Abstract
Background, aim, and scope
The success of phytoextraction depends upon the identification of suitable plant species that hyperaccumulate heavy metals and produce large amounts of biomass using established agricultural techniques. In this study, the Mediterranean saltbush Atriplex halimus L., which is a C4 perennial native shrub of Mediterranean basin with an excellent tolerance to drought and salinity, is investigated with the main aim to assess its phytoremediation potential for Pb and Cd removal from contaminated soils. In particular, the influence of soil salinity in metal accumulation has been studied as there is notable evidence that salinity changes the bioavailability of metals in soil and is a key factor in the translocation of metals from roots to the aerial parts of the plant.
Materials and methods
Three pot experiments were conducted under greenhouse conditions for a 10-week period with A. halimus grown in soil artificially polluted with 20 ppm of Cd and/or 800 ppm of Pb and irrigated with three different salt solutions (0.0%, 0.5%, and 3.0% NaCl). Soil measurements for soil characterization were performed with the expiration of the first week of plant exposure to metals and NaCl, and at the end of the experimental period, chlorophyll content, leaf protein content, leaf specific activity of guaiacol peroxidase (EC 1.11.1.7), shoot water content, biomass, and Cd and Pb content in the plant tissues were determined. Additionally, any symptoms of metal or salt toxicity exhibited by the plants were visually noted during the whole experimental period.
Results
The experimental data suggest that increasing salinity increases cadmium uptake by A. halimus L. while in the case of lead there was not a clear effect of the presence of salt on lead accumulation in plant tissues. A. halimus developed no visible signs of metal toxicity; only salt toxicity symptoms were observed in plants irrigated with 3% NaCl solutions. Chlorophyll content, leaf protein content, shoot water content, and biomass were not negatively affected by the metals; instead, there was even an increase in the amount of photosynthetic pigments in plants treated with both metals and salinity. The specific activity of guaiacol peroxidase seems to have a general tendency for increase in plants treated with the metals in comparison with the respective controls but a statistically significant difference exists only in plants treated with the metal mixture and saline conditions.
Discussion
The data revealed that lead and cadmium accumulation in plant tissues was kept generally at low levels. Salinity was found to have a positive effect on cadmium uptake by the plant and this may be related to a higher bioavailability of the metal in soil due to decreased Cd sorption on soil particles. On the other hand, salinity did not influence in a clear way the uptake of Pb by the plant probably because of lead’s limited mobility in soils and plant tissues. Cd and Pd usually decrease the chlorophyll content and biomass and change water relations in plants; however, A. halimus was found not to be affected indicating that it is a Cd- and Pb-tolerant plant. Guaiacol peroxidase activity as one of the parameters expressing oxidative damage and extent of stress in plants was not generally found to be significantly affected under the presence of metals in most plants suggesting that the extent of stress in plants was minimal, while only for plants treated with the metal mixture and low salinity the enzyme activity was elevated confirming that this enzyme serves as an antioxidative tool against the reactive oxygen species produced by the metals.
Conclusions
Atriplex halimus L. is a Pb- and Cd-tolerant plant but metal concentrations achieved in plant tissues were kept generally at low levels; however, metal accumulation in shoots, especially for Cd, considered together with its high biomass production, rapid growth, and deep root system able to cope with poor structure and xeric characteristics of several polluted soils suggest that this plant deserves further investigation.
Recommendations and perspectives
Phytoextraction by halophytes is a promising alternative for the remediation of heavy metal contaminated sites affected by salinity since saline depressions often indicate sites of industrial effluents accumulation, contaminated by heavy metals, including Pb and Cd. Halophytes are also promising candidates for the removal of heavy metals from non-saline soils. Furthermore, the use of such plants can be potentially viewed as an alternative method for soil desalination where salt is removed from the soil instead of being washed downwards by water or other solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Heavy metals, including Cd and Pb, in soils are a serious concern given their widespread distribution due to human activities such as mining, energy production and agricultural activities, and the potential human and ecological risks posed by them. Long-term environmental safety dictates the removal of these metals. While conventional technologies have proven to be effective in small areas, they are not only costly but they also cause soil disturbances, and they are not readily accepted by the general public (Martin and Ruby 2004; Saifullah et al. 2009). Phytoremediation and more specifically phytoextraction, which involves the use of plants to remove metals from the soil into their above-ground biomass which can be harvested using conventional agricultural techniques, has been posed as a cost-effective, environment-friendly alternative restoration strategy for the cleanup of heavy metal contaminated soils (Palmer et al. 2001; US EPA 2001; Memon and Schröder 2009; Butcher 2009). However, the success of phytoextraction depends upon the identification of suitable plant species that tolerate and hyperaccumulate heavy metals and produce large amounts of biomass using established agricultural techniques.
