Abstract
Application of phosphatic (P) fertilizers and biosolids is known to enhance cadmium (Cd) contamination in saline soils. Increased concentration of dissolved chloride (Cl−) in soil solution significantly influences Cd bioavailability in P fertilizer- or biosolid-amended soils. Arbuscular mycorrhizal (AM) fungi have an ability to protect plants against salinity and heavy metals by mediating interactions between toxic ions and plant roots. The effects of Glomus mosseae (AM) and NaCl and Cd stresses on Cd uptake and osmolyte and phytochelatin (PCs) synthesis in Cajanus cajan (L.) Millsp. (pigeonpea) were studied under greenhouse conditions. Two genotypes [Sel 85 N (tolerant) and ICP 13997 (sensitive)] were subjected to NaCl (4 and 6 dS m−1) and Cd (CdCl2, 25 and 50 mg kg−1 dry soil) treatments. NaCl and Cd applied individually as well as in combination caused dramatic reductions in plant biomass and induced membrane peroxidation, ionic perturbations, and metabolite synthesis in both genotypes, although Sel 85 N was less affected than ICP 13997. Cadmium uptake was enhanced when NaCl was added along with Cd. The protection of growth in Sel 85 N was associated with restricted accumulation of Na+, Cl−, and Cd2+ and higher concentrations of stress metabolites (sugars, proteins, free amino acids, proline, glycine betaine). Cd led to a significant increase in biothiols (NP-SH) and glutathione (GSH), with a larger pool of NP-SH which strongly induced accumulation of phytochelatins, whereas no significant effects in their concentrations were detectable under NaCl stress. The interactive effects of NaCl and Cd on all parameters were larger than those of individual treatments. Fungal inoculations improved plant growth and reduced accumulation of toxic ions. Higher stress metabolite synthesis and PCs observed in AM plants of Sel 85 N indicated the role of an efficient AM symbiosis capable of attenuating NaCl and Cd stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental deterioration has become an increasing threat to sustainable crop production (Munns 2005; Katerji and others 2009). Abiotic stresses such as soil salinization and heavy-metal contamination are worldwide agricultural and ecoenvironmental problems posing a serious threat to global agricultural production (Gabrijel and others 2009; Grewal 2010). More than 800 million hectares of land throughout the world are salt-affected (Munns and Tester 2008), which is equal to more than 6% of the world’s total land area (FAO 2008). In agricultural soils degraded by salinity, due to inappropriate water quality used for irrigation, phosphatic (P) fertilizers, sewage sludge, and biosolids are frequently applied to improve soil quality. Biosolids are the organic materials resulting from the sewage treatment process and a significant source of nutrients (nitrogen, phosphorus) that are used especially as fertilizer or soil amendment to sustainably improve and stimulate growth of a number crops (Weggler-Beaton and others 2000). However, biosolids are known to be rich in sodium chloride (NaCl) and also contain heavy metals such as cadmium (Gabrijel and others 2009). Soil salinity has been shown to significantly enhance the mobility and bioavailability of Cd in soils and, thus, causes increased Cd uptake and concentrations in crops grown on P fertilizer or biosolid-borne soils (Shafi and others 2009; Raziuddin and others 2011). Even though salinity–heavy-metal interactions are not mechanistically fully understood, it has been shown previously that Cd sorption to soil constituents is reduced by the application of Cl−, and this effect has been ascribed to the formation of soluble Cd–Cl (cadmium-chloro) complexes (Gabrijel and others 2009). Chloro-complexation of Cd and the resulting improved diffusion of Cd through soil to the plant roots and possibly uptake of Cd–Cl complexes are suspected to be the reasons for the Cl− effect on Cd uptake (Lopez-Chuken and others 2010). Therefore, in many arid and semiarid regions, it may be presumed that saline water irrigation containing high levels of chloride might aggravate heavy-metal pollution problems resulting in enhanced Cd uptake by crops.
Both Cd and salt stress can pose several problems for plant growth and development as well as inhibit leaf photosynthesis (Shafi and others 2010; Raziuddin and others 2011). Cadmium becomes toxic to plants through irreversible changes to protein conformation by forming metal thiolate bonds, and through alteration of cell wall and membrane permeability (Zeng and others 2009). Furthermore, both stresses in combination cause higher plasma membrane permeability and elicit lipid peroxidation (Muhling and Lauchli 2003), which can culminate in poor growth and low biomass production (Monteiro and others 2009; Zhou and others 2010).
The biochemical pathways involved in these processes offer a battery of biomarkers that provide mechanistic end points of toxicity. Relating these end points to traditional responses (growth) can help in understanding the overall process of stress-induced senescence and the protection strategies adopted by plants against stresses (Monteiro and others 2009). One important physiologic and basic strategy employed by higher plants to resist abiotic stresses is the adjustment of osmosis through production and subsequent transportation of organic osmoprotectants in plant cells (Xu and others 2010). Many plant species accumulate significant amounts of these stress metabolites such as proline, glycine betaine, total soluble sugars, and free amino acids in response to stress conditions (Garg and Manchanda 2009). Plants cope with cellular damage of toxic metals by complexation with phytochelatins (PCs), which has been identified as an important mechanism for detoxifying toxic metals such as Cd in various plant species (Vazquez and others 2009). Phytochelatins form complexes with metals through their cysteine sulfhydryl groups and these metal complexes are subsequently compartmentalized in the vacuole (Vazquez and others 2009; Garg and Aggarwal 2011). These stress metabolites play a crucial role in osmoregulation under stress, but their relative contribution varies among species, genotypes, and even between different organs within the same plant (Siddiqui and others 2009). The combined effect of NaCl and Cd is not simply an additive influence, yet only a few researchers have tried to elucidate their effects on plant growth and development. There are a few reports on osmolyte accumulation under individual NaCl and Cd stresses, however, little is reported about the interactive influences between salinity and Cd stresses on osmolyte and PC synthesis. To fill in this gap, the relative contributions of increasing NaCl concentrations in the soil solution to Cd uptake and allocation in different plant organs were studied in Cajanus cajan (L.) Millsp. (pigeonpea) genotypes.
