Abstract
Heavy metals (HM) are a unique class of toxicants because they cannot be broken up into nontoxic forms. Excess HM causes stunted growth, upsets mineral nutrition, and affects membrane structure and permeability. High tolerance to HM toxicity is based on reduced metal uptake or increased internal sequestration in a genotype. Arbuscular mycorrhizal (AM) fungi are important rhizospheric microorganisms that occur in metal-contaminated soils and perhaps detoxify the potential effects of metals. The aim of this work was to study the role of the AM fungus Glomus mosseae in the alleviation of cadmium (Cd) and lead (Pb) toxicities in Cajanus cajan (L.) Millsp. (pigeonpea) genotypes. The effects of interactions between Cd (25 and 50 mg/kg) and Pb (500 and 800 mg/kg) on plant dry mass, nitrogen metabolism, and production of phytochelatins (PCs) and glutathione (GSH) were monitored with and without AM fungus in genotypes Sel-85N (relatively tolerant) and Sel-141-97 (sensitive). Cd treatments were more toxic than Pb, and their combinations led to synergistic inhibitions to growth and nitrogen-fixing potential (acetylene reduction activity [ARA]) in both genotypes. However, the effects were less deleterious in Sel-85N than in Sel-141-97. Exposure to Cd and Pb significantly increased the levels of PCs in a concentration- and genotype-dependent manner, which could be directly correlated with the intensity of mycorrhizal infection (MI). Stimulation of GSH production was observed under Cd treatments, although no obvious effects on GSH levels were observed under Pb treatments. The metal contents (Cd, Pb) were higher in roots and nodules when compared with that in shoots, which was significantly reduced in the presence of AM fungi. The results indicated that PCs and GSH might function as potential biomarkers for metal toxicity, and microbial inoculations showed bioremediation potential by helping pigeonpea plants to grow in multimetal contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Man’s energy and chemical consumption, metal smelting, mining, and manufacturing processes often result in environmental contamination with a mixture of potentially toxic metals. The main sources of heavy-metal pollution in agricultural soils are fertilizer impurities and the use of refuge-derived compost and sewage disposal and municipal and industrial waste. Soil contamination with heavy metals has become a worldwide problem, leading to losses in agricultural yield and hazardous health effects as they enter the food chain (Salt and others 1995; Shalaby 2003). Most of the metal precipitates readily in the soil. Metals that are immobilized are considered more hazardous to plants than those that can be dissolved. Higher levels of metal pollutants such as cadmium (Cd) and lead (Pb) can have lethal effects on plant growth. Plants respond favorably to the application of phosphate fertilizers, which often contain cadmium (Cd). Therefore, agricultural soils all over the world are slightly to moderately contaminated by Cd (Vassilev 2002; Rodriguez-Ortiz and others 2006).
Under field conditions, plants are often affected by more than one metal. As reported by Baker and others (1990), Cd never occurs in isolation in the natural environment but mostly as a “guest” metal in Pb/Zn mineralization. Cd is a nonessential element for plants, and excessive amounts in soil can result in injuries such as chlorosis and growth inhibition leading to plant death (Kahle 1993). This heavy metal is also known to affect photosynthesis, nitrogen metabolism, and water and nutrient uptake (Czuba and Kraszewski 1994; Sanita di Toppi and Gabrielli 1999; Labri and others 2002). There are several reports documenting oxidative stress following exposure to high concentrations of Cd (Smeets and others 2005; Dong and others 2006; Lin and others 2007; Lopez-Millan and others 2009). Pb toxicity leads to decreases in germination, length, and dry mass of roots and shoots (Mishra and Choudhari 1998; Obroucheva and others 1998; Munzuroglu and Geckil 2002); disturbed mineral nutrition (Walker and others 1977; Burzynski 1987; Paivoke 2002); reduction in cell division (Wierzbicka 1994; Eun and others 2000); and inhibition of photosynthetic activity (Ahmed and Tajmir-Riahi 1993; Rashid and others 1994; Poskuta and others 1996; Boucher and Carpentier 1999; Jabeen and others 2009). When Cd and Pb enter the cells, even in minute amounts, they produce various toxic effects such as growth retardation (Uveges and others 2002), changes in enzyme activity (Banaszak and others 2001), inhibition of chlorophyll biosynthesis (Miranda and Ilangiovan 1996), and disturbance of respiration (Romanowsk and others 2002) and mineral nutrition and water balance (Hagemeyer and others 1986; Ouariti and others 1997). Both these heavy metals enter plants via the root system where they are found primarily in root cap cells and mucilage as well as in the root hair zone, in the apoplast of the rhizodermis, and in five to six outer layers of the cortex cells (Seregin and others 2003). Then, these metals move from the root cortex to the stele and from there through the xylem to the shoots, though the phloem is not involved (Tudoreanu and Phillips 2004). The interactions between the two metals may be synergistic or antagonistic (Ting and others 1991; John and others 2008). Due to the strong adsorptive ability of Pb in soil, Pb remains fixed in soil, forming stable compounds that are not easily moved. The Cd replaced by Pb improves the phytoavailability of Cd. Thus, Pb is always inferior to Cd in competition, and the phytoavailability of Pb decreases with the presence of Cd (Chen and others 2007). John and others (2008) showed that there was a higher accumulation of Cd than Pb in the shoots of Cucumis sativus, which confirms that there is less uptake of Cd in the presence of Pb. They also indicated that exposure of Lemna polyrrhiza to different concentrations of Cd and Pb results in an increase in growth and in pigment, proline, protein, and sugar content at lower concentrations; at higher concentrations these parameters decreased. The effect of Cd on the inhibition of growth and development was more significant than that of Pb. These interactions among metals obviously complicate studies of the effects of metals on plant growth and other processes and need to be investigated thoroughly.
Plants, like other organisms, have adaptive mechanisms whereby they are able to respond to both nutrient deficiencies and toxicities. One response of plants to heavy-metal stress is the induction of phytochelatins (PCs), a family of related peptides that have the structure (γ-Glu-Cys) n -Gly (where n > 1) and are clearly related to the tripeptide glutathione (GSH), for which n = 1. PC synthesis is rapidly induced in the presence of a wide range of heavy metals (Grill and others 1987; Maitani and others 1996) and is involved in the cellular detoxification mechanism responsible for the ability to form stable metal-phytochelatin complexes. Therefore, it is the formation of stable complexes that is important for metal tolerance rather than the ability to synthesize PCs (Yen and others 1999).