In this study, the Mediterranean saltbush Atriplex halimus L., which is a C4 perennial native shrub of Mediterranean basin with an excellent tolerance to drought and salinity, is investigated. Saltbushes are dominant in many arid and semi-arid regions of the world, particularly in habitants that combine relatively high soil salinity with aridity and have been used as a resource for livestock or for the rehabilitation of degraded lands like sand dunes, saline/alkaline soils, mine waste, badlands, shallow soils, etc. (Le Houérou 1992; Ortíz-Dorda et al. 2005). A. halimus was chosen for phytoextraction research because its presence is often reported on mining areas contaminated with heavy metals and because of its high biomass production—it branches out almost from the base, can reach 1–3 m high and up to 3 m in diameter—and its deep root system is able to cope with poor structure of several polluted substrates (Lutts et al. 2004; Ortíz-Dorda et al. 2005). Moreover, it is a plant well adapted to arid and salt-affected areas where few species can develop. It also grows in non-saline soils and for these reasons together with its favorable crude protein content, it has been studied and extensively used as livestock fodder reserves in arid and semi-arid counties (Ortíz-Dorda et al. 2005; Osman et al. 2006). In addition, it can be cultivated with saline irrigation water which is a purposeful feature since often high-quality irrigation water is not available for application to crops in arid regions and brackish waters must be used. Saltwater irrigation is becoming an increasingly important practice because the quality of irrigation waters is decreasing as water supplies for agriculture become restricted due to urban needs and climate change (Wahla and Kirkham 2008). Furthermore, halophyte species are of special interest since these plants are naturally present in environments characterized by an excess of toxic ions, mainly sodium and chloride, and can also tolerate other stresses such as chilling, freezing, heat, and drought while it has been speculated that salt-tolerant plants may also be heavy metal tolerant and, further, may be able to accumulate metals (Thomas et al. 1998; Jordan et al. 2002; Lutts et al. 2004). Therefore, halophytes have been suggested to be better adapted to coping with environmental stresses, including heavy metals than salt-sensitive crop plants commonly chosen for phytoextraction studies (Ghnaya et al. 2005, 2007). Thus, halophytes are expected to receive more attention of phytoremediation researchers in the near future.
The main purpose of this work was to assess the phytoremediation potential of the Mediterranean saltbush for lead and cadmium removal from contaminated soils. In order to achieve that goal, the accumulation of Pb and Cd via root uptake from contaminated soil has been investigated. Moreover, the influence of soil salinity in metal accumulation has been studied as there is notable evidence that salinity changes the bioavailability of metals in soil and is a key factor in the translocation of metals from roots to the aerial parts of the plant (Otte 1991; Fitzgerald et al. 2003; Weggler et al. 2004; Wahla and Kirkham 2008; Manousaki et al. 2008). Furthermore, the response of plant growth, water content, chlorophyll content and leaf protein content to Pb and Cd and saline environment were also investigated in order to obtain a better understanding of the tolerance of A. halimus to heavy metals. Furthermore, the activity of guaiacol peroxidase (as ‘stress enzyme’) was used as biomarkers to evaluate the intensity of stress in the Atriplex plants.
2 Materials and methods
2.1 Plant material
Cuttings of Atriplex halimus L. collected from mature plants growing on the Wall of the Old City of Heraklion (Crete, Greece) were propagated in sand of a mist propagator inside a greenhouse for 20 days. To enhance rooting, hormones (auxins) were used. The rooted cuttings were transferred individually into plastic pots (21.5 cm in height and 24 cm in diameter) filled with the same amount of organic substrate produced in Germany with the trade name Blumenerde, Capriflor which was a blend of weakly decomposed white sphagnum peat, high grade frozen through black sphagnum peat, green compost, and wood fibers, with pH (H2O) 5.5–6.5, amount of added N:P:K 12:12:17 fertilizer 1.5 kg/m3, organic C from biological origin 47%, organic N 1%, total amount of nitrogen 200–300 mg/l, and with concentration of other nutrients: P2O5 200–500 mg/l, K2O 300–800 mg/l, MgO 100–300 mg/l. The adaptation period was extended to 11 months in order to allow the root system to mature.
2.2 Pot experiments
At the beginning of the experimental phase, plants were divided into three pot experiments with A. halimus grown in soil polluted with: 20 ppm (per dry weight of soil) of Cd; 800 ppm of Pb; 20 ppm of Cd; and 800 ppm of Pb. Additionally, one set of control plants was used for all experiments. Every set consisted of three experimental groups-treatments, with 0% salty watering, 0.5% salty watering, and 3% salty watering, and with six plants per group-treatment resulting in 72 plants in total. The complete experimental design is shown in Table 1. The experiments were conducted under greenhouse conditions with the plants grown in soil which was artificially polluted with the metals in the above concentrations as aqueous solutions of Pb(NO3)2 and Cd(NO3)2·4H2O in one dose at the beginning of the experiments. The experimental period was 10 weeks (September to November) and the photoperiod was 10.5–12.5 h. Temperature and humidity were measured three times during daytime and minimum and maximum temperatures were measured during the night. The ranges and average values are provided in Table 2. Plants were watered every 2–3 days, depending on the evaporative demand, with approximately 200 ml of tap water or salty water (according to the treatment, see Table 1) in order to avoid leakage of water from the pots while plastic trays were placed under each pot. The salty water was prepared from edible sea salt and tap water. Any symptoms of metal or salt toxicity exhibited by plants were visually noted during the whole experimental period.