AM symbiosis occurs in almost all habitats, including disturbed soils, and aids in ecosystem restoration (Khade and Adholeya 2007; Miransari 2010). The AM fungi most commonly observed in agroecosystems and disturbed soils are Glomus sp., with G. mosseae the most efficient fungus in terms of plant performance and protection offered against the detrimental effects of soil stresses (Zhang and others 2006; Porras-Soriano and others 2009). AM fungi can alleviate the unfavorable effects of stresses such as heavy metals, salinity, and drought on plant growth due to the formation of extensive hyphal networks (Garg and Chandel 2010). AM symbioses reduce oxidative damage and bring stress tolerance under salinity and Cd stress (Azcon and others 2009; Wu and others 2010). Legume crops are sensitive to NaCl and Cd stresses as compared to cereals and forage grasses and frequently encounter strong inhibition of biomass production (Grewal 2010). However, reports on the interactions between AM fungi and NaCl and Cd stresses in combination in legume species are lacking. Therefore, it is important to investigate the effect of AM inoculation on osmolyte synthesis and PC accumulation under combined stresses of NaCl and Cd which might offer potential knowledge for improving stress tolerance.
The study was conducted to assess whether mycorrhizal colonization might help plants better adapt to soil salinity and Cd stress by regulating metal uptake in their tissues through PC complexation and relatively higher osmolyte synthesis than non-AM plants.
Pigeonpea (Cajanus cajan [L.] Millsp.) is a major pulse crop of the semiarid tropics that is traditionally cultivated as an annual crop in Africa, Asia, and Australia (Sairam and others 2009). The crop represents about 5% of world legume production (Hillocks and others 2000), with about 90% being produced in India (Odeny 2007). Due to its deep rooting habit, pigeonpea offers little competition to other crops and is therefore much used in intercropping systems with cereals such as maize, sorghum, millet, or other legumes. This crop shows genetic diversity and has an advantage over other plant species because it can also respond to AM fungi along with soil-borne nitrogen-fixing rhizobacteria simultaneously. Both microorganisms are active in root cortical cells but do not seem to compete for infection sites. Thus, growth and productivity of pigeonpea are dependent on the synergistic interactions of microsymbionts, that is, AM fungi and rhizobia (Franzini and others 2009). Moreover, under saline and heavy-metal conditions, enhancement of growth in legume plants has been related partly to mycorrhiza-mediated enhancement of mineral nutrition (Kaya and others 2009) and decreased uptake of Na and Cd (Garg and Aggarwal 2011; Manchanda and Garg 2011).
Despite extensive research on the various mechanisms conferring tolerance to NaCl and Cd stresses, little is known about the role of soil microsymbionts such as AM in ensuring survival of pigeonpea under these stresses. In this study, one tolerant and one sensitive genotype of Cajanus cajan (L.) Millsp. (pigeonpea) colonized by Glomus mosseae were used to study their response to growth, cadmium uptake, and osmolyte and PC synthesis under NaCl and Cd toxicities.
Material and Methods
Plant Growth/Cultivation Conditions
The experiments were conducted in a greenhouse from mid-June to October 2010 in the Department of Botany, Panjab University, Chandigarh, India (30.5°N, 76.5°E and elevation 305–366 m). The minimum temperature was 22°C (night) and maximum temperature 37°C (day). The relative humidity varied between 55 and 92% in the morning and between 42 and 81% in the afternoon.
Plant Material and Symbiotic Inoculants (Rhizobial and Mycorrhizal)
Two genotypes of pigeonpea, tolerant (Sel 85 N) and sensitive (ICP 13997) to both NaCl and Cd stresses, were used as experimental plants. The seeds were washed with water and surface sterilized by dipping in 15% H2O2 solution for 8 min, then washed several times with deionized water to remove any traces of chemical that could interfere in seed germination. Seeds were pretreated with specific rhizobial inoculum of Sinorhizobium fredii AR-4 and were kept at room temperature for drying. Seeds were also inoculated with fungal inoculum of Glomus mosseae, placed at 15-cm pot depth prior to sowing of seeds into the pots to facilitate fungal colonization of plant roots (approximately 300 spores/100 g of soil). Rhizobial and mycorrhizal inocula were obtained from the Division of Microbiology, Indian Agricultural Research Institute (IARI), New Delhi, India.
Experimental Design and Treatments
The soil (mixture of sand and loam in a 1:1 ratio) was obtained from the nearby agricultural fields. It was fumigated thoroughly with 0.1% formaldehyde under air-tight plastic sheets for 5 days and the fumigant allowed to dissipate for 10 days (Giri and others 2007). Three plants were maintained in each pot containing 7 kg of soil mixture, with pH 7.2, electrical conductivity (ECe) of 1.0 dS m−1, organic carbon content of 0.68% (Walkley 1947), total N content of 0.42% (Nelson and Sommers 1973), 20 ppm of P (Chapman and Pratt 1961) and 3.9 mg Cd kg−1 dry soil (Ouzounidou and others 1992). Fifteen-day-old plants were exposed to salt and Cd treatments. The experimental layout was a 3 × 3 × 2 complete factorial combination comprising three saline treatments [1.0, 4, and 6 dS m−1 ECe corresponding to 0, 40, and 60 mM NaCl, respectively (Richards 1954)] and three Cd treatments (applied as CdCl2 at 0, 25, and 50 mg Cd kg−1 of dry soil), individually as well as in combinations, and two mycorrhizal inoculations (with and without treatments). Each treatment was replicated six times in a randomized block design. Roots and leaves from control and treated plants were harvested 60 days after treatment (DAT) for analysis of various physiological and biochemical parameters.
Measurements and Analysis
Determination of Biomass
Harvested plants were divided into shoots and roots, oven-dried at 70°C for 72 h until they attained constant weight, and weighed. Dried samples were ground to powder and stored for mineral analysis.