Soil microorganisms are known to play a key role in the mobilization and immobilization of metal cations, thereby changing their availability to plants. Metal tolerance in soil microorganisms has been studied in the context of removing metals from polluted soils (bioremediation), but also in providing a biological understanding of adaptation of living organisms to extreme environments (Khade and Adholeya 2007). Arbuscular mycorrhizal (AM) associations are integral functioning parts of plant roots and are widely recognized as enhancing plant growth at severely disturbed sites, including those contaminated with heavy metals. They are reported to be present on the roots of plants growing on heavy-metal-contaminated soils and play an important role in metal tolerance and accumulation (Pawlowska and others 1996; Del Val and others 1999; Hildebrandt and others 1999; Gaur and Adholeya 2004; Andrade and others 2005). In mycorrhizal symbiosis, increases or reductions in metal content in the host plant have been observed depending on growth conditions as well as on the fungi and plant species involved (Weissenhorn and Leyval 1995; Joner and Leyval 1997). AM fungi can alleviate metal toxicity by enhancing nutrient supply and/or improving water relations (Meharg and Cairney 2000). Other possible metal tolerance mechanisms in the AM symbiosis include dilution of the metal ions by increased root or shoot growth, increased metal:phosphorus ratio, exclusion by precipitation of polyphosphate granules, and compartmentalization into plastids (de Andrade and others 2008; Azcón and others 2009).
With respect to PC production, Turnau and others (1993) observed high concentrations of nitrogen (N) and sulfur (S) as well as Cd in the vacuoles of an AM fungi associated with Pteridium aquinilnum collected from Cd-contaminated soil, suggesting the existence of thiol-binding peptides in this class of fungi. The presence of metal-binding peptides in AM plants was reported by Galli and others (1994), who found an increase in thiol compounds related to the mycorrhizal status in response to copper (Cu) stress in maize plants (de Andrade and others 2008).
In general, it has been shown that legume crops are less tolerant to Cd toxicity compared to cereals and grasses (Inouhe and others 1994; Mazen 1995). However, there is evidence of intraspecific genetic variation in the tolerance of legumes to heavy metals (Belimov and others 2003). Although significant differences in biomass concentrations of Cd in plant biomass have been found among cultivars of peanut, soybean, and navybean (Bell and others 1997), little is known about cultivar-dependent variability in the uptake of both Cd and Pb (individual as well as combined) in legume species.
Pigeonpea [Cajanus cajan (L.) Millspaugh] is a short-lived perennial shrub that is traditionally cultivated as an annual crop in developing countries. Based on the vast natural genetic variability in local germplasm and the presence of numerous wild relatives, Van der Maesen (1980) and Saxena (2008) reported that India is probably its primary center of origin and has the largest area (around 3.6 M ha) under pigeonpea cultivation. Pigeonpea is a hardy, widely adapted, and drought-tolerant crop with a large temporal variation for maturity. These traits allow its cultivation in a range of environments and cropping systems. Its roots help to release soil-bound phosphorus, making it available for plant growth. With so many benefits at a low cost, pigeonpea has become an ideal crop for sustainable agriculture.
The aim of this work was to study the effects of the interactions between Cd and Pb and the AM fungus Glomus mosseae on growth, metal distribution, and production of PCs and GSH in Cajanus cajan (L.) Millsp. (pigeonpea) genotypes with different metal tolerances.
Materials and Methods
Experimental Design and Growth Conditions
Greenhouse experiments were conducted to study the relative response of two genotypes of pigeonpea [Cajanus cajan (L.) Millsp.] in terms of growth and phytochelatin and glutathione production under heavy-metal conditions. The seeds were obtained from Pulse Laboratory, and specific strains of Sinorhizobium fredii AR-4 and Glomus mosseae were obtained from the Department of Microbiology, Indian Agricultural Research Institute (I.A.R.I), New Delhi, India. The minimum temperature ranged from 22 to 29°C and the maximum temperature ranged from 30 to 37°C. The morning relative humidity was between 55 and 92% and afternoon relative humidity was between 42 and 81%.
Preparation of Pots
The soils were fumigated with methyl bromide under air-tight plastic sheets and the fumigant was allowed to dissipate for 1 week (Al-Raddad 1991). Each pot was filled with 7 kg of soil mixture; the pH of the soil was 7.2, electrical conductivity was 1.0 dSm−1, and the P content was 20 ppm. The pots were watered well before sowing to check for proper drainage from the pots.
Sowing and Raising of Plants
The seeds were washed with water and surface-sterilized by dipping in a 15% H2O2 solution for 8 min, then they were washed several times with sterile water to remove any trace of chemical that could interfere with seed germination. Seeds were pretreated with specific rhizobial inoculum of Sinorhizobium fredii AR-4 and were kept at room temperature to dry. Seeds were simultaneously inoculated with AM cultures of Glomus mosseae. The AM spores were applied at ten spores per seed (approximately 1500 spores/100 g of media). Seeds were inoculated by placing the AM inoculum in the hole under the seed and covering the soil. Eight seeds per pot were sown at uniform depth and distance. After the establishment of seedlings, they were thinned so that there were only three plants of uniform size in each pot.
Treatments
The pots were supplemented with heavy-metal solutions of Pb (500 and 800 mg/kg dry soil) and Cd (25 and 50 mg/kg dry soil) individually as well as in combinations 15 days after emergence (DAE). Two pots from each of the treatments were selected at random for sampling at 60 DAE. Six replicates were sampled for recording the observations.
Sampling Technique
The plants were carefully separated from the pots by gently washing out the soil with running tap water in order to avoid damage to the nodules. The sticking soil, along with the plants, were then transferred to a sieve (mesh) and the roots with attached nodules were washed clear of the adhering soil. The detached roots and nodules were collected from the sieve and combined with the rest of the plant material. For dry weight (DW) measurements, the samples were dried in an oven at 70°C for a few days until they reached constant weight.
Statistical Analysis
The significance of differences between mean values obtained from the experiments was determined by using Dunnett’s multiple-range test. The standard errors were also calculated using SPSS software (SPSS, Inc., Chicago, IL, USA).