2.3 Soil measurements
For soil characterization, with the expiration of the first week of plant exposure to metals and NaCl, two randomly selected plants per treatment were harvested and the soil samples of each pot were air dried at room temperature, ground in a ceramic mortal to pass through a 2-mm-mesh sieve (Restch Test Sieve, Germany), homogenized, and stored in polyethylene bags for subsequent analysis. Soil pH values were measured in a 1:5 (w/v) soil-to-water ratio. Electrical conductivity (EC) was determined in the soil saturation extract. Organic matter (OM) was determined by the Walkley–Black method (Nelson and Sommers 1996). For the determination of CaCO3, CO2 released by addition of HCl was measured with a calcimeter (Calcimeter Scheibler). Τotal Cd and Pb contents of the soil samples were determined using microwave (Anton Paar, Multiwave) assisted acid digestion of soils (US Environmental Protection Agency 1994 method 3051) and DTPA-extractable Cd and Pb concentrations were analyzed according to the method of Lindsay and Norvell (1978) with the use of a Leeman Labs PS1000AT inductively coupled plasma atomic emission spectroscope (ICP-AES). All analyses were conducted in triplicates per sample.
2.4 Chlorophyll content measurement
The chlorophyll content was measured at the end of the experiments by the method of Harborne (1984). Fresh leaves (0.4 g) were selected randomly from the middle part of each plant, washed with deionized water, and homogenized in a porcelain mortar with 80% acetone solution. The homogenate was centrifuged at 16,000 ×g for 1 min twice and the measurement of chlorophyll content was done by direct determination of the absorbance at wavelengths 663 and 646 nm at the clear supernatant fraction using UV–Vis spectrophotometer (Shimadzu 1240 UV–Vis).
2.5 Guaiacol peroxidase activity and protein determination
The last day of the experiments, 1 g of fresh leaves was homogenized in 0.05 M cold phosphate buffer (pH 5.8) for the extract preparation according to the method of Erdelský and Frič (1979). The homogenate was filtered through four layers of cheese cloth and centrifuged at 16,000 ×g for 25 min. Supernatant was used for the determination of protein content and activity of guaiacol peroxidase. Protein content was measured using Modified Lowry Protein Assay Kit from Pierce (Rockford, IL, USA; product no. 23240) based on the Lowry method using bovine serum albumin as a standard. Activity of guaiacol peroxidase (GPX; EC 1.11.1.7) was assayed according to Erdelský and Frič (1979). The reaction mixture was prepared by adding 2.7 ml of 0.05 M phosphate buffer (pH 5.8), 0.1 ml of plant enzyme extract, and 0.1 ml of guaiacol (15 mg/ml). To start the reaction, 0.1 ml of 1% H2O2 was added to the mixture with a final volume of 3 ml. The increase of absorbance due to oxidation of guaiacol to tetraguaiacol was monitored for 3 min at 470 nm in a UV–Vis spectrophotometer (Shimadzu 1240 UV–Vis). Enzyme activity unit was expressed as the change in absorbance per minute (∆A 470/min). The specific activity of enzyme is expressed in terms of units per milligram of extracted proteins. Each sample was measured in triplicates.
2.6 Water content and biomass measurement
With the expiration of the 10-week experimental period, shoots were harvested and roots were carefully taken out of the soil, washed with tap water and twice with deionized water in order to remove any dust deposits and surface soil, respectively, and their fresh weights (FW) were determined. Dry weights (DW) were determined after oven drying for 48 h at 70°C and cooled down to room temperature. Finally, all dry samples were milled, air dried, and stored until metal content determination in the plant tissue. Water content (WC, %) was calculated according to the formula:
2.7 Determination of lead and cadmium in the plant tissue
Metal content analysis in the plant tissues was performed by ICP-AES (Leeman Labs PS1000AT) according to the modified method of Soon (1998). For the sample preparation, 0.5 g of dried ground plant tissue was ashed in the muffle furnace for 16 h at 480°C. Ash was dissolved in 10 ml of 2 N HCl (on a hot plate (~100°C)), solution was filtered, diluted with deionized water to 50 ml, and the cadmium and lead content were determined.