Arbuscular Mycorrhizal (AM) Colonization
Arbuscular mycorrhizal (AM) colonization was estimated by using the modified Phillips and Hayman (1970) procedure. The roots were cut into small pieces of approximately 1.0 cm which were then dipped in KOH solution for 24 h, placed in HCl solution for 15–30 min for neutralization, and then stained with glycerol-trypan blue solution (0.05%) for 24–36 h. Each root piece was examined under a compound light microscope for AM colonization, and data regarding the presence and/or absence of mycorrhizal structures, that is, arbuscules/vesicles, were recorded and the percentage occurrence of these structures was calculated as follows:
Electrolyte Leakage (Relative Membrane Permeability)
The electrolyte leakage was determined to assess membrane permeability as described by Zwiazek and Blake (1991). The plant samples were washed with deionized water to remove surface contamination. A total of 2.5 g of fresh plant material was placed in 25 ml of deionized water for 10 min and, subsequently, the electrolytic conductivity of the bathing solution was measured with a conductivity meter. The plant material was then heated to boiling. The bathing solution was cooled to room temperature, and electrolytic conductivity was measured again. The electrolyte leakage was calculated according to the following equation:
Lipid Peroxidation (MDA Content)
Lipid peroxidation was estimated by measuring the formation of malondialdehyde (MDA) with the thiobarbituric acid (TBA) reaction as described by Heath and Packer (1968). Plant samples (0.4 g) were homogenized with 2 ml of 5% trichloroacetic acid (TCA) and centrifuged at 10,000×g for 15 min. After centrifugation, 1 ml of supernatant was mixed with 1 ml of 0.5% TBA in 20% TCA and incubated in a boiling water bath for 30 min. Thereafter, it was immediately cooled on ice to stop the reaction and centrifuged at 10,000×g for 5 min. The absorbance at 532 and 600 nm was determined, and MDA concentration was estimated by subtracting the nonspecific absorption at 600 nm from the absorption at 532 nm, using an absorbance coefficient of extinction of 156 mM−1 cm−1.
Ion Determinations
Sodium (Na + ) Sodium content was estimated by flame photometry using the method of Chapman and Pratt (1961). Ten milliliters of acid mixture consisting of nitric acid, sulfuric acid, and perchloric acid (9:4:1) was added to 2–5 g of ground plant samples and kept at 120°C overnight. The samples were then maintained at 70°C on a hot plate for 30 min; the temperature was increased to 120°C for 30 min and finally to 250°C until 3–4 ml of sample was left. The final volume of 50 ml was maintained with deionized water and samples were left overnight. The next day they were filtered using filter paper (Whatman No. 1), and sodium content was estimated on a flame photometer. A blank was run without plant samples.
Chloride (Cl −) Plant samples of equal mass were extracted with 1 N HNO3 (mol l−1) as described by Storey (1995) and Cl− was determined by a modified method of silver titration as described by Chen and others (2001). Excess AgNO3 solution (0.025 N) was used to precipitate chloride of aqueous extracts and excess Ag+ was estimated by NH4SCN titration. NH4Fe(SO4)2 was used as a color indicator for isoionic point determination. Chloride concentration was calculated using the formula:
where N AgNO3 and N NH4SCN are the concentrations (mol l−1) of AgNO3 and NH4SCN solutions, DW (g) is the dry weight of plant tissue, V 1 (ml) is the total volume of AgNO3 solution in Cl– extracts, and V 2 (ml) is the total volume of NH4SCN solution used for excess Ag+ precipitation.
Cadmium (Cd 2+ ) After harvest, roots and leaves were thoroughly washed with deionized water. The root samples were then immersed in 20 mM ethylenediaminetetraacetic acid (EDTA) solution for 10 min to remove adsorbed metal on the surfaces, rewashed with deionized water, and dried in an oven (Lin and others 2007). Cd content was determined in plant material (250 mg) by the method of Ouzounidou and others (1992). Dried soil samples and plant material were wet digested in a HNO3–HClO4 acid mixture (5:2 v/v) at 125–135°C for 6 h (AOAC 1990). Cd content was determined by atomic absorption spectrophotometry (AAS, Hitachi 1800-type spectrophotometer) and expressed on a dry weight basis.
Chlorophyll Determination
Leaf chlorophyll concentration (Chl a and b) was measured as described by Hiscox and Israelstam (1979). Leaves were cut into uniform discs (100 mg), suspended in 10 ml of dimethyl sulfoxide (DMSO), and then incubated at 65°C for 1 h. The extinction value of chlorophyll was measured at dual wavelengths of 645 and 663 nm on a Double Beam UV-190 spectrophotometer (Labnics Equipment) using DMSO as a blank. The amount of total chlorophyll was calculated from the extinction coefficients following the equations of Arnon (1949):
Relative Water Content (RWC)
RWC of fully expanded leaves (0.5 g) from the top of the plant was used to assess the relative tolerance of mycorrhizal and nonmycorrhizal plants to stress using the following equation (Schonfeld and others 1988):
where FW is leaf fresh weight, DW is leaf dry weight after 24 h at 70°C, and TW is leaf turgid weight after submergence in deionized water for 4 h.
Stress Metabolites
Total soluble sugars (TSS) The sugars were estimated by the method of Irigoyen and others (1992). Five hundred milligram of freshly harvested plant samples was crushed in 5 ml of 95% (v/v) ethanol. The insoluble fraction of the extract was washed twice with 5 ml of 70% ethanol. All soluble fractions were centrifuged at 3,500×g for 10 min. TSS were analyzed by reacting 0.1 ml of the alcoholic extract with 3 ml of freshly prepared anthrone (150 mg anthrone + 100 ml 72% [w/v] H2SO4) and placing the solution in a boiling water bath for 10 min. After cooling, the absorbance at 625 nm was determined on a Double Beam UV-190 spectrophotometer (Labnics Equipment). A standard curve was prepared using graded concentrations of glucose.
Total proteins The total proteins were determined using the Bio-Rad assay (Bradford 1976). One hundred milligram of plant samples was homogenized in 5 ml of phosphate buffer (pH 7.0). The extract was centrifuged at 15,000×g for 30 min. The residue was re-extracted with 3 ml of phosphate buffer and the supernatants were pooled. The above extract (0.1 ml) was added to 5 ml of protein reagent and the contents were mixed thoroughly. The absorbance was taken at 595 nm against a reagent blank prepared from 0.1 ml of appropriate buffer and 5 ml of protein reagent. The protein concentration was quantified using bovine serum albumin (BSA) as a standard.
Free amino acids The method of Lee and Takahashi (1966) was adopted for the estimation of total free amino acids. Dried plant samples (100 mg) were homogenized in 5 ml of 80% ethanol, refluxed for 15 min on a steam bath, and centrifuged. The residue was further refluxed twice with 80% ethanol. The supernatants were pooled together for free amino acid estimations. The extract (0.2 ml) was added to 3.8 ml of ninhydrin reagent. The contents were heated in a boiling water bath for 12 min, cooled to room temperature, and spectrophotometrically analyzed. The purplish blue color was read at 570 nm. The quantity of total free amino acids was calculated from the reference curve prepared by using glycine (5–50 mg) and expressed as mg amino acids per mg tissue dry weight.
