plants showed severe chlorosis and tissue damage, whereas the treated plants showed no symptoms of tissue damage. The control plants exhibited significant shoot damage at −3.5°C, whereas ethyl acetate treated plants showed no symptoms of injury. The control plants failed to recover from freeze-induced tissue damage, whereas the treated plants continued to grow after a lag phase of 8–10 days. At −4.5°C, all treated plants started showing chlorophyll degradation; however, chlorotic symptoms were much more severe in the ANE treated plants than plants treated with the ethyl acetate subfraction. At this temperature, the control plants showed 100% mortality, whereas it was 60 and 40% in ANE and ethyl acetate subfractions, respectively (Fig. 3a).
Ascophyllum nodosum induced freezing tolerance in greenhouse grown Arabidopsis thaliana (a) phenotypic responses of plants treated with (i) water control (ii) ANE (1.0 g L−1) and (iii) ethyl acetate subfractions (1.0 g L−1) to a temperature of −2.5°C for 24 h in "Peat pellet freezing assay". (b) Dose dependant responses of plants treated with ANE (1.0 g L−1) and its ethyl acetate subfractions (0.5, 1.0, 2.0 g L−1) in the electrolyte leakage assay. Each value represents the average of ten replicates. Bars represent standard error
Electrolyte leakage assay
The temperature causing 50% leakage of electrolytes from leaf cells (LT50) was determined for 15 samples per treatment for each temperature regime in three independent experiments. The LT50 values for the control plants and plants treated with various concentrations of ANE or its organic fraction are shown in Fig. 3b. At −3.5°C, the percent of ion leakage in control plants had already exceeded the LT50 values (63%), whereas the corresponding value for ANE treated plants was only 12% and the corresponding ion leakage values were 7.4, 5.6, and 11.4% for 0.5, 1.0 and 2.0 g L−1 ethyl acetate fraction, respectively. ANE-treated plants showed 15% electrolyte leakage at −4.5°C while it was 24.4, 8.9 and 43.3% for the respective concentrations of ethyl acetate sub-fraction at the concentration of 0.5, 1.0, and 2.0 g L−1, respectively. The percentage ion leakage of ANE treated plants was 49% at −5.5°C, while in the ethyl acetate fraction (1.0 g L−1) treatment it was as low as 37% (Fig. 3b). At this temperature, other concentrations of ethyl acetate fractions had exceeded the LT50 values and displayed ion leakage percentages of 60.8 and 66.9% for 0.5 and 2.0 g L−1, respectively. (Fig. 3b).
Estimation of chlorophyll
Freezing-induced chlorophyll damage was significantly lower in ANE-treated plants as compared to water controls (Fig. 4). The total chlorophyll content of ANE-treated plants after exposure to freezing stress was 0.91 mg g−1 fresh weight, whereas it was 0.36 mg g−1 fresh weight of leaf tissue in control plants (data not shown). The results indicated that the ANE-treated plants possess a threefold higher capacity to protect chlorophyll against freezing-induced damage as compared to water controls. The higher chlorophyll retention by ANE-treated plants during post-freezing recovery was directly reflected in the content of chlorophyll a and b, with no significant change in their relative proportions (Fig. 4).
Ascophyllum nodosum reduced freezing-induced chlorophyll degradation in Arabidopsis thaliana leaves. Comparison of chlorophyll a and b content of treated and untreated controls 48 h after freezing treatment. Each value represents the average of 15 replicates. Bars represent standard error. Differential expression patterns of Arabidopsis chlorophyllase genes (AtCLH1 and AtCLH2) in (i) controls (ii) ANE and (iii) ethyl acetate subfraction treated plants (inset)
The ability of ANE and its ethyl acetate fraction to retain the chlorophyll (or reduce degradation) was correlated with the reduced expression of two chlorophyllase genes AtCLH1 and AtCLH2 during post-freezing recovery (Fig. 4).
Quantification of freezing damage by viability staining
ANE and its ethyl acetate fraction imparted greater tolerance against freezing-induced tissue damage in A. thaliana. The area of damaged tissue was significantly lower in treated plants as compared to water controls (Fig. 5a). At −3.5°C, 94% of the leaf area of control plants exhibited tissue damage, compared to 25 and 17% in ANE and ethyl acetate fraction, respectively. The percentage area of tissue damage was 49 and 35%, respectively, for the ANE and ethyl acetate fractions at a temperature of −4.5°C, whereas it was 97% for control plants (Fig. 5b). These results are in agreement with the electrolyte leakage assay used to quantify the tissue damage at different temperature treatments.