3 Results
3.1 Soil properties
Selected properties of the soil samples are presented in Table 3. Soil pH ranges from 5.3 to 5.9 with a small tendency to decrease with increasing salinity as expected since it is well known that an increase of salts such as NaCl, NaSO4, or Ca(NO3)2 in soil results in a progressive decrease of soil pH (Thomas 1996). Moreover, the increase of soil salinity with the use of NaCl aqueous solutions as irrigation water is shown by the increase of electrical conductivity from 1.3 to 2 in the treatments without NaCl (0/0, Cd/0, Pb/0, Pb + Cd/0) to 3.0–3.8 in the treatments with 0.5% NaCl (0/0.5, Cd/0.5, Pb/0.5, Pb + Cd/0.5), and reaching 9.3–11.9 mS/cm in the treatments with 3% NaCl (0/3, Cd/3, Pb/3, Pb + Cd/3). Furthermore, the measured organic matter was 58–60% as expected since the experimental soil was organic substrate and the total CaCO3 was found to be zero. The average total concentrations of Cd and Pb in all samples are in accordance with the spiked concentrations of the metals confirming the experimental design. From the DTPA-extractable metals used for the determination of metal phytoavailability, the measured concentrations show that the entire amount of metals added in the soil was available for plant uptake; the results are arguable especially for a soil with a high organic content, therefore it is believed that in this case the DTPA method failed to provide information for metal bioavailability although one-step extraction methods with single extractants, such as DTPA, are usually chosen to evaluate a particular controlling mechanism such as desorption by increasing salinity or presence of other metals (Rauret 1998).
3.2 Metal accumulation in plant tissues
The experimental data revealed that lead and cadmium concentrations achieved in plant tissues were kept generally in low levels. For cadmium, its concentration in roots ranged from 1.9 to 4.9 ppm for the plants exposed only to cadmium and 2.1–4.5 ppm for the plants exposed to cadmium and lead (Fig. 1). Cd concentration in shoots ranged from 0.9 to 2 ppm for the plants grown on soil polluted only with cadmium and 1.8–2.7 ppm for the plants grown on soil polluted with both metals, but the toxic level of Cd in leaves of plants which is 5–30 ppm dry weight (Orcutt and Nilsen 2000) was never exceeded. Interestingly, increased salinity resulted in increased cadmium uptake by A. halimus L. Accumulation in roots of plants treated only with cadmium increased from 1.9 ppm in the treatment without salt addition to 3.4 ppm in the treatment with 0.5% salinity, and reached 4.9 ppm for plants treated with 3% soil salinity. Correspondingly, the accumulation in roots of plants treated with the metal mixture increased with increasing salinity, but there was not a clear effect of the presence of salt to cadmium accumulation in plant shoots. Moreover, the same information was obtained by examining the total removal of cadmium (phytoextraction) by the plant as shown in Fig. 2, which is calculated by multiplying shoot metal concentration by tissue dry weight and can provide further information for the effectiveness of the plant as a cleanup tool for phytoextraction applications since it takes under consideration the effect of the metal and salt as stressors to the biomass production.
In all treatments with lead, the metal was mostly accumulated in Atriplex roots with 75–95% of the total accumulated lead by the whole plant to be found in root tissues (Fig. 3). Lead concentrations in shoots were kept in low levels not exceeding the concentrations considered to be toxic to plants which are 30–300 ppm dry weight for lead (Orcutt and Nilsen 2000). Moreover, salinity did not influence in a clear way the uptake of Pb by the plant. Only in case of plants treated with the metal mixture was there an increase of Pb shoot concentration observed with increasing salinity while the same conclusion is reached by examining the total removal of lead (phytoextraction) by the plant (Fig. 4).
3.3 Correlation between metal and salt concentrations and physiological response
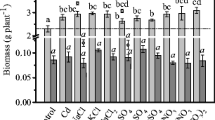
A. halimus L. developed no visible signs of metal toxicity. Only salt toxicity symptoms were observed in plants treated with 3% saline watering after the sixth experimental week, confirming the decreased shoot water content (Fig. 6) and finally the reduced survival up to 50% of these plants (detailed data not shown), and for this reason, the determination of chlorophyll and protein content and GPX activity was not performed in these plants. On the contrary, the low soil salinity (0.5% saline watering) seems to have a positive effect on biomass (Fig. 5) and shoot water content (see Fig. 6) of Atriplex plants confirming their halophytic nature, but the differences are not statistically significant. Chlorophyll content in all experiments was not negatively affected by the metals; instead, there seems to be an increase of the amount of photosynthetic pigments in all plants treated with lead but a statistically significant difference exists only in plants treated with both metals and low salinity (Fig. 7). Moreover, the shoot water content was not found to be affected by the presence of metals (see Fig. 6). The growth of plants expressed as biomass dry weight (see Fig. 5) was not found to be influenced by cadmium while it seems that there was an increase in the treatments with lead and a decrease in treatments with the metal mixture, but again these are not statistically different. Furthermore, the leaf protein content was not found to be negatively affected by the metals, but there was a slight decrease in leaf proteins concentrations for all plants watered with 0.5% NaCl but the differences are not significant (detailed data not shown). The specific activity of guaiacol peroxidase again seems to have a general tendency to increase in plants treated with the metals and a tendency to decrease under the influence of salt stress in comparison with the respective controls, but a statistically significant difference exists only in plants treated with both metals and saline conditions (Fig. 8).