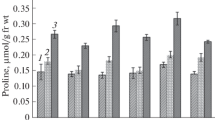
Proline content Free proline content was determined following the method of Bates and others (1973). Proline estimation is based on the formation of a brick-red-colored proline–ninhydrin complex in acidic medium. Plant samples (0.5 g) were homogenized in 5 ml of sulfosalicylic acid (3%) using a mortar and pestle, the homogenate was filtered, and the filtrate was used for estimation of proline content. Two milliliters of the extract was transferred to a test tube and to which 2 ml of glacial acetic acid and 2 ml of ninhydrin reagent were added, and then the preparation was heated at 100°C for 30 min. Six milliliters of toluene was added to the preparation and then transferred to a separating funnel. The chromophore-containing toluene was separated and its absorbance read at 520 nm in a Double Beam UV-190 spectrophotometer (Labnics Equipment) against a toluene blank. The concentration of proline was estimated by referring to a standard curve made from known concentrations of l-proline.
Glycine betaine content Glycine betaine estimation was carried out following the method of Greive and Grattan (1983). Betaine makes a betaine periodite complex with iodide in acidic medium, which absorbs at 360 nm in the UV range. Finely ground plant samples (0.5 g) were mechanically shaken with 20 ml of deionized water for 48 h at 25°C and the extracts were diluted 1:1 with 2 N sulfuric acid. A 0.5 ml aliquot was cooled in ice water for 1 h and cold potassium iodide–iodine reagent (0.2 ml) was added to it. The samples were stored at 0–4°C for 16 h and were centrifuged at 10,000×g for 15 min at 0°C. The supernatant was carefully aspirated. The periodite crystals were dissolved in 9 ml of 1,2-dichloroethane (reagent grade). After 2.0–2.5 h, the absorbance was measured at 365 nm with a Double Beam UV-190 spectrophotometer (Labnics Equipment). Reference standards of glycine betaine (50–200 mg/ml) were prepared in 2 N sulfuric acid and the procedure for sample estimation was followed.
Determination of Glutathione
Glutathione was estimated following the method of Anderson (1985). Fresh plant samples (0.5 g) were homogenized in 2 ml of 5% (w/v) sulfosalicylic acid at 0–4°C. The homogenate was centrifuged at 10,000×g for 10 min. To 0.5 ml of supernatant, 0.5 ml of 0.1 M (pH 7.0) reaction buffer, 0.5 mM EDTA, and 50 μl of 3 mM 5′dithio-bis-(2-nitrobenzoic acid) (DTNB) were added. After 5 min, the absorbance for determination of GSH was read at 412 nm on a Double Beam UV-190 spectrophotometer (Labnics Equipment). A standard curve was prepared from varying concentrations of reduced glutathione.
Determination of Total Nonprotein Thiols/Biothiols
Total nonprotein thiols were determined as described by Del Longo and others (1993). The supernatant (100 μl) (described above) was transferred to a microfuge tube, to which 0.5 ml of reaction buffer [0.1 M phosphate buffer (pH 7.0), 0.5 mM EDTA] and 0.5 ml of DTNB (1 mM) were added. The reaction mixture was incubated for 10 min and spectrophotometrically read at 412 nm using a Double Beam UV-190 spectrophotometer (Labnics Equipment). Values were corrected against a blank containing no extract. A standard curve was prepared from varying concentrations of cysteine to calculate the nonprotein thiol content in samples.
For theoretical determination of phytochelatins (PCs), the difference between total nonprotein thiols and GSH was considered to represent PCs (Bhargava and others 2005).
Statistical Analysis
Data presented are the mean values based on six biological repeats ± standard error (SE) per treatment. All results were subjected to one-way analysis of variance (ANOVA) using SPSS ver. 16.0 (SPSS, Inc., Chicago, IL, USA). Dunnett’s multiple-comparison test was performed at P < 0.05 on each of the significant variables measured.
Results
Biomass Production and AM Colonization
Increasing NaCl and cadmium (Cd) concentrations in the soil solution brought significant decreases in shoot and root dry matter (Table 1) of both the genotypes, with a greater reduction in ICP 13997 than in Sel 85 N. Even the control plants of the two genotypes differed substantially in growth, as plants of ICP 13997 were only about half the size of Sel 85 N (Table 1). The results clearly indicated that the combination of two stresses (NaCl + Cd) led to a further decline in these growth traits. It was observed that there was a much greater reduction in root dry weight (RDW) than in shoot dry weight (SDW) with increase in soil salinity and Cd contamination; this resulted in a decline in the root-to-shoot ratio (RSR) (Table 1). Inoculation with the AM fungus Glomus mosseae alleviated the negative effects of NaCl and conferred partial tolerance under Cd stress and salt + metal combinations in Sel 85 N. Mycorrhizal plants of Sel 85 N exhibited higher biomass accumulation than ICP 13997 under all stress treatments. Percentage AM colonization (Table 1) was significantly affected by NaCl and Cd treatments as well as by their combinations. No mycorrhizal colonization was found in the uninoculated plants. Irrespective of genotype, root colonization (Table 1) was observed under unstressed conditions and gradually decreased as the salinity and Cd stresses increased, when applied individually and in combination. Significant differences in root colonization were observed between the two genotypes, and AM colonization was much lower for ICP 13997 than for Sel 85 N.
Ionic Relations
Sodium (Na+) and Chloride (Cl–) Concentrations
A clear demarcation between the two genotypes in their tolerance and sensitivity was evident, even under control conditions. ICP 13997 was sensitive to stresses, as characterized by high accumulation of Na+, Cl–, and Cd2+ (Table 2), which was significantly accentuated by salinity and Cd treatments. The interactive effects of NaCl and Cd (NaCl × Cd) on Na+ and Cl– content were more pronounced when compared with the effects of NaCl and Cd alone. In both genotypes, roots were the most preferential site of Na+ accumulation; however, Cl– concentration was higher in leaves than in roots. Under stressed conditions, AM symbiosis notably decreased Na+ and Cl– concentrations in both genotypes, with a higher decline in Sel 85 N than ICP 3997 under both stresses applied singly and in combinations.