Methodology
Mycorrhizal Infection
Mycorrhizal infection (MI) was calculated according to Phillips and Hayman (1970) as follows:
Metal Determination
Harvested plant samples were divided into roots and shoots, carefully washed with deionized water after rinsing with tap water, and then dried at 105°C for 20 min and then at 70°C in an oven until completely dry. The dried plant samples were ground to powder after their dry weights were determined. Soil samples were air-dried and ground using a mortar and pestle and then were sieved through a 0.149-mm sieve (Wei and Zhou 2004). The plant and soil samples were digested in a solution containing 3:1 HNO3:HClO4 solution (Wang and Zhou 2003). The concentrations of heavy metals were determined using an atomic absorption spectrophotometer (AAS, Hitachi 180-180 type) with certified reference materials for quality assurance purposes. Reagent blanks and internal standards were used to ensure accuracy and precision in Cd and Pb analysis.
Determination of Glutathione
Glutathione was estimated following the method of Anderson (1985). Fresh leaf samples (0.5 g) were homogenized in 2 ml of 5% (w:v) sulfosalicylic acid under cold conditions. The homogenate was centrifuged at 10,000 rpm for 10 min. To 0.5 ml of supernatant, 0.6 ml of 100 mM (pH 7.0) phosphate buffer and 40 μl of 5′,5′-dithiobis-2-nitrobenzoic acid (DTNB) were added. After 2 min the absorbance was read at 412 nm on a UV–Vis spectrophotometer. A standard curve was prepared from varying concentrations of reduced glutathione.
Determination of Total Nonprotein Thiols
Total nonprotein thiols were determined as described by Del Longo and others (1993). One hundred microlitres of the supernatant (described above) was placed in a microfuge tube to which 0.5 ml of reaction buffer [0.1 M phosphate buffer (pH 7.0) and 0.5 mM EDTA] and 0.5 ml of DTNB (1 mM) were added. The reaction mixture was incubated for 10 min and absorbance was read at 412 nm using the UV–Vis spectrophotometer. Values for the absorbance were corrected by preparing a blank without extract. A standard curve was prepared from varying concentrations of cysteine to calculate nonprotein thiol content in samples. For theoretical determination of PCs, the difference between total nonprotein thiol and GSH was considered to represent PCs (Bhargava and others 2005).
Results
Percent Mycorrhizal Infection (MI)
MI was calculated for the roots of inoculated plants of both the genotypes (Sel-85N and Sel-141-97) under heavy-metal stress (Table 1). No MI was found in the uninoculated plants. A gradual reduction in MI was observed when the roots were exposed to increasing concentrations of Cd and Pb in the soil. However, the decline was greater in cases of Cd compared to Pb treatments. As the concentration of the metals increased, MI decreased. When plants were exposed to a combination of Cd and Pb, the effects were synergistic and MI further decreased. Although the results showed a decrease in MI with increasing levels of heavy-metal toxicity, it was observed that MI was relatively tolerant to heavy-metal stress and significant infection was observed even at high metal concentrations under single as well as combined applications. Mycorrhizal symbiosis was comparatively more effective in Sel-85N than in Sel-141-97.
Cadmium (Cd) and Lead (Pb) Concentrations
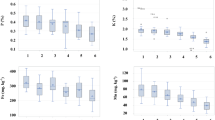
Plants absorbed both Cd and Pb but the absorption of Cd was less compared to Pb depending upon the concentrations of metals applied (Figs. 1, 2) The rate of accumulation of Cd and Pb was higher at lower concentrations in all the plant organs (roots, nodules, and shoots). However, at higher concentrations the absorption was almost stagnant due to the toxicity caused to the plant.
Effects of arbuscular mycorrhizal (AM) inoculation on cadmium content (μg g−1 DW) in pigeonpea genotypes under cadmium (Cd) and lead (Pb) stress, singly and in combinations. Values are means ± SE of six replicates. Asterisk denotes significant difference between exposed and control plants (p < 0.05) as determined by Dunnett’s multiple-comparison test (a nodules, b roots, c leaves)
Effects of arbuscular mycorrhizal (AM) inoculation on lead content (μg g−1 DW) in pigeonpea genotypes under cadmium (Cd) and lead (Pb) stress, singly and in combinations. Values are means ± SE of six replicates. Asterisk denotes significant difference between exposed and control plants (p < 0.05) as determined by Dunnett’s multiple-comparison test (a nodules, b roots, c leaves)
The contents of Cd and Pb were highest in roots, significantly higher in the nodules, and lowest in the shoots. The genotype Sel-85N, which was tolerant to metal stress and supported higher plant biomass, also had relatively lower metal contents than Sel-141-97. In the presence of Pb, the uptake of Cd significantly increased in all plant parts, whereas the absorption of Pb decreased. The presence of the fungal symbiont in the rooting medium significantly arrested the uptake of Cd and Pb into the root system and further translocations into the aboveground parts (shoots).
Dry Weights of Roots and Shoots (g/plant)
In general, the heavy metals Cd and Pb had significant effects on the growth of both genotypes but the effect of Cd was more detrimental than that of Pb. Heavy-metal stress significantly decreased root and shoot dry weights (Table 1). As the concentration of metals increased, dry weights of roots and shoots further decreased. Shoots seemed to withstand Cd and Pb stress more than the roots and percent decline in root biomass was significantly more than shoots. The combined treatments with both metals proved to be synergistic and plant dry weights were further reduced. Because Cd treatments were more toxic, the presence of Pb might have increased the phytoavailability of Cd, thereby inducing higher negative effects. Higher concentrations of Cd and Pb further hampered the growth of the plants. This decline was less significant in Sel-85N, indicating a higher tolerance as compared to Sel-141-97 (single-metal treatments and their combinations). Although Cd content was lower than Pb, the toxicity of Cd was much higher resulting in higher declines in plant biomass compared to that with Pb. However, with the introduction of Glomus mosseae in the rooting medium, there was a significant improvement in the growth of the plants. Because mycorrhizal infection was tolerant to different concentrations of metals, their effective symbiosis might have been responsible for causing tolerance in both genotypes.