Effect of Cd, Pb, and soil salinity on chlorophyll content (per gram of fresh weight of leaves) of Atriplex halimus L. Values shown are means (n = 3) with minimum and maximum values. Asterisk denotes significant differences (p < 0.05) when compared with respective control according to the two-sample t test for difference of means
Effect of Cd, Pb, and soil salinity on guaiacol peroxidase activity in leaves of Atriplex halimus L. Values shown are means (n = 3) with minimum and maximum values. Asterisk denotes significant differences (p < 0.05) when compared with respective control according to the two-sample t test for difference of means
4 Discussion
4.1 Metal accumulation in Atriplex tissues
The data revealed that lead and cadmium concentrations achieved in plant tissues were kept generally at low levels (see Figs. 1 and 3). However, these results are not in agreement with previous hydroponic experiments with young plants of A. halimus in our lab (Kadukova et al. 2004) and by other research groups (Lutts et al. 2004) which showed that, although higher concentrations of both metals were found in roots of plants as in this study, significantly high concentrations were also measured in the above-ground tissues and together with the fact that there was no reduction of biomass suggested that A. halimus could be a possible Cd and Pb hyperaccumulator. These findings have not been confirmed by experiments using soil as substrate which take into consideration the bioavailability of the metals in soil condition and by observing no growth inhibition on a long-term basis. Moreover, it is known that extrapolation of results from hydroponic studies to phytoextraction of metals from soils could be misleading even when using the same plants due to the much greater metal bioavailability in the hydroponic solution compared to metals in soil and to the effects of biosorption and other passive assimilation processes that take place under hydroponic growth; thus, hydroponics indicate the metal tolerance of the plant and that uptake is possible rather than providing estimates of the actual concentrations and the distinction between hyperaccumulator and non-hyperaccumulator plants on the basis of metal concentration in the biomass (US EPA 2001; Nedelkoska and Doran 2000; Boominathan and Doran 2003). Besides these, different results are believed to be mainly due to the different age and subsequent developmental stages of the plant since the previously mentioned studies were performed with young plants or seedlings while this study was performed with 11-month-old full-grown plants and generally the age and the growth stages of plants are factors affecting metal uptake by the plant (Orcutt and Nilsen 2000; Fitzgerald et al. 2003; Qadir et al. 2005).
The ideal plant species to remediate a heavy metal contaminated soil would be a high biomass-producing crop that can both tolerate and accumulate the contaminant of interest (Pulford and Watson 2003), yet hyperaccumulator plants are usually small with a shallow root system and low biomass production and the technology for their large-scale cultivation is not fully developed; therefore, their use is rather limited (Raskin et al. 1997; Palmer et al. 2001; Pulford and Watson 2003; Saifullah et al. 2009). In contrast, plants with good growth usually exhibit low metal accumulation or the metals are accumulated mainly in the roots besides having a low tolerance to heavy metals. Therefore, if such a combination is not possible, a trade-off between hyperaccumulation and lower biomass must be made (Pulford and Watson 2003). The concentrations of metals achieved in plant tissues of A. halimus, especially for cadmium, considered together with its high biomass production and its metal tolerance suggest that it could be used for phytoextraction research. Although roots accumulated higher concentrations of cadmium than shoots, average Cd accumulation in shoots was found 68% of the total accumulated cadmium by the whole plant (see Fig. 2) due to the high above-ground biomass production by the plant, an important feature for phytoextraction applications.
Furthermore, soil pH, organic matter, salinity, presence of other metals, and calcium concentrations constitute important factors affecting metal bioavailability in the soil since the transfer of metals between the readily available and less-available phases of the soil is significantly influenced by the competition for surface exchange sites by other cations (especially H+) and by the presence of binding surfaces such as the organic matter (Martin and Kaplan 1998; Naidu et al. 2003; Fitzgerald et al. 2003; Rieuwerts et al. 2006). Salinity was found to have a positive effect on cadmium uptake by Atriplex plants (see Fig. 1), and this is probably related to higher bioavailability of the metal in soil since the increased metal mobility and thus bioavailability and phytoaccumulation upon the addition of NaCl may be due to (1) displacement of metals from binding sites in the soil matrix by Na+, (2) solubilization of organic matter to which the metals are bound, and (3) as recent studies have shown, formation of soluble chloro-complexes of Cd which tend to shift Cd from solid to solution phase (Bingham et al. 1983; Smolders and McLaughlin 1996; Smolders et al. 1998; Norvell et al. 2000; Weggler et al. 2004; Wahla and Kirkham 2008). In addition to increasing transport to roots, these chloro-complexes may also be taken up directly by plant roots through different mechanisms than those responsible for uptake of unassociated Cd+2 (Smolders et al. 1998). Nevertheless, salinity did not influence in a clear way the uptake of Pb by Atriplex plants (see Fig. 3) probably because lead, unlike Cd which is generally mobile in soils and plant tissues, is known to be extremely insoluble in the normal range of soil pH and moreover its translocation from roots to aerial shoots is limited due to binding at root surfaces and cell walls (Raskin et al. 1997; Balsberg Påhlsson 1989; Lasat 2002; Butcher 2009; Saifullah et al. 2009); thus, lead in most plants is accumulated in roots as also observed in this study. Furthermore, salinity is shown to be a key factor in the translocation of metals from roots to the aerial parts of plants (Otte 1991; Fitzgerald et al. 2003; Manousaki et al. 2008), but the present results do not support such a case.