Cadmium (Cd) Accumulation
A proportional increase in Cd uptake was observed in pigeonpea plants in an organ-, genotype-, and dose-dependent manner (Table 2). Increased concentrations of Cl− ions in the soil solution led to mobilization of Cd and increased accumulation Cd in the roots but not its translocation from roots to leaves. Significant relationships between the presence of Cl− ions in the soil solution and Cd uptake by the plants were observed as the stress levels increased in both genotypes. Cadmium content showed a positive and significant correlation with Cl– ions in the roots (r = 0.69**, Sel 85 N; r = 0.81**, ICP 13997) and in the leaves (r = 0.51**, Sel 85 N; r = 0.79**, ICP 13997). ICP 13997 accumulated higher concentrations of Cd in the roots and in leaves than Sel 85 N. The colonization of pigeonpea plants with G. mosseae significantly reduced Cd uptake by roots and subsequent translocation into leaves under Cd and NaCl + Cd stress conditions; hence, the concentration of Cd in inoculated plants was remarkably lower than in uninoculated plants. This was more apparent in Sel 85 N than in ICP 13997.
Relative Membrane Permeability, Lipid Peroxidation, Photosynthetic Pigments, and Relative Water Content
NaCl and Cd stresses generated oxidative stress in roots and leaves (data not shown) resulting in electrolyte leakage and membrane peroxidation and reduced concentrations of major photosynthetic pigments (chlorophyll a, b, Chl a/b) and leaf hydration (Table 3) in a concentration- and genotype-dependent manner. NaCl supply had a stimulatory effect on Cd mobilization at the root–soil interface which accounted for synergistic induction of membrane damage and chlorophyll degradation when compared to NaCl or Cd treatments alone. In NaCl + Cd-exposed pigeonpea plants colonized by G. mosseae, smaller perturbations in membrane leakage and peroxidation, Chl pigment concentration, and RWC were observed. The application of AM inoculum to stressed plants reduced oxidative damage resulting in decreased membrane leakage, MDA production, and chlorophyll degradation in Sel 85 N when compared to ICP 13997 under NaCl, Cd, and NaCl + Cd treatments.
Stress Metabolites
Total Soluble Sugars (TSS), Proline, and Glycine Betaine Content
The organic solutes, (Figs. 1, 2, 3) accumulated preferentially in stressed and injured plant organs (roots) of both the genotypes and became much more apparent with increasing levels of NaCl and Cd. Concentrations of stress metabolites (TSS, proline, and glycine betaine) remarkably increased in Sel 85 N when compared with ICP 13997 under all the NaCl and Cd treatments. The additional effect of NaCl + Cd in terms of increasing TSS, proline, and glycine betaine was greater than that of NaCl and Cd individually. Mycorrhizal symbiosis helped colonized plants to attenuate the adverse effects of salt, Cd, and NaCl + Cd interactions which further enhanced the synthesis and accumulation of TSS, proline and glycine betaine in roots and leaves of Sel 85 N more than those of ICP 13997.
Effect of arbuscular mycorrhizal (AM) inoculations on total soluble sugars (µg g−1 FW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Effect of arbuscular mycorrhizal (AM) inoculations on free proline (mg g−1 FW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Effect of arbuscular mycorrhizal (AM) inoculations on glycine betaine (µg g−1 DW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Total Proteins and Free Amino Acids
The reduction of root and leaf (data not given) proteins (Fig. 4) correlated with the augmentation of salt and Cd concentrations in soil which was clearly evident in ICP 13997 compared to Sel 85 N. This was supported by a progressive and substantial increase in free amino acid content (Fig. 5) under increasing stress treatments. The combination of NaCl + Cd, compared with NaCl and Cd alone, was found to be synergistic, which further increased protein degradation resulting in accumulation of free amino acids. Mycorrhizal symbiosis led to a smaller decline in total protein content and as a result Sel 85 N had comparatively more proteins and free amino acids under salt and heavy-metal conditions.
Effect of arbuscular mycorrhizal (AM) inoculations on total proteins (µg g−1 FW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Effect of arbuscular mycorrhizal (AM) inoculations on free amino acids (mg g−1 DW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Nonprotein Thiols (NP-SH), Glutathione (GSH), and Phytochelatin (PC) Accumulation
The plants supplemented with Cd exhibited a steady and consistent increase in NP-SH, GSH, and PCs in the roots (Figs. 6, 7, 8) of Sel 85 N, indicating its intrinsic ability to tolerate cellular metal load compared with ICP 13997. Although thiols and GSH synthesis increased with the level of metal stress imposed, the increase in thiols was greater than that in GSH. The treatment of Cd strongly registered an increase in PC synthesis in roots and they were the dominant biothiols under Cd exposure. The study also revealed that levels of NP-SH, GSH, and PCs were not affected by plants supplemented with only NaCl. The NaCl + Cd treatment increased the production of NP-SH, GSH, and PCs in Sel 85 N more than treatment with either salt or Cd alone. Accumulation of thiols, GSH, and PCs in roots and leaves (data not given) of both genotypes was affected by mycorrhizal colonization. The levels of these metabolites were comparable in unstressed (control) nonmycorrhizal and mycorrhizal plants as well as in salt-stressed plants, while a considerable increase in their levels was observed in mycorrhizal plants grown in the presence of Cd alone and NaCl + Cd.