Rhizobial Symbiosis
The specific strain of Sinorhizobium fredii AR-4 could induce significantly higher nodulation in both genotypes. The nodule number, nodule dry weight per plant, leghemoglobin, and nitrogenase activity under control and different concentrations of heavy metal in AM-inoculated and uninoculated pigeonpea plants are presented in Table 2. In general, nodules seemed to be highly sensitive to heavy-metal stress and showed significant reduction in their numbers and dry weights, even at the lower heavy-metal concentration of 25 mg Cd/kg dry soil and 500 mg Pb/kg dry soil. In Sel-141-97, the higher heavy-metal concentration of 50 mg of Cd and 800 mg of Pb were progressively deleterious and very few nodules could be counted. However, the reduction in the nodule number per plant and dry weight in Sel-85N was much less, and a comparatively higher number of nodules and greater mass were noticed, even at the highest heavy-metal concentration of 50 mg of Cd and 800 mg of Pb. When plants were exposed to the combination of Cd and Pb, root mass decreased. This led to less root hair which further decreased nodule formation, and there was less leghemoglobin content and nitrogenase activity in the plants. The color of the nodules turned brown due to loss in leghemoglobin protein. The presence of higher levels of Cd and Pb in the nodules led to a drastic decline in the nitrogen-fixing potential of nodules (ARA). AM-inoculated plants had greater plant growth which was accompanied by less decline in nitrogen fixation potential compared to uninoculated plants. The mycorrhizal association displayed a synergistic effect on plant-Rhizobium symbiosis and the plants seemed more symbiotically efficient when AM fungi were present in the root system of all the plants.
Phytochelatins (PCs) and Glutathione (GSH) Production
Nonprotein thiols (NPT) increased with increasing concentrations of both Cd and Pb (Fig. 3). The levels of PCs and GSH also increased significantly with exposure to Cd, whereas exposure to lower concentrations of Pb alone had no obvious effect but at high concentrations Pb had some effect on PCs and GSH levels in both genotypes (Figs. 4, 5). As Cd content was higher in nodules as discussed earlier, PCs were also found to be higher in nodules than in roots and shoots. Although Pb treatments alone had little effect on PC production, Pb combined with Cd yielded higher PC activity compared to that from single Cd treatments, probably because of lead’s role in increasing Cd availability in the soil. The degree of the effect of the combination was dependent on the concentration of both Cd and Pb. Because the presence of AM fungus reduced the absolute contents of both metals significantly, it had a direct role in further increasing the production of PCs and GSH, and the production of these biomarkers made the plants more adaptable to the stress conditions.
Effects of arbuscular mycorrhizal (AM) inoculation on nonprotein thiols (μmol g−1 FW) in pigeonpea genotypes under cadmium (Cd) and lead (Pb) stress, singly and in combinations. Values are means ± SE of six replicates. Asterisk denotes significant difference between exposed and control plants (p < 0.05) as determined by Dunnett’s multiple-comparison test (a nodules, b roots, c leaves)
Effects of arbuscular mycorrhizal (AM) inoculation on glutathione production (μmol g−1 FW) in pigeonpea genotypes under cadmium (Cd) and lead (Pb) stress, singly and in combinations. Values are means ± SE of six replicates. Asterisk denotes significant difference between exposed and control plants (p < 0.05) as determined by Dunnett’s multiple-comparison test (a nodules, b roots, c leaves)
Effects of arbuscular mycorrhizal (AM) inoculation on phytochelatin production (μmol g−1 FW) in pigeonpea genotypes under cadmium (Cd) and lead (Pb) stress, singly and in combinations. Values are means ± SE of six replicates. Asterisk denotes significant difference between exposed and control plants (p < 0.05) as determined by Dunnett’s multiple-comparison test (a nodules, b roots, c leaves)
Discussion
The data from studies have clearly shown that Cd and Pb strongly accumulate in roots, more so than in nodules, but substantially lower amounts were observed in leaves (An 2004; Lima and others 2006; Bidar and others 2007). It was also found that accumulation of Pb in roots was greater than Cd. These results were in agreement with the data obtained by other authors for different plant species (Burzynski 1987; Trivedi and Erdei 1992; Burzynski and Buczek 1998; Kim and others 2002). It was proposed by Brennan and Shelley (1999) that a very high accumulation of Pb in roots might be connected with precipitation of the metal at the root surface as amorphous Pb-phosphate. It was noteworthy that transport of Cd to shoots was higher compared to Pb. These data suggest that the mechanism of translocation of Pb and Cd from roots to shoots is different (Kim and others 2002; Brekken and Stennes 2004; Duman and others 2007; Bidar and others 2009).
The constant increase of lead and cadmium in different plant parts resulted in a general reduction in the growth of Cajanus cajan genotypes. Greater decline in plant biomass was observed in the presence of Cd than Pb, although the uptake of the former was significantly less than that of the latter. The reduction in growth of C. cajan provided evidence that elements like Cd or Pb, if present in excess, are responsible for producing toxic effects that reduce plant growth and development (Dixit and others 2001; Vange and others 2004; Hassan and others 2005; Mobin and Khan 2007; Anjum and others 2008). This growth reduction might be due to competition for the same uptake systems between Cd and Pb and other divalent ions required for plant development (Alcantara and others 1994; Lozano-Rodriguez and others 1997; Rivetta and others 1997). Also, Cd and Pb affect plant growth by inhibiting cell division (Paradiso and others 2008).
Legumes and their associated rhizobial bacteria are important components of the biogeochemical cycles in agricultural and natural ecosystems. Nitrogen fixation in nodules is sensitive to heavy-metal stress that affects soil biological activity and plant metabolism. The presence of Cd in the growth media reduces nodule formation and impairs nodule functioning in several legumes (Zornoza and others 2002; Carpena and others 2003; Chen and others 2003; Younis 2007). The results of the present study also revealed that nodule differentiation was affected under Cd and Pb stress. This differentiation was manifested by the appearance of brown nodules due to loss in leghemoglobin pigment. Senescence of legume root nodules is a complex process that implies the structural breakdown of both the bacteroides and the host cells. A positive correlation exists between leghemoglobin content and nitrogenase activity (Dakora 1995; Comba and others 1998). The inhibition of ARA by heavy metals could result from the effects of the heavy metals on bacteroid oxygen uptake because bacteroid respiration provides the energy and reducing power that nitrogenase needs for efficient nitrogen fixation (Balestrasse and others 2004, 2006; LiQin and others 2008; Shvaleva and others 2010).