Ascophyllum nodosum reduced freezing-induced leaf damage in Arabidopsis thaliana. a The extent of freezing-induced tissue damage in controls and treated plants as revealed by trypan blue staining. b Comparison of the area of tissue damage in the trypan blue-stained leaves using the image processing and analysis software Image-j®. Each value represents the average of 15 replicates. Bars represent standard error
Light microscopy
The histological observations of plastic-embedded leaf sections under the light microscope revealed that ANE and its organic fractions imparted higher tolerance against freezing injury at the cellular level (Fig. 6b). At −3.5°C, the control plants displayed severe cellular injury and tissue damage. The cell structure was completely disrupted and no cell layers could be clearly differentiated. In addition, the vascular tissue was completely degenerated, the cytosolic contents contracted, and cells flattened (Fig. 6b). In contrast, plants treated with ANE or its ethyl acetate subfraction exhibited less cellular disruption or tissue disorganization as compared to water controls. The mesophyll cells in the leaf sections of treated plants showed cell enlargement as compared to untreated controls (Fig. 6b, Table 1). Consequently, control plants had a maximum number of cells per microscopic field (55 ± 1.75) as compared to ANE (18 ± 0.68) and its ethyl acetate fraction (27 ± 1.02) (Table 1). Increased cell density in control plants was expected due to freezing-induced ion leakage which resulted in cell contraction (Fig. 6b).
Ascophyllum nodosum protects cellular structure during freezing stress in Arabidopsis thaliana. a Position and orientation of leaf sections sampled from Arabidopsis leaves for histological studies. b Comparison of freezing-induced tissue damage in the stained plastic embedded leaf sections from (i) control plants or treated with (ii) ANE (1.0 g L−1) or (iii) ethyl acetate subfraction (1.0 g L−1). c Fluorescence images of sections stained with Nile red showing membrane integrity in plants treated with (v) ANE (1.0 g L−1) or (vi) ethyl acetate subfraction (1.0 g L−1) as compared to (iv) water controls. Magnification Bar 10 μm
Nile red staining of leaf sections revealed that the plants treated with ANE or its ethyl acetate fraction maintained the integrity of plasma membrane and tissue organization compared to control plants (Fig. 6c). In ethyl acetate fraction, the lipid bilayer was intact both in plasmalemma as well as the chloroplast membrane. ANE-treated plants showed less tissue degradation, whereas control plants displayed complete degradation (Fig. 6c).
Quantification of the pectin methyl esterase (PME) enzymatic activity
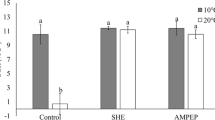
The gel diffusion assay for the quantification of PME activity showed no difference between treated and control plants (Supplementary Figure 1). This result indicated that the cell enlargement in treated plants during the post freezing recovery period (after a freezing treatment of −2.5°C for 24 h) may be the result of cell wall elongation through a PME independent route.
Molecular analysis of A. nodosum mediated freezing tolerance
The expression of key candidate genes known to be associated with the freezing tolerance of A. thaliana was analyzed. The expression of the low-temperature stress marker genes COR15A (Lin and Thomashow 1992), COR78/RD29A (Yamaguchi-Shinozaki and Shinozaki 1993), transcription factor DREB1A/CBF3 (C-repeat Binding Factor 3) (Stockinger et al. 1997), negative regulator ESK1 (Eskimo 1) (Xin and Browse 1998) and a biosynthetic gene FAD8 (Fatty Acid Desaturase 8) (McConn et al. 1994) were investigated.
A 1.8-fold induction of the transcription factor, CBF3 was observed in ethyl acetate treated plants at −2°C and a two-fold increase was observed at thawing (Fig. 7a). The COR genes, RD29A, and COR15A exhibited a 2.63- and 1.96-fold increase (respectively) in the transcript level at −2°C as revealed by real-time PCR studies (Fig. 7b, c). During the post freezing recovery period, RD29A showed a decreasing trend in treated plants as compared to control, whereas there was no significant difference in the expression of COR15A between treated and control plants (Fig. 7b, c). Additionally, semi-quantitative PCR analysis revealed that the expression of the negative regulator, ESK1, and the fatty acid desaturase gene FAD8 were not affected by treatment with ANE and its ethyl acetate fraction (Fig. 7d).