Effect of arbuscular mycorrhizal (AM) inoculations on nonprotein thiols (NPT) (nmol g−1 FW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Effect of arbuscular mycorrhizal (AM) inoculations on glutathione content (GSH) (nmol g−1 FW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Effect of arbuscular mycorrhizal (AM) inoculations on phytochelatins (PCs) (nmol g−1 FW) in roots of pigeonpea genotypes under salt (NaCl) and cadmium (Cd) stress, individually and in combination. Values are means based on six biological repeats ± standard error (SE). Asterisk denotes significant difference between exposed and control plants (P < 0.05), as determined by Dunnett’s multiple-comparison test
Discussion
Plant growth inhibition is a classical parameter commonly used in the assessment of the effects of stress on plants. Apart from being an important indicator of toxicity at an individual level, growth inhibition is a nonspecific manifestation of alterations in physiobiochemical characteristics that are produced by plants as a specific response to a particular stress (Monteiro and others 2009). The present study revealed that rising root zone salinity (NaCl) and cadmium (Cd) toxicity caused a significant depression of growth in two pigeonpea genotypes; however, the two genotypes responded differently in terms of tolerance to stress. Stress-exposed plants of ICP 13997 suffered from more severe stress effects than Sel 85 N. Plant biomass declined proportionately with increased uptake of NaCl and Cd from the soil solution into plant organs. Similar observations have been reported on chickpea and lettuce (Monteiro and others 2009; Grewal 2010). The addition of NaCl to Cd-loaded soils caused severe toxicity to ICP 13997 plants when compared to Sel 85 N, resulting in further reduction in SDW and RDW when compared with the stresses from NaCl and Cd individually. The detrimental effects of NaCl and Cd were more severe on RDW than SDW, thereby resulting in a decline in the root-to-shoot ratio (RSR). Our data agreed with that of Grewal (2010), Feng and others (2010), Shafi and others (2010), and Raziuddin and others (2011) that RDW was considerably decreased as soil stresses intensified. In the present study, colonization with the AM fungus Glomus mosseae significantly improved plant biomass production in plants under NaCl and Cd stresses as well as their combinations, indicating an enhancement of stress tolerance by mycorrhizal plants. Mycorrhizal colonization increased the fitness of the host plants and as a result growth improvement in stressed AM-inoculated plants was highly significant, with Sel 85 N being more tolerant than ICP 13997. The stimulatory effect of AM inoculation on development of plants under stresses from NaCl and Cd individually has also been observed by Sheng and others (2008), Garg and Manchanda (2009), and Hajiboland and others (2010). However, reports on interaction of the effects of AM, NaCl, and Cd in legumes were lacking. The results of the present study showed that increasing salt and Cd concentrations reduced colonization of pigeonpea plants. Reduction in mycorrhizal colonization has been reported by Porras-Soriano and others (2009), Wu and others (2010), and Garg and Aggarwal (2011).
Significantly increased tissue concentrations of sodium (Na+), chloride (Cl–), and cadmium (Cd2+) in analyzed plant material are common indicators of ionic imbalance (Aydi and others 2008) and were noted in the present study as well. Increasing salinity in combination with water deficit and ion toxicity (enhanced Na+, Cl–, and Cd2+) resulted in decreased plant growth and dry matter accumulation. A greater distribution of Na+, Cl–, and Cd2+ was observed in both organs of ICP 13997 under increasing concentrations of NaCl and Cd. Stress-induced increases in Na+, Cl–, and Cd2+ have been reported by Aydi and others (2008), Monteiro and others (2009), and Roy and others (2010). The NaCl + Cd treatments increased Na+, Cl–, and Cd2+ contents in both genotypes compared to NaCl and Cd alone due to heightened Cd solubility and increased transport of Cd within roots in the presence of Cl–, as reported by Shao and others (2008), Lopez-Chuken and others (2009), and Manousaki and Kalogerakis (2009). Our results showed that Na+, Cl−, and Cd2+ concentrations were reduced in plants inoculated with the AM fungus, G mosseae, even at high stress levels. The inoculation treatment also displayed a higher ability for accumulating Na+ and Cd2+ in roots with lesser partitioning of Na+ and Cd2+ to shoots when exposed to NaCl + Cd treatments. Giri and others (2007) and Andrade and Silveira (2008) suggested that Na+ and Cd2+ might have been retained in the intraradical AM fungal hyphae or were compartmentalized in the root cell vacuoles.
Significant increases in electrolyte leakage and MDA content and decreases in chlorophyll (Chl) content and RWC were recorded in organs under NaCl + Cd stresses. Similar results have been reported under individual NaCl and Cd stresses (Monteiro and others 2009; Sairam and others 2009; Feng and others 2010; Khan and others 2010). Synergistic effects of NaCl and Cd stresses were observed under their combined treatments. AM symbiosis improved membrane integrity, Chl a, b concentrations, Chl a/b ratio, and water status under NaCl + Cd stresses. Similar observations have been reported by Beltrano and Ronco (2008), Colla and others (2008), Andrade and others (2009), and Hajiboland and others (2010) for some plant species.
Our results showed that exposure to NaCl and Cd stresses induced the accumulation of TSS, glycine betaine, and especially proline in roots and leaves of both pigeonpea genotypes. Alterations in the contents of these metabolites were also observed in the studies of Palma and others (2009) and Hajlaoui and others (2010). The combined stresses (NaCl + Cd) caused a further increase in the concentrations of TSS, free proline, and glycine betaine compared to individual stresses, as has also been reported by Xu and others (2010). In the present study, mycorrhizal inoculation promoted the accumulation of these stress metabolites, thereby alleviating the adverse effects of NaCl and Cd stresses individually as well as in combination. Therefore, the better growth of AM-inoculated Sel 85 N plants compared to uninoculated plants exposed to stresses may be the result of increased osmolyte (TSS, proline, and glycine betaine) concentrations. The studies of Kaya and others (2009) and Garg and Manchanda (2009) reported accumulation of these metabolites in mycorrhiza-stressed plants (individual NaCl and Cd).
NaCl and Cd stresses induced protein degradation and subsequent accumulation of amino acids in roots and leaves of pigeonpea genotypes when applied individually and in combination. Decreased total protein level is a potential indicator of proteolysis, a senescence parameter that was seen in the sensitive genotype ICP 13997. Protein concentration was positively correlated with mycorrhizal colonization in Sel 85 N under both NaCl and Cd stresses and their combination. Stimulation of de novo synthesis of proteins by mycorrhization has been observed under salt or metal stresses (Khudsar and others 2001; Andrade and Silveira 2008; Azevedo Neto and others 2009; Roy and others 2010).
A significant increase in nonprotein thiol (NP-SH) and GSH levels was observed particularly in Sel 85 N under increasing Cd and NaCl + Cd treatments. However, no substantial changes in the concentration of NP-SH were detectable under NaCl stress. This suggests active participation of biothiols in the detoxification of Cd, which is a common response to toxic metals. Enhanced levels of NP-SH and GSH improve Cd tolerance (DalCorso and others 2008; Sobrino-Plata and others 2009; Kolb and others 2010). Cadmium stress induced the rapid biosynthesis of PCs, which play an important role in reduced Cd translocation to shoots, and the results are consistent with those of Vazquez and others (2009). The salt + Cd treatments substantially increased the accumulation of PCs, resulting in increased Cd tolerance in Sel 85 N compared to ICP 13997. In the present study, mycorrhizal symbiosis remarkably enhanced accumulation of NP-SH, GSH, and PCs subjected to Cd as well as NaCl + Cd treatments, which shows that mycorrhizal colonization may not only help plants better adapt against soil salinity and Cd stress, but AM-inoculated plants may be more efficient in regulating metal ions by PC complexation and higher osmolyte synthesis. Although there is general lack of information concerning Cd tolerance strategies operating in mycorrhizal plants, tolerance of heavy-metal toxicity in AM symbiosis is based on nutritional mechanisms, including heavy-metal chelation through the production of chelating molecules such as metallothioneins (MTs) and PCs (Rivera-Becerril and others 2002; Rivera-Becerril 2003). The accumulation of thiol groups above the GSH levels leading to PC synthesis suggested that mycorrhizal roots of pigeonpea might possess a heavy-metal chelation pathway contributing to stress tolerance in metal-contaminated soils (Garg and Aggarwal 2011).