The results of the present study showed the induction of PCs by both Cd and Pb, although Cd was found to be a stronger inducer of the production of PCs than Pb probably because of the higher toxic effect of Cd observed in the plants. Phytochelatin synthase is one of the key enzymes in PC synthesis and is responsible for the metal-regulated synthesis of PCs. This enzyme requires free metal ions such as Cd2+ and Pb2+ for activity (Chen and others 1997; Li and others 2006; Mishra and others 2006; Pal and Rai 2010). Therefore, the results obtained in the present study further demonstrate that the synthesis of PCs is closely linked to metal uptake and accumulation. This observation is consistent with reports on plants exposed to unequal concentrations of excess Cd or Pb (Zenk 1996; Gisbert and others 2003; Kang and others 2007; Jabeen and others 2009; Pal and Rai 2010). When Cd and Pb were combined, reciprocal synergistic effects of Cd and Pb with respect to the production of PCs were observed. The synergistic effects observed in the present study are likely to be connected with the effects of intracellular metals simultaneously activating the catalytic activities of PCs in the cytoplasm. Furthermore, the present results obtained using different concentrations of Cd and Pb in combination suggests that there are interactive effects of Cd and Pb with respect to the production of PCs.
Glutathione (GSH) is a major low-molecular-weight thiol compound in most plants (Kunert and Foyer 1993; Foyer and others 1997). It is a major nonprotein thiol in plant cells and also represents a storage form of reduced sulfur (S). It is not only a central compound in S metabolism but also a major component of the cellular antioxidative defense system against heavy-metal toxicity (May and others 1998a, b; Gong and others 2005; Pitschke and others 2006; Blum and others 2007; Szalai and others 2009). Glutathione acts as a disulfide reductant to protect thiol groups on enzymes (Noctor and others 2002). Sulfate assimilation pathways provide plants with cysteine which is further used for protein synthesis and as a source of reduced sulfur for the biosynthesis of a number of S-containing compounds, including GSH. It has previously been shown that cysteine synthesis is improved with the addition of S sources and that the surplus of Cys limits the synthesis of GSH (Saito and others 1994; Nikiforova and others 2003). Therefore, as observed in the present study, GSH synthesis of plant cells is dependent on and regulated by the S supply of the plants. It has been suggested that GSH acts as a first line of defense against metal toxicity by complexing/influxing metals before the induced synthesis of PCs arrives at the effective level (Singhal and others 1987; Freedman and others 1989). Furthermore, GSH is the direct precursor/substrate for the synthesis of PC (Anjum and others 2008; Singh and others 2008). It has also been reported that elevated levels of GSH synthesis appear to be an intrinsic response of plants to stress and may be involved in signal transduction of environmental stress (May and others 1998a). Based on these results, GSH may serve as a biomarker for metal toxicity in plants, especially for Cd. This indicates that under metal stress, pigeonpea plants have a positive responsive mechanism to regulate GSH synthesis with an adaptation to metal stress and for PC synthesis. The results suggest that GSH may have a dual role in response to metal stress.
Pigeonpea plants developed chlorosis and necrosis when grown in heavy-metal-contaminated soil not inoculated with AM fungi, but these plants resisted the adverse soil conditions when they were inoculated with AM fungi. High amounts of heavy metals in the soil can decrease plant growth and nutrient uptake and it is known that AM fungi protect plants against the toxicity of heavy metals (Rabie 2005; Abou-Shanab and others 2008). Extrapolation of the data shows that nodule formation and percentage of mycorrhizal infection in pigeonpea plants were greatly reduced with increasing Cd and Pb concentrations (Wang and others 2007). Nevertheless, Cd was more toxic than Pb to inoculated plants. This finding is in line with results of Hildebrandt and others 1999 and Vogel-Mikus and others (2005) who reported that sensitivity of AM symbionts to heavy-metal-contaminated soil was expressed as a reduction in spore germination, hyphal growth, or root colonization, while sensitivity of N2-fixing bacteria was expressed as decreased nodule formation and bacterial numbers (Andrade and others 2004). AM symbiosis is known to support nitrogen fixation by providing legumes with phosphorus (P) and other immobile nutrients such as copper and zinc that are essential for nitrogen fixation (Clark and Zeto 2000; Scheublin and van der Heijden 2006). However, reports on the role of AM fungi in improving nodulation and nitrogen metabolism under Cd and Pb stress are scarce. The present study showed that the presence of AM fungi enhanced the number of nodules, their dry weights, leghemoglobin content, and nitrogenase activity in both genotypes. There are a few reports on the effects of mycorrhizal fungi on Cd and Pb uptake by plants. We recorded less Cd and Pb in the shoots of mycorrhizal plants compared with nonmycorrhizal plants at the highest Cd and Pb levels. Galli and others (1994) suggested a possible retention of heavy metals by fungal mycelia involving adsorption to cell walls, thereby minimizing metal translocation to the shoots. This hypothesis was corroborated by Joner and others (2000) who demonstrated that AM mycelia had a high metal sorption capacity. However, the lower Cd and Pb concentrations found in mycorrhizal plants compared to those in nonmycorrhizal plants may be a consequence of the dilution effects caused by their greater growth, as suggested by Plenchette and others (1983), Jamal and others (2002), Yu and others (2005), and Vosatka and others (2006). AM attenuates the toxic effect of metals, retaining them in the fungal structure with the subsequent restriction of metal transfer to the plant (Joner and Leyval 1997); Vivas and others 2003). We noticed a qualitative and quantitative increase in plant organ phytochelatin synthesis that correlated with fungal presence. Heavy-metal stress has recently been shown to increase the transcription of a phytochelatin synthetase gene in mycorrhizal pigeonpea roots, in which the expression of genes was also enhanced (Rivera-Becerril and others 2002, 2005). In general, it is currently accepted that the presence of heavy metals in the soil induces changes in the pattern of plant gene and protein expression (Robinson and others 1994; Prasad 1995; Hajduch and others 2001). Recent findings by Repetto and others (2003) showed that mycorrhizal symbioses also modulate the expression of several plant proteins. The significance of these changes and their relation to plant tolerance are not clear yet.
Conclusion
Specific intraspecific variation was found between the tested genotypes of Cajanus cajan in their tolerance to Cd and Pb in the presence of toxic Cd and Pb concentrations when the plants were grown in contaminated soil. The differential effects between Cd and Pb tolerance, heavy-metal concentrations, and more efficient biomass production indicate the existence of independent genetic controls of these traits and represent a useful model for studying mechanisms of heavy-metal tolerance in pigeonpea genotypes.