4.2 Physiological response of Atriplex halimus L.
The presence of metals usually affects adversely plant health. It has been shown that cadmium and lead in plants interfere with and inhibit various physiological processes such as plant–water relationships, chlorophyll biosynthesis, transpiration rates, enzyme activities, nutrient uptake, root elongation, biomass production, and growth (Balsberg Påhlsson 1989; Das et al. 1997; Orcutt and Nilsen 2000; Cheng 2003). However, A. halimus was not found to be affected by the presence of metals, corroborating previous studies (Lutts et al. 2004; Kadukova et al. 2004) that it is a cadmium- and lead-tolerant plant. Biomass, shoot water content, and chlorophyll content were not negatively affected by the metals; instead, there was even a case of chlorophyll increase in plants treated with both metals and salinity (see Fig. 7). These findings are in accordance with many studies which showed that small amounts of lead in plant tissues may have a stimulation effect on some plants (Liu et al. 2000; Balsberg Påhlsson 1989 and references within). Moreover, salinity as a stressor induces a wide range of detrimental effects at the cell and whole plant level; however, A. halimus L. as a halophyte developed salinity toxicity symptoms only after exposure to very high salinity levels that simulated seawater irrigation for a long period of time, while in treatments with the low saline irrigation the biomass and shoot water content seems to have a general tendency to increase but the differences are not statistically significant at least for the duration of the present experiment. Bajji et al. (1998) and Blumenthal-Goldschmidt and Poljakoff-Mayber (1968) also reported that low NaCl concentrations promoted A. halimus growth confirming its halophytic nature.
Furthermore, heavy metals and salinity are known to cause oxidative damage to plants through the formation of reactive oxygen species (ROS) which cause damage to biomolecules such as membrane lipids, proteins, etc. To resist oxidative damage, plants have an antioxidant defense system comprising of antioxidant enzymes such as peroxidases, superoxide dismutases and catalases, non-enzymatic constituents such as ascorbate and reduced glutathione which scavenge ROS leading to adaptation and ultimate survival of plants during periods of stress (Radotic at al. 2000; Verma and Dubey 2003; Zhu et al. 2004). Peroxidases are widely accepted as ‘stress enzymes’ and it has been shown that, under sublethal salinity and metal toxicity conditions, the level of peroxidase activity can be used as a potential biomarker to evaluate the intensity of stress in plants (Radotic at al. 2000; Verma and Dubey 2003). The present results suggest that the presence of Pb and Cd did not influence the activity of guaiacol peroxidase as one of the enzymatic protectors against peroxidation reactions suggesting that the extent of stress in plants was minimal while only for plants treated with the metal mixture and low salinity the enzyme activity was elevated (see Fig. 8), confirming stress conditions in plants and suggesting that GPX serves as a tolerance tool against Pb- and Cd-induced oxidative damage in A. halimus L. On the contrary, the enzyme activity seems to have a general tendency to decrease in plants irrigated with low salt concentrations but without a statistically significant difference from the corresponding controls, findings in accordance with Bajji et al. (1998) who found decreased guaiacol peroxidase activity in A. halimus leaves in response to 150 mM NaCl under hydroponic growth but increased enzyme activities in response to higher stress intensity and concluded that, in A. halimus, guaiacol peroxidases do not seem to be directly involved in the salt tolerance at least at the leaf level. Furthermore, the leaf protein content was not affected by the metals or low saline irrigation, findings again parallel to those of Bajji et al. (1998) who found decreased protein concentration in roots while in leaves it presented a minimal value in response to 300 mM NaCl but in response to higher salinities it was only slightly lower than controls.
5 Conclusions
The Mediterranean saltbush was found to be a lead- and cadmium-tolerant plant but Pb and Cd concentrations achieved in above-ground tissues were kept generally at low levels; however, its potential use for phytoextraction or generally phytoremediation purposes is not prohibitive. Further research is needed in order to investigate its removal efficiency under higher bioavailable metal levels in soil (either in different soil types or with the use of soil amendments) and in different developmental stages of the plant. Salinity was found to have a positive effect on cadmium uptake by A. halimus, but did not influence with a clear trend the uptake of Pb. Biomass, shoot water content, chlorophyll content, and leaf protein concentrations were not negatively affected by the metals; instead, there was even an increase in the amount of photosynthetic pigments in plants treated with both metals and salinity. Guaiacol peroxidase activity as one of the parameters expressing oxidative damage and extent of stress in plants was not generally found to be significantly affected under the presence of metals in most plants suggesting that the extent of stress in plants was minimal while only for plants treated with the metal mixture and low salinity the enzyme activity was elevated confirming that this enzyme serves as an antioxidative tool against the reactive oxygen species produced by the metals. Furthermore, the unchanged enzyme activity under the presence of salt suggests that, in A. halimus, guaiacol peroxidases do not seem to be directly involved in the salt tolerance at least at the leaf level.