Conclusions
The results of this study showed that the mycorrhizal fungus Glomus mosseae was an effective inoculant with regard to stimulating growth and alleviating salt and heavy-metal toxicities through increased osmolyte synthesis in Cajanus cajan (L.) Millsp. genotypes. Variation between the genotypes could be the result of an inherent difference in the tendency to accumulate toxic ions in the plant. The enhanced stress tolerance bestowed by AM fungus was at least partly due to its influence in arresting NaCl-induced Cd uptake by root tissues.
References
Anderson ME (1985) Determination of glutathione and glutathione disulfides in biological samples. Methods Enzymol 113:548–570
Andrade SAL, Silveira APD (2008) Mycorrhiza influence on maize development under Cd stress and P supply. Braz J Plant Physiol 20(1):39–50
Andrade SAL, Gratao PL, Schiavinato SMA, Silveira APD, Azevodo RA, Mazzafera P (2009) Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing concentrations. Chemosphere 75:1363–1370
AOAC (1990) Official method of analysis of the association of official analytical chemists, 15th edn, vol 1. Association of Analytical Chemists, Arlington
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenyloxidase in Beta vulgaris. Plant Physiol 24:1–15
Aydi S, Sassi S, Abdelly C (2008) Growth, nitrogen fixation and ion distribution in Medicago truncatula subjected to salt stress. Plant Soil 312:59–67
Azcon R, Medina A, Roldan A, Biro B, Vivas A (2009) Significance of treated agrowaste residue and autochthonous inoculates (arbuscular mycorrhizal fungi and Bacillus cereus) on bacterial community structure and phytoextraction to remediate soils contaminated with heavy metals. Chemosphere 75:327–334
Azevedo Neto ADA, Prisco JT, Gomes-Filho E (2009) Changes in soluble amino-N, soluble proteins and free amino acids in leaves and roots of salt-stressed maize genotypes. J Plant Interact 4(2):137–144
Bates LS, Waldran RP, Teare ID (1973) Rapid determination of free proline for water studies. Plant Soil 39:205–208
Beltrano J, Ronco MG (2008) Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus claroideum: effect on growth and cell membrane stability. Braz J Plant Physiol 20(1):29–37
Bhargava P, Srivastava AK, Urmil S, Rai LC (2005) Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J Plant Physiol 162:1220–1225
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
Chapman HD, Pratt PF (1961) Methods of analysis for soil plant and waters. Division of Agricultural Sciences, University of California, Berkley
Chen S, Li J, Wang S, Huttermann A, Altman A (2001) Salt, nutrient uptake and transport and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees Struct Funct 15:186–194
Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera CM, Rea E (2008) Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol Fertil Soils 44:501–509
Dal Corso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: Staking all on metabolism and gene expression. J Integr Plant Biol 50(10):1268–1280
Del Longo OT, Gonzalez CA, Pastori GM, Tripps VS (1993) Antioxidant defenses under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol 34:1023–1028
FAO (2008) FAO land and plant nutrition management service. http://www.fao.org/ag/agl/ahll/spush. Accessed 13–15 May 2008
Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci Hortic 123:521–530
Franzini VI, Azcon R, Mendes FL, Aroca R (2009) Interactions between Glomus species and Rhizobium affect the nutritional physiology of drought-stressed legume hosts. J Plant Physiol 167(8):614–619
Gabrijel O, Davor R, Zed R, Marija R, Monika Z (2009) Cadmium accumulation by muskmelon under salt stress in contaminated organic soil. Sci Total Environ 407:2175–2182
Garg N, Aggarwal N (2011) Effects of interactions between cadmium and lead on growth, nitrogen fixation, phytochelatin, and glutathione production in mycorrhizal Cajanus cajan (L.) Millsp. J Plant Growth Regul 30:286–300
Garg N, Chandel S (2010) Arbuscular mycorrhizal networks: process and functions. A review. Agron Sustain Dev 30(3):581–599
Garg N, Manchanda G (2009) Role of arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Millsp. (pigeonopea). J Agron Crop Sci 195(2):110–123
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol 54:753–760
Greive CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary–amino compounds. Plant Soil 70:303–307
Grewal HS (2010) Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agric Water Manag 97:148–156
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327
Hajlaoui H, Ayeb NE, Garrec JP, Denden M (2010) Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Indust Crops Prod 31:122–130
Heath RL, Packer I (1968) Photoperoxidation in isolated chloroplast I, Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hillocks RJ, Minja E, Nahdy MS, Subrahmanyam P (2000) Diseases and pests of pigeonpea in eastern Africa. Int J Pest Manag 46:7–18
Hiscox TD, Israelstam GF (1979) A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1331–1334
Irigoyen JJ, Emerich DW, Sanchez-Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Katerji N, Mastrorilli M, van Hoorn JW, Lahmer FZ, Hamdy A, Owies T (2009) Durum wheat and barley productivity in saline-drought environments. Eur J Agron 31:1–19
Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA (2009) The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants at high salinity. Sci Hortic 121:1–6
Khade SW, Adholeya A (2007) Feasible bioremediation through arbuscular mycorrhizal fungi imparting heavy metal tolerance: a retrospective. Biorem J 11(1):33–43
Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MA (2010) Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant 32:121–132
Khudsar T, Mahmooduzzafar Iqbal M (2001) Cadmium-induced changes in leaf epidermis, photosynthetic rate and pigment concentrations in Cajanus cajan. Boil Plant 44(1):59–64
Kolb D, Muller M, Zelling G, Zechmann (2010) Cadmium induced changes in subcellular glutathione contents within glandular trichomes of Cucurbita pepo L. Protoplasma 243:87–94
Lee YP, Takashi T (1966) An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem 14:71–77