References
Abou-Shanab RA, Ghanem K, Ghanem N, Al-Kolaibe A (2008) The role of bacteria on heavy metal extraction and uptake by plants growing on multi-metal-contaminated soils. World J Microbiol Biotechnol 24:253–262
Ahmed A, Tajmir-Riahi HA (1993) Interaction of toxic metals ions Cd2+, Hg2+ and Pb2+ with light-harvesting proteins of chloroplast thylakoid membranes: an FTIR spectroscopic study. J Inorg Biochem 40:235–243
Alcantara E, Romera FJ, Canete M, De La Guardia MD (1994) Effects of heavy metals on both induction and function of root Fe (III) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. J Exp Bot 45:1893–1898
Al-Raddad A (1991) Response of bean, broad bean and chickpea plants to inoculation with Glomus species. Sci Hortic 146:195–200
An YJ (2004) Soil ecotoxicity assessment using cadmium sensitive plants. Environ Sci Technol 127:21–26
Anderson ME (1985) Determination of glutathione and glutathione disulfides in biological samples. Meth Enzymol 113:548–570
Andrade SAL, Abreu CA, Abreu MF, Silveira APD (2004) Influence of lead additions on arbuscular mycorrhiza and Rhizobium symbiosis under soybean plants. Appl Soil Ecol 26:123–131
Andrade SAL, Jorge RA, da Silveira APD (2005) Cadmium effect on the association of jackbean (Canavalia ensiformis) and arbuscular mycorrhizal fungi. Sci Agric 62(4):389–394
Anjum NA, Umar S, Ahmad A, Iqbal M (2008) Responses of components of antioxidant system in moongbean (Vigna radiata L. Wilezek) genotypes to cadmium. Common Soil Sci Plant Anal 39:2469–2483
Azcón R, Perálvarez Mdel C, Roldán A, Barea JM (2009) Arbuscular mycorrhizal fungi, Bacillus cereus and Candida parapsilosis, from a multicontaminated soil alleviate metal toxicity in plants. Microb Ecol 59(4):668–677
Baker AJM, Ewart K, Hendry GAF, Thorpe PC, Walker PL (1990) The evolutionary basis of cadmium tolerance in higher plants. In: 4th international conference on environmental contamination, Barcelona, pp 23–29
Balestrasse KB, Gallego SM, Tomaro ML (2004) Cadmium induced senescence in nodules of soybean (Glycine max. L.) plants. Plant Soil 262:373–381
Balestrasse KB, Gallego SM, Tomaro ML (2006) Oxidation of the enzymes involved in nitrogen assimilation plays an important role in the cadmium-induced toxicity in soybean plants. Plant Soil 284:187–194
Banaszak A, Napieralska A, Wozny A (2001) Inhibition of greening of Lemna minor by Pb2+. Biology 56:111–116
Belimov AA, Safronova VI, Tsyganov VE, Borisov AY, Kozhemyakov AP, Tikhonovich IA, Stepanok VV, Martenson AM, Gianinazzi–Pearson V (2003) Genetic variability in tolerance to cadmium and accumulation of heavy metals in pea (Pisum sativum L.). Euphytica 131:25–35
Bell MJ, Mclaughlin MJ, Wright GC, Cruickshank J (1997) Inter and intra-specific variation in accumulation of cadmium by peanut, soybean, and navybean. Aus J Agr Res 48:1151–1160
Bhargava P, Srivastava AK, Urmil S, Rai LC (2005) Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J Plant Physiol 162:1220–1225
Bidar G, Garcon G, Pruvot C, Dewaele D, Cazier F, Douay F, Shirali P (2007) Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environ Pollut 147:546–553
Bidar G, Pruvot C, Garcon G, Verdin A, Shirali P, Douay F (2009) Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environ Sci Pollut Res 16:42–53
Blum R, Beck A, Korte A, Stengel A, Letzel T, Lendzian K, Grill E (2007) Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J 49:740–749
Boucher N, Carpentier R (1999) Hg2+, Cu2+, and Pb2+ induced changes in photosystem-II photochemical yield and energy storage in isolated thylakoid membranes: a study using simultaneous and photoacoustic measurements. Photosynth Res 59:167–174
Brekken A, Stennes E (2004) Seasonal concentrations of cadmium and zinc in native pasture plants: consequences for grazing animals. Sci Total Environ 326:181–195
Brennan MA, Shelley ML (1999) A model of the uptake, translocation and accumulation of lead (Pb) by maize for the purpose of phytoextraction. Ecol Eng 12:271–297
Burzynski M (1987) The influence of lead and cadmium on the absorption and distribution of potassium, calcium, magnesium and iron in cucumber seedlings. Acta Physiol Plant 9:229–238
Burzynski M, Buczek J (1998) Uptake and assimilation of ammonium ions by cucumber seedlings from solutions with different pH and addition of heavy metals. Acta Soc Bot Pol 67(2):197–200
Carpena RO, Vazquez S, Esteban E, Fernandez-Pascual M, de Felipe MR, Zornoza P (2003) Cadmium-stress in white lupin: effects on nodule structure and functioning. Plant Physiol Biochem 41:911–919
Chen J, Zhou J, Goldsbrough PB (1997) Characterization of phytochelatin synthase from tomato. Physiol Plant 101:165–172
Chen YX, He YF, Yang Y, Yu YL, Zhen SJ, Tian GM, Luo YM, Wong MH (2003) Effect of cadmium on nodulation and N2 fixation of soybean in contaminated soils. Chemosphere 50:781–787
Chen S, Sun T, Chao L, Guo G (2007) Interaction between cadmium, lead and potassium fertilizer (K2SO4) in a soil-plant system. Environ Geochem Health 29:435–446
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23:867–902
Comba ME, Benavides MP, Tomaro ML (1998) Effect of salt stress on antioxidant defence system in soybean root nodules. Aust J Plant Physiol 25:665–671
Czuba M, Kraszewski A (1994) Long term cadmium exposure accelerates oxidant injury: significance of bound/free water states during long-term metal stress. Ecotoxicol Environ Saf 29(3):330–348
Dakora FD (1995) A functional relationship between leghemoglobin and nitrogenase based on novel measurements of the two proteins in legume root nodules. Ann Bot 75:49–54
de Andrade SAL, da Silveira APD, Jorge RA, de Abreu MF (2008) Cadmium accumulation in sunflower plants influenced by arbuscular mycorrhiza. Int J Phytoremed 10(1):1–13
Del Longo OT, Gonzalez CA, Pastori GM, Tripps VS (1993) Antioxidant defences under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol 34:1023–1028