Discussion
The results presented in this paper show that extracts of A. nodosum impart significant tolerance to freezing stress in A. thaliana. The treatments were given as root irrigation which caused an enhancement of tolerance at the above ground parts. The results suggests that treatment with ANE and/or its organic subfractions induce specific systemic physiological responses leading to improved freezing tolerance at the whole plant level. The plants grown in Petri dishes survived lower temperatures as compared to the plants grown in peat pellets. This may be attributed to differences in the rate of ice nucleation among the plants grown in the in vitro and in vivo conditions.
Chlorophyll degradation during the post freezing recovery process is one of the morphological markers of freezing-induced tissue damage in plants (Huner et al. 1993). Freezing-induced chlorophyll damage was significantly lower in ANE-treated plants as compared to controls. ANE-treated plants exhibited three-fold higher chlorophyll content as compared to control plants. ANE-induced chlorophyll retention might be, atleast in part, due to the reduction in the activity of chlorophyllases, a group of enzymes involved in chlorophyll degradation during leaf senescence. Biotic stresses such as pathogen attack and abiotic stress such as salinity, low temperature and excess light trigger chlorophyllases (Karpinski et al. 2003; Kariola et al. 2005). Our studies on the expression of the two chlorophyllases AtCLH1 and AtCLH2 revealed that ANE and lipophylic components of A. nodosum affected the expression of these genes (Fig. 4).
A number of gene products, such as COR15A (Lin and Thomashow 1992; Nakayama et al. 2007) or their modified polypeptides have been shown to protect chloroplast during low-temperature stress. Our gene expression studies showed significant and sustained induction of COR15A transcripts in ANE and ethyl acetate treated plants during freezing stress. Arabidopsis COR15A encodes a chloroplast stromal protein that shows cryoprotective activity (Nakayama et al. 2007). It protects freeze-labile enzymes in the chloroplast stroma and is capable of associating with chloroplast membranes, thus imparting protection against low-or-freezing-temperature stresses (Steponkus et al. 1998; Nakayama et al. 2007). Taken together, ANE and its organic subfractions improve chlorophyll retention by, at least in part, a reduction in stress-induced chlorophyllase activity and induction of COR15A during low-or-freezing-temperature stresses.
Histological observations of stained leaf sections under the light microscope revealed that ANE and its ethyl acetae subfractions preserved cellular integrity under freezing stress. Moreover, treated plants exhibited a significant cell enlargement, which may be due to cellular adaptation in response to rehydration and cell expansion during the recovery phase. In higher plants, the cell wall has a pivotal role in cell expansion and enlargement. Pectin methyl esterases (PMEs) are the group of enzymes which regulate the plasticity of cell wall in higher plants. As a result, they are capable of affecting the plant cell wall response during abiotic stresses. However, the gel diffusion assay for quantification of PME activity exhibited no difference in activity between ANE-treated and control plants (Supplementary Figure 1). This indicates that the cell enlargement in ANE-treated plants during the post freezing recovery period may be the result of a PME-independent mechanism.
Genetic studies suggest that cold acclimation and subsequent freezing tolerance is a multigenic, quantitative trait with additive effects (Thomashow 1994). Freezing temperatures activate a number of cold-inducible genes which code for dehydrins, lipid transfer proteins, translation elongation factors, and the late-embryogenesis-abundant proteins (Jones and Inouye 1994; Nishida and Murata 1996). The largest class of cold-inducible genes that have been correlated with tolerance to freezing stress are the ‘Cold Responsive’ or COR genes. Arabidopsis COR genes that are required for freezing-tolerance responses include: COR15A, COR78/RD29, COR47, and COR6.6 (Thomashow 1999). Induction of COR genes is mediated by a transcription factor known as the CRT/DRE Binding Factor (CBF) (Stockinger et al. 1997). CBF binds to the CRT/DRE elements in the promoter of COR genes and initiates their transcription. ESK1, a negative regulator independent of the CBF regulon also has a major role in freezing tolerance of Arabidopsis. ESK1 acts as a switch and controls the transcript levels of many downstream cold-regulated genes (Xin and Browse 1998). ANE and its ethyl acetate subfraction affected the expression of key stress marker genes. RD29A, a key regulator for drought, salinity and low temperature, and CBF, a critical transcription factor for low-temperature tolerance, showed increased levels of expression in ANE and ethyl acetate treated plants. Realtime PCR analysis revealed that ANE and ethyl acetate fractions induced significant levels (two-fold increase over control) of CBF3 expression in treated plants during freezing stress (−2°C) and the post-freezing thawing period (Fig. 7). Induction of CBF3 leads to the activation of COR genes, like RD29A, COR15A, a gene family involved in low-temperature tolerance (Stockinger et al. 1997). Thus, activation of CBF3 and its target gene RD29A by ANE and its organic subfractions could have resulted in the active induction of a series of downstream genes which in turn imparted freezing tolerance to extract treated plants.