6 Recommendations and perspectives
Within the field of phytoextraction, halophytes like A. halimus L. have been suggested to be better adapted to coping with environmental stresses, including heavy metals, compared to salt-sensitive crop plants commonly chosen for phytoextraction studies; thus, halophytes are promising candidates for the removal of heavy metals not only from heavy metal polluted soils but also from heavy metal contaminated sites affected by salinity since saline depressions often constitute accumulation sites of industrial effluents contaminated by heavy metals, including Pb and Cd (Ghnaya et al. 2005, 2007). Furthermore, the use of salt-accumulating halophytes can be viewed as an alternative phytoremediation method of soil desalination where salt is removed from the soil to the point that the soil can be returned to agricultural productivity.
References
Bajji M, Kinet J-M, Lutts S (1998) Salt effects on roots and leaves of Atriplex halimus L. and their corresponding callus cultures. Plant Sci 137:131–142
Balsberg Påhlsson A-M (1989) Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water Air Soil Pollut 47:287–319
Bingham FT, Strong JE, Sposito G (1983) Influence of chloride salinity on cadmium uptake by Swiss chard. Soil Sci 135:160–165
Blumenthal-Goldschmidt S, Poljakoff-Mayber A (1968) Effects of substrate salinity on growth and on submicroscopic structure of leaf cells of Atriplex halimus L. Aust J Bot 16:469–478
Boominathan R, Doran PM (2003) Cadmium tolerance and antioxidative defenses in hairy roots of the cadmium hyperaccumulator Thlaspi caerulescens. Biotechnol Bioeng 83:158–167
Butcher DJ (2009) Phytoremediation of lead in soil: recent applications and future prospects. Appl Spectrosc Rev 44:123–139
Cheng S (2003) Effects of heavy metals on plants and resistance mechanisms. Environ Sci Pollut Res 10(4):256–264
Council of the European Communities (1986) Council directive of 12 June 1986 on the protection of the environment and in particular of the soil, when sewage sludge is used in agriculture. Official J European Communities no. L181, pp 6–12
Das P, Samantaray S, Rout GR (1997) Studies of cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Erdelský K, Frič F (1979) Praktikum a analytické metódy vo fyziológii rastlín (Prakticum and analytical methods in plant physiology), SPN Bratislava
Fitzgerald E, Caffrey J, Nesaratnam S, McLoughlin P (2003) Copper and lead concentrations in salt marsh plants on the Suir Estuary, Ireland. Environ Pollut 123:67–74
Ghnaya T, Nouairi I, Slama I, Messedi D, Grignon C, Adbelly C, Ghorbel MH (2005) Cadmium effects on growth and mineral nutrition of two halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum. J Plant Physiol 162:1133–1140
Ghnaya T, Slama I, Messedi D, Grignon C, Ghorbel MH, Adbelly C (2007) Effects of Cd+2 on K+, Ca2+ and N uptake in two halophytes Sesuvium portulacastrum and Mesembryanthemum crystallinum: consequences on growth. Chemosphere 67:72–79
Harborne JB (1984) Chlorophylls. In: Phytochemical methods, 2nd edn. Chapman and Hall, London, pp 214–221
Jordan FL, Robin-Abbott M, Maier RM, Glenn EP (2002) A comparison of chelator-facilitated metal uptake by a halophyte and a glycophyte. Environ Toxicol Chem 21:2698–2704
Kadukova J, Papadontonakis N, Naxakis G, Kalogerakis N (2004) Lead accumulation by the salt-tolerant plant Atriplex halimus. In: Moutzouris C, Christodoulatos C, Dermatas D, Koutsospyros A, Skanavis C, Stamou A (eds) e-Proceedings of the International Conference on Protection and Restoration of the Environment VII, June 28–July 1, Mykonos, Greece
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31:109–120
Le Houérou HN (1992) The role of saltbushes (Atriplex spp.) in arid land rehabilitation in the Mediterranean Basin: a review. Agroforest Syst 18:107–148
Lindsay WL, Norvell WA (1978) Development of a DTPA test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Liu D, Jiand W, Liu C, Xin C, Hou W (2000) Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresour Technol 71:273–277
Lutts S, Lefèvre I, Delpèrèe C, Kivits S, Dechamps C, Robledo A, Correal E (2004) Heavy metal accumulation by halophyte species Mediterranean saltbush. J Environ Qual 33:1271–1279
Manousaki E, Kadukova J, Papadantonakis N, Kalogerakis N (2008) Phytoextraction and phyto-excretion of Cd by Tamarix smyrnensis growing on contaminated non saline and saline soils. Environ Res 106:326–332
Martin HW, Kaplan DI (1998) Temporal changes in cadmium, thallium and vanadium mobility in soil and phytoavailability under field conditions. Water Air Soil Pollut 101:399–410
Martin TA, Ruby MV (2004) Review of in situ remediation technologies for lead, zinc and cadmium in soil. Remed J 14:35–53