Del Val C, Barea JM, Azcon-Aguilar C (1999) Diversity of arbuscular mycorrhizal fungus populations in heavy metal contaminated soils. Appl Environ Microbiol 65:718–723
Dixit V, Pandey V, Shyam R (2001) Differential oxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109
Dong J, Wu FB, Zhang GP (2006) Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 64:1659–1666
Duman F, Cicek M, Sezen G (2007) Seasonal changes of metal accumulation and distribution in common club rush (Schoenoplectus lacustris) and common reed (Phragmites australis). Ecotoxicology 16:457–463
Eun SO, Youn HS, Lee Y (2000) Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol Plant 110:357–365
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Freedman JH, Ciriolo MR, Peisach J (1989) The role of glutathione in copper metabolism and toxicity. J Biol Chem 264:5598–5605
Galli U, Schuepp H, Brunold C (1994) Heavy metal binding by mycorrhizal fungi. Physiol Plant 92:364–368
Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86:528–534
Gisbert C, Ros R, de Haro A, Walker DJ, Bernal MP, Serrano R, Navarro-Avino J (2003) A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem Biophys Res Commun 303:440–445
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Grill E, Winnacker EL, Zenk MH (1987) Phytochelatins, a class of heavy metal binding peptides from plants are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84:439–443
Hagemeyer J, Kahle H, Breckle SW, Waisel Y (1986) Cadmium in Fagus sylvatica L. trees and seedlings: leaching, uptake and interconnection with transpiration. Water Air Soil Pollut 29:347–359
Hajduch M, Rakwal R, Agrawal GK, Yonekura M, Petrova A (2001) High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: drastic reduction/fragmentation of ribulose-1,5-biphosphate carboxylase/oxygenase and induction of stress-related proteins. Electrophoresis 22:2824–2831
Hassan JM, Wang Z, Zhang G (2005) Sulphur alleviates growth inhibition and oxidative stress caused by cadmium toxicity in rice. J Plant Nutr 28:1785–1800
Hildebrandt U, Kaldorf M, Bothe H (1999) The zinc violet and its colonization by arbuscular mycorrhizal fungi. J Plant Physiol 154:709–717
Inouhe M, Ninomiya S, Tohoyama H, Joho M, Murayama T (1994) Differential characteristics of root in the cadmium-tolerance and Cd-binding complex formation between mono- and dicotyledonous plants. J Plant Res 107:201–207
Jabeen R, Ahmad A, Iqbal M (2009) Phytoremediation of heavy metals: physiological and molecular mechanisms. Bot Rev 75:339–364
Jamal A, Ayub N, Usman M, Khan AG (2002) Arbuscular mycorrhizal fungi enhance zinc and nickel uptake from contaminated soil by soybean and lentil. Int J Phytorem 4:205–221
John R, Gadgil K, Ahmad P, Sharma S (2008) Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil Environ 54(6):262–270
Joner EJ, Leyval C (1997) Uptake of 109Cd by roots and hyphae of a Glomus mosseae/Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol 135:352–360
Joner EJ, Briones R, Leyval C (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 226:227–234
Kahle H (1993) Response of roots of trees to heavy metals. Environ Exp Bot 33:99–119
Kang SH, Singh S, Kim JY, Lee W, Mulchandani A, Chen W (2007) Bacteria metabolically engineered for enhanced phytochelatin production and cadmium accumulation. Appl Environ Microbiol 73:6317–6320
Khade SW, Adholeya A (2007) Feasible bioremediation through arbuscular mycorrhizal fungi imparting heavy metal tolerance: a retrospective. Bioremed J 11:1–33
Kim YY, Yang YY, Lee Y (2002) Pb and Cd uptake in rice roots. Physiol Plant 116:368–372
Kunert KJ, Foyer CH (1993) Thiol/disulfide exchange in plants. In: De Kok LJ (ed) Sulphur nutrition and assimilation in higher plants. SPB Academic Publishing, The Hague, pp 139–151
Labri A, Morales F, Abadia A, Gogorcena Y, Lucena JJ, Abadia J (2002) Effects of Cd and Pb in sugarbeet plants grown in nutrient solution: induced Fe deficiency and growth inhibition. Funct Plant Biol 29:1453–1464
Li J, Guo J, Xu W, Ma M (2006) Enhanced cadmium accumulation in transgenic tobacco expressing the phytochelatin synthase gene of Cynodon dactylon L. J Integr Plant Biol 48:928–937
Lima AI, Pereria SI, Figueira EM, Caldeira GC, Caldiera HD (2006) Cadmium detoxification in roots of Pisum sativum seedlings: relationship between toxicity levels, thiol pool alterations and growth. Environ Exp Bot 55:149–162
Lin RZ, Wang XR, Luo Y, Du WC, Guo HY, Yin DQ (2007) Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 69:89–98
LiQin C, YiFei G, LiMin Y, QinQuan W (2008) Synergistic defensive mechanism of phytochelatins and antioxidative enzymes in Brassica chinensis L. against Cd stress. Chin Sci Bull 53(10):1503–1511
Lopez-Millan A, Sagardoy R, Solonas M, Abadia A, Abadia J (2009) Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot 65:376–385
Lozano-Rodriguez E, Hernandez LE, Bonay P, Carpena-Ruiz RO (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 306:123–128
Maitani T, Kubota H, Sato K, Yamada T (1996) The composition of metals bound to class III metallothionein (phytochelatin and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiol 110:1145–1150
May MJ, Vernoux T, Leaver C, Van-Montagu M, Inze D (1998a) Glutathione homeostatis in plants: implications for environmental sensing and plant development. J Exp Bot 49:649–667
May MJ, Vernoux T, Sanchez-Fernandez R, Van Montagu M, Inze D (1998b) Evidence for posttranscriptional activation of γ-glutamylcysteine synthetase during plant stress responses. Proc Natl Acad Sci USA 95:12049–12054
Mazen AMA (1995) Assessment of heavy metal accumulation and performance of some physiological parameters in Zea mays L. and Vicia faba L. grown on soil amended by sewage sludge resulting from sewage water treatment in the state of Qatar. Qatar Univ Sci J 15:353–359
Meharg AA, Cairney JWG (2000) Co-evolution of mycorrhiza symbionts and their host to metal contaminated environments. Adv Ecol Res 30:69–112