The CBF-independent negative regulator, ESK1 (Xin and Browse 1998) was not involved in the ANE-induced freezing tolerance. Treatments that activate the CBF regulon may not influence esk pathway (Xin and Browse 1998). Additionally, the expression of the fatty acid desaturase gene FAD8 was not affected by ANE treatment. FAD is a polygenic family with at least eight genes (FAD1–8) that are actively cross-linked in fatty acid desaturation of lipid bilayers (Upchurch 2008) and some member in this polygenic family other than FAD8 may be active and responsible for lipid unsaturation in ANE-induced freezing tolerance in Arabidopsis.
Discovery of chemical compounds that induce freezing tolerance in crop plants would be beneficial to agriculture, especially in the northern hemisphere. However, there has been only limited success in the identification of chemical factors that impart freezing tolerance. For example, application of choline chloride in wheat seedlings (Horváth and Van Hasselt 1985) and ethanolamine in tomato seedlings (Ilker et al. 1976) have been shown to improve freezing tolerance and recovery from freezing injury (Ilker et al. 1976; Horváth and Van Hasselt 1985). However, the effect of these chemicals on animal and human health and the environment is not clear; therefore, the use of natural compounds and extracts from safe sources like A. nodosum would be ideal. The results presented in this paper show that extracts of A. nodosum induce specific systemic physiological responses resulting in significant improvement of tolerance to low temperature. The 1H NMR spectra, fatty acid, and sterol analysis of the bioactive organic fraction of ANE showed that it is dominated by lipophilic components and that these components represented ~40% (w/w) of the bioactive organic fraction. These components have not been previously reported in the literature to elicit freezing tolerance when directly applied to the plants. Further studies are needed to identify the chemical components in the organic fractions that elicit freezing tolerance.
Abbreviations
- NMR:
-
Nuclear magnetic resonance
- FYM:
-
Farmyard manure
- COR:
-
Cold responsive
- ANE:
-
Ascophyllum nodosum extract
- TMS:
-
Tetramethylsilane
- NIH:
-
National Institutes of Health
- PME:
-
Pectin methyl esterase
- CBF:
-
CRT/DRE binding factor
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts; polyphenoloxidse in Beta vulgaris. Plant Physiol 24:1–15
Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G (2003) Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421:268–272
Blunden G (1991) Agricultural uses of seaweeds and seaweed extracts. In: Guiry MD, Blunden G (eds) Seaweed resources in Europe: uses and potential. Wiley, Chichester, pp 65–81
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Biologists, Rockville, pp 1158–1249
Burchett S, Fuller MP, Jellings AJ (1998) Application of seaweed extract improves winter hardiness of winter barley cv Igri. Abstracts—plant cell topics. York Meeting
Crouch I, Van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Downie B, Dirk LMA, Hadfield KA, Wilkins TA, Bennett AB, Bradford KJ (1998) A gel diffusion assay for quantification of pectin methylesterase activity. Anal Biochem 264:149–157
Featonby-Smith BC, van-Staden J (1983) The effect of seaweed concentrate on the growth of tomatoes in nematode infested soil. Scient Hort 20:137–146
Gilmour SJ, Hajela RK, Thomashow MF (1988) Cold acclimation in Arabidopsis thaliana. Plant Physiol 87:745–750
Guy CL (2003) Freezing tolerance of plants: current understanding and selected emerging concepts. Can J Bot 81:1216–1223
Horváth I, Van Hasselt PR (1985) Inhibition of chilling-induced photooxidative damage to leaves of Cucumis sativus L. by treatment with amino alcohols. Planta 164:83–88
Huner NPA, Oquist G, Hurry VM, Krol M, Falk S, Griffith M (1993) Photosynthesis, photoinhibition and low temperature acclimation tolerant plants in cold. Photosynth Res 37:19–39
Ilker R, Warring AJ, Lyons JM, Breidenbach RW (1976) The cytological responses of tomato seedling cotyledons to chilling and the influence of membrane modifications upon these responses. Protoplasma 90:229–252
Jones PG, Inouye M (1994) The cold shock response: a hot topic. Mol Microbiol 11:811–818