Lin R, Wang X, Luo Y, Du W, Guo H, Yin D (2007) Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 69:89–98
Lopez-Chuken UJ, Young SD, Sanchez-Gonzalez MN (2009) The use of chlorocomplexation to enhance Cd uptake by Zea mays and Brassica juncea: evaluating a ‘free ion activity model’ and implications for phytoremediation. Int J Phytorem 12(7):680–696
Lopez-Chuken UJ, Young SD, Guzman-Mar JL (2010) Evaluating a ‘biotic ligand model’ applied to chloride-enhanced Cd uptake by Brassica juncea from nutrient solution at constant Cd2+ activity. Environ Technol 31(3):307–318
Manchanda G, Garg N (2011) Alleviation of salt-induced ionic, osmotic and oxidative stresses in Cajanus cajan nodules by AM inoculation. Plant Biosyst 145(1):88–97
Manousaki E, Kalogerakis N (2009) Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environ Sci Pollut Res 16:844–854
Miransari M (2010) Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol (Stuttg) 12(4):563–569
Monteiro MS, Santos C, Soares AMVM, Mann RM (2009) Assessment of biomarkers of cadmium stress in lettuce. Ecotoxicol Environ Saf 72:811–818
Muhling KH, Lauchli A (2003) Interaction of NaCl and Cd stress on compartmentation pattern of cations, antioxidant enzymes and proteins in leaves of two wheat genotypes differing in salt tolerance. Plant Soil 253(1):219–231
Munns R (2005) Genes and salt tolerance: bringing them together. Plant Cell 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nelson DW, Sommers LE (1973) Determination of total nitrogen in plant material. Agron J 65:109–112
Odeny DA (2007) The potential of pigeonpea (Cajanus cajan (L.) Millsp.) in Africa. Nat Res Forum 31:297–305
Ouzounidou GE, Eleftheriou P, Karataglis S (1992) Ecophysiological and ultrastructural effects of copper in Thlaspi ochroleucum (cruciferae). Can J Bot 70:947–957
Palma F, Lluch C, Iribane C, Garcia-Garrido JM, Garcia Tejera NA (2009) Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul 58:307–316
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Porras-Soriano A, Soriano-Martin MS, Porras-Piedra A, Azcon R (2009) Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol 166:1350–1359
Raziuddin F, Hassan G, Akmal M, Shah SS, Mohammad F et al (2011) Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pakistan J Bot 43(1):333–340
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. U. S. Department of Agriculture Handbook 60
Rivera-Becerril F (2003) Physiological and molecular responses to cadmium in mycorrhizal and non mycorrhizal pea. Ph.D thesis, Universite de Bourgogne, Dijon
Rivera-Becerril F, Calantzis C, Turnau K, Caussanel JP, Belimov AA, Gianinazzi S et al (2002) Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. J Exp Bot 53:1177–1185
Roy BK, Prasad R, Gunjan (2010) Heavy metal accumulation and changes in metabolic parameters in Cajanus cajan grown in mine soil. J Environ Biol 31(5):567–573
Sairam RK, Kumutha D, Ezhilmathi K, Chinnusamy V, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol Plant 53(3):493–504
Schonfeld MA, Johnson RC, Carver BF, Mornhinweg DW (1988) Water relations in winter wheat as drought resistance indicator. Crop Sci 28:526–531
Shafi M, Bakht J, Hassan MJ, Raiziuddin M, Zhang G (2009) Effect of cadmium and salinity stresses on growth and antioxidant enzyme activities of wheat (Triticum aestivum L.). Bull Environ Contam Toxicol 82:772–776
Shafi M, Zhang G, Bakht J, Khan MA, Islam UE, Khan MD et al (2010) Effect of cadmium and salinity stresses on root morphology of wheat. Pakistan J Bot 42(4):2747–2754
Shao G, Chen M, Wang W, Zhang G (2008) The effect of salinity pretreatment on Cd accumulation and Cd-induced stress in BADH-transgenic and nontransgenic rice seedling. J Plant Growth Regul 27:205–210
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plant under salt stress. Mycorrhiza 18:287–296
Siddiqui MH, Mohammad F, Khan MN (2009) Morphological and physio-biochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J Plant Interact 4(1):67–80
Sobrino-Plata S, Ortego-Villasante C, Flore-Caceres M, Escobar C, Campo FFD, Hernandez LE (2009) Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77:946–954
Storey R (1995) Salt tolerance, ion relations and the effects of root medium on the response of Citrus to salinity. Aust J Plant Physiol 22:101–114
Vazquez S, Goldsbrough P, Carpena RO (2009) Comparative analysis of the contribution of phytochelatins to cadmium and arsenic tolerance in soybean and white lupin. Plant Physiol Biochem 47:63–67
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils: effects of variations in digestion conditions and of organic soil constituents. Soil Sci 63:251–263
Weggler-Beaton K, McLaughlin MJ, Graham RD (2000) Salinity increases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Aust J Soil Res 38:37–45
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304
Xu J, Yin H, Liu X, Li X (2010) Salt affects plant Cd-stress responses by modulating growth and Cd accumulation. Planta 231:449–459
Zeng X, Ma LQ, Qiu R, Tang Y (2009) Responses of non-protein thiols to Cd exposure in Cd hyperaccumulator Arabis paniculata French. Environ Exp Bot 66:242–248
Zhang XH, Lin AJ, Chen BD, Wang YS, Smith SE, Smith FA (2006) Effects of Glomus mosseae on the toxicity of heavy metals to Vicia faba. J Environ Sci 18(4):721–726
Zhou YQ, Huang SZ, Yu SL, Gu JG, Zhao JZ, Han YL et al (2010) The physiological responses and sub-cellular localization of lead and cadmium in Iris pseudacorus L. Ecotoxicology 19:69–76
Zwiazek JJ, Blake TJ (1991) Early detection of membrane injury in black spruce (Picea mariana). Can J For Res 21:401–404
Acknowledgment
The authors are deeply grateful to University Grants Commission (UGC), New Delhi, India, for providing the financial support required for undertaking this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, N., Chandel, S. Role of Arbuscular Mycorrhizal (AM) Fungi on Growth, Cadmium Uptake, Osmolyte, and Phytochelatin Synthesis in Cajanus cajan (L.) Millsp. Under NaCl and Cd Stresses. J Plant Growth Regul 31, 292–308 (2012). https://doi.org/10.1007/s00344-011-9239-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-011-9239-3