Miranda MG, Ilangiovan K (1996) Uptake of lead by Lemna gibba L. influence on specific growth rate and basic biochemical changes. Bull Environ Contam Toxicol 56:1000–1007
Mishra A, Choudhari MA (1998) Amelioration of lead and mercury effects on germination and rice seedling growth by antioxidants. Biol Plant 41:469–473
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MN (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44(1):25–37
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Munzuroglu O, Geckil H (2002) Effects of metals on seed germination, root elongation, and coleoptiles and hypocotyls growth in Triticum aestivum and Cucumis sativus. Arch Environ Contam Toxicol 43:203–213
Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R (2003) Transcriptome analysis of sulphur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J 33:633–650
Noctor G, Gomez LA, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Obroucheva NV, Bystrova EI, Ivanov VB, Anupova OV, Seregin IV (1998) Root growth responses to lead in young maize seedlings. Plant Soil 200:55–61
Ouariti O, Boussama N, Zarrouk M, Cherif A, Ghorbal MH (1997) Cadmium and copper induced changes in tomato membrane lipids. Phytochemistry 45:1343–1350
Paivoke AEA (2002) Soil lead alters phytase activity and mineral nutrient balance of Pisum sativum. Environ Exp Bot 48:61–73
Pal R, Rai JPN (2010) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160:945–963
Paradiso A, Berardino R, de Pinto MC, Sanita di Toppi L, Storelli MM, Tommasi F, Gara LG (2008) Increase in ascorbate- glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49(3):362–374
Pawlowska TE, Blaszkowski J, Ruhling A (1996) The mycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza 6:499–505
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Pitschke A, Forzani C, Hirt H (2006) Reactive oxygen species signaling in plants. Antioxid Redox Signal 8:1757–1764
Plenchette C, Furlan V, Fortin JA (1983) Responses of endomycorrhizal plants grown in a calcined montmollironite clay to different levels of soluble phosphorus. II. Effect on nutrient uptake. Can J Bot 61:1384–1391
Poskuta JW, Parys E, Romanowaska E (1996) Toxicity of lead to photosynthesis, accumulation of chlorophyll, respiration and growth of Chlorella pyrenoidosa. Protective role of dark respiration. Acta Physiol Plant 18:165–171
Prasad MNV (1995) Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot 35:525–545
Rabie GH (2005) Contribution of arbuscular mycorrhizal fungus to red kidney and wheat plants tolerance grown in heavy metal-polluted soil. Afr J Biotechnol 4(4):332–345
Rashid A, Camm EL, Ekramoddoullah KM (1994) Molecular mechanism of action of Pb and Zn2+ on water oxidizing complex of photosystem II. FEBS Lett 350:296–298
Repetto O, Bestel-Corre G, Dumas-Gaudot E, Berta G, Gianinazzi-Pearson V, Gianinazzi S (2003) Targeted proteomics to identify cadmium-induced protein modification in Glomus mosseae-inoculated pea roots. New Phytol 157:555–567
Rivera-Becerril F, Calantzis C, Turnau K, Caussanel JP, Belimov AA, Gianinazzi S, Strasser JR, Gianinazzi-Pearson V (2002) Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. J Exp Bot 371:1177–1185
Rivera-Becerril F, van Tuinen D, Martin-Laurent F, Metwally A, Dietz KJ, Gianinazzi S, Gianinazzi-Pearson V (2005) Molecular changes in Pisum sativum L. roots during arbuscular mycorrhiza buffering of cadmium stress. Mycorrhiza 16:51–60
Rivetta A, Negrini N, Cocucci M (1997) Involvement of Ca2+ calmodulin in Cd2+ toxicity during the early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ 20:600–608
Robinson NJ, Urwin PE, Robinson PJ, Jackson PJ (1994) Gene expression in relation to metal toxicity and tolerance. In: Basra AJ (ed) Stress induced gene expression in plants. Harwood Academic Publishers, Amsterdam, pp 209–248
Rodriguez-Ortiz JC, Valdez-Cepeda RD, Lara-Mireles JL, Rodriguez-Fuentes H, Vazquez-Alvarado RE, Magallanes-Quintanar R, Garcia-Hernandez JL (2006) Soil nitrogen fertilization effects on phytoextraction of cadmium and lead by tobacco (Nicotiana tabaccum L.). Biorem J 10(3):105–114
Romanowsk E, Igamberdiev U, Parys E, Gardestron P (2002) Stimulation of respiration by Pb2+ in detached leaves and mitochondria of C3 and C4 plants. Physiol Plant 116:148–154
Saito K, Kurosawa M, Tatsuguchi K, Takagi Y, Murakoshi I (1994) Modulation of cysteine biosynthesis in the chloroplasts of transgenic tobacco overexpressing cysteine synthesis (O-acetylserine(thiol)lyase). Plant Physiol 106:887–895
Salt DEM, Blaylock NP, Kumar V, Dushenkov BD, Ensley I, Chet RI (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Sanita di Toppi L, Gabbrielli R (1999) Response of cadmium in higher plants. Environ Exp Bot 41:105–130
Saxena KB (2008) Genetic improvement of pigeon pea—a review. Trop Plant Biol 1:159–178
Scheublin TR, van der Heijden MGA (2006) Arbuscular mycorrhizal fungi colonize non-fixing nodules of several legume species. New Phytol 172:732–738
Seregin IV, Ivanov VB, Shpigun LK (2003) Distribution and toxic effects of cadmium and lead on maize roots. Russ J Plant Physiol 51(4):525–533
Shalaby AM (2003) Responses of arbuscular mycorrhizal fungal spores isolated from heavy metal-polluted and unpolluted soil to Zn, Cd, Pb and their interactions in vitro. Pak J Biol Sci 6(16):1416–1422
Shvaleva A, de La Peña TC, Rincón A, Morcillo CN, de la Torre VSG, Lucas MM, Pueyo JJ (2010) Flavodoxin overexpression reduces cadmium-induced damage in alfalfa root nodules. Plant Soil 326:109–121
Singh S, Anjum NA, Khan NA, Nazar R (2008) Metal-binding peptides and antioxidant defence system in plants: significance in cadmium tolerance. In: Khan NA, Singh S (eds) Abiotic stress and plant responses. IK International, New Delhi, pp 159–189
Singhal RK, Anderson ME, Meister A (1987) Glutathione, a first line