Memon AR, Schröder P (2009) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res 16:162–175
Naidu R, Gupta VVSR, Rogers S, Kookana RS, Bolan NS, Adriano DC (2003) Bioavailability of metals in the soil plant environment and its potential role in risk assessment. In: Naidu R, Gupta VVSR, Kookana RS, Rogers S, Adriano D (eds) Bioavailability, toxicity and risk relationships in ecosystems. Science Publishers, Enfield, pp 21–57
Nedelkoska TV, Doran PM (2000) Hyperaccumulation of cadmium by hairy roots of Thlaspi caerulescens. Biotechnol Bioeng 67:607–615
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Sparks DL (ed) Methods of soil analysis, Part 3, chemical methods—SSSA book series no. 5. Soil Science Society of America and American Society of Agronomy, Madison, pp 995–1007
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Orcutt DM, Nilsen ET (2000) Phytotoxicity and soil pollution: heavy metals and xenobiotics. In: The physiology of plants under stress, soil and biotic factors. Wiley, New York, pp 481–517
Ortíz-Dorda J, Martínez-Mora C, Correal E, Simón B, Cenis JL (2005) Genetic structure of Atriplex halimus populations in the Mediterranean Basin. Ann Bot—London 95:827–834
Osman AE, Bahhady F, Hassan N, Ghassali F, Al Ibrahim T (2006) Livestock production and economic implications from augmenting degraded rangeland with Atriplex halimus and Salsola vermiculata in northwest Syria. J Arid Environ 65:474–490
Otte ML (1991) Contamination of coastal wetlands with heavy metals: factors affecting uptake of heavy metals by salt marsh plants. In: Rozema J, Verkleij JAC (eds) Ecological responses to environmental stresses. Kluwer Academic, Netherlands, pp 126–133
Palmer CE, Warwick S, Keller W (2001) Brassicaceae (Cruciferae) family, plant biotechnology, and phytoremediation. Int J Phytorem 3:245–287
Pulford ID, Watson C (2003) Phytoremediation of heavy metal-contaminated land by trees—a review. Environ Int 29:529–540
Qadir M, Schubert S, Steffens D (2005) Phytotoxic substances in soils. In: Hillel D (ed) Encyclopedia of soils in the environment. Elsevier, Oxford, pp 216–222
Radotic K, Ducic T, Mutavdzic D (2000) Changes in peroxidase activity and isoenzymes in spruce needles after exposure to different concentration of cadmium. Environ Exp Bot 44:105–113
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Rauret G (1998) Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 46:449–455
Rieuwerts JS, Ashmore MR, Farago ME, Thornton I (2006) The influence of soil characteristics on the extract ability of Cd, Pb and Zn in upland and moorland soils. Sci Total Environ 366:864–875
Saifullah ME, Qadir M, de Caritat P, Tack FMG, Du Laing G, Zia MH (2009) EDTA-assisted Pb phytoextraction. Chemosphere 74:1279–1291
Smolders E, McLaughlin ML (1996) Effect of Cl on Cd uptake by Swiss Chard in nutrient solutions. Plant Soil 179:57–64
Smolders E, Lambregts RM, McLaughlin MJ, Tiller KG (1998) Effect of soil solution chloride on cadmium availability to Swiss chard. J Environ Qual 27:426–431
Soon YK (1998) Determination of cadmium, chromium, cobalt, lead and nickel in plant tissue. In: Kaltra P (ed) Handbook of reference methods for plant analysis. CRC, London, pp 193–198
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis, part 3, chemical methods—SSSA book series no. 5. Soil Science Society of America and American Society of Agronomy, Madison, pp 475–489
Thomas JC, Malick FK, Endreszl C, Davies EC, Murray KS (1998) Distinct responses to copper stress in the halophyte Mesembryanthemum crystallinum. Physiol Plant 102:360–368
US Environmental Protection Agency (1994) Test methods for evaluating soils and wastes. SW 846. http://www.epa.gov/sw-846/main.htm
US Environmental Protection Agency (2001) Phytoremediation of contaminated soil and ground water at hazardous waste sites. EPA/540/S-01/500. http://www.epa.gov/ada/download/issue/epa_540_s01_500.pdf
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wahla IH, Kirkham MB (2008) Heavy metal displacement in salt-water-irrigated soil during phytoremediation. Environ Pollut 155:271–283
Weggler K, McLaughlin MJ, Graham RD (2004) Effects of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Environ Qual 33:496–504
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Acknowledgments
The project was funded by the European Social Fund and National Resources—EPEAEK II—IRAKLITOS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Peter Schröder and Jean-Paul Schwitzguébel
Rights and permissions
About this article
Cite this article
Manousaki, E., Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environ Sci Pollut Res 16, 844–854 (2009). https://doi.org/10.1007/s11356-009-0224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0224-3