Kamal-Eldin A, Määttä K, Toivo J, Lampi A-M, Piironen V (1998) Acid-catalyzed isomerization of fucosterol and Δ5-avenasterol. Lipids 33:1073–1077
Kariola T, Brader G, Li J, Palva ET (2005) Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17:282–294
Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM (2003) Light perception in plant disease defense signalling. Curr Opin Plant Biol 6:390–396
Lin C, Thomashow MF (1992) A cold-regulated Arabidopsis gene encodes a polypeptide having potent cryoprotective activity. Biochem Biophys Res Commun 183:1103–1108
McConn M, Hugly S, Browse J, Somerville C (1994) A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. Plant Physiol 106:1609–1614
Metting B, Zimmerman WJ, Crouch T, van-Staden J (1990) Agronomic uses of seaweed and microalgae. In: Akatsuka I (ed) Introduction of applied phycology. SPB Academic, Hague, pp 589–627
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nabati DA (1991) Response of two grass species to plant growth regulators, fertilizer N, chelated Fe, salinity and water stress. Ph.D. dissertation. Virginia Polytechnic Institute and State University, Blacksburg
Nabati DA, Schmidt RE, Parrish DJ (1994) Alleviation of salinity stress in Kentucky bluegrass by plant growth regulators and iron. Crop Sci 34:198–202
Nakayama K, Okawa K, Kakizaki T, Honma T, Itoh H, Inaba T (2007) Arabidopsis COR15A is a chloroplast stromal protein that has cryoprotective activity and forms oligomers. Plant Physiol 144:513–523
Nishida I, Murata N (1996) Chilling sensitivity in plants and Cyanobacteria: the crucial contribution of membrane lipid. Annu Rev Plant Physiol Plant Mol Biol 47:541–568
Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11:1695–1708
Sharma P, Sharma N, Deswal R (2005) The molecular biology of the low-temperature response in plants. Bioessays 27:1048–1059
Spurlock BO, Skinner MS, Kattine AA (1966) A simple rapid method for staining epoxy- embedded specimens for light microscopy with the polychromatic stain paragon-1301. Am J Clin Path 46:252
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31
Steponkus PL, Uemura M, Joseph RA, Gilmour SJ, Thomashow MF (1998) Mode of action of the COR15A gene on the freezing tolerance of Arabidopsis thaliana. Proc Natl Acad Sci USA 95:14570–14575
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcription activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94:1035–1040
Temple WD, Bomke AA (1989) Effects of Kelp (Macrocystis integrifolia and Eklonia maxima) foliar applications on bean crop growth. Plant Soil 117:85–92
Thomashow MF (1994) Arabidopsis thaliana as a model for studying mechanisms of plant cold tolerance. In: Meyerowitz E, Somerville C (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, New York, pp 807–834
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30:967–977
Wilson S (2001) Frost management in cool climate vineyards. In: University of Tasmania Research Report—UT 99/1. Grape and Wine Research and Development Corporation
Xin Z, Browse B (1998) Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc Natl Acad USA 95:7799–7804
Yamaguchi-Shinozaki K, Shinozaki K (1993) Arabidopsis DNA encoding two desiccation-responsive rd29 genes. Plant Physiol 101:1119–1120
Yan J (1993) Influence of plant growth regulators on turfgrass polar lipid composition, tolerance to drought and salinity stresses, and nutrient efficiency. Ph.D. dissertation. Virginia Polytechnic Institute and State University, Blacksburg
Acknowledgments
BP’s lab is supported by the grants from Atlantic Canada Opportunities Agency (ACOA), Natural Sciences and Engineering Research Council of Canada (NSERC), Nova Scotia Department of Agriculture & Marketing (NSDAF) and Acadian Seaplants Limited. PR is grateful to Kalyani Prithiviraj, Nova Scotia Agricultural College for her help with real time PCR experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2009_920_MOESM1_ESM.tif
Supplementary Figure 1. Pectin methyl esterase activity in plants treated with ANE (1.0 g L-1) orethyl acetate sub-fractions (1.0 g L-1) and untreated controls. (TIFF 6357 kb)
Rights and permissions
About this article
Cite this article
Rayirath, P., Benkel, B., Mark Hodges, D. et al. Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana . Planta 230, 135–147 (2009). https://doi.org/10.1007/s00425-009-0920-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0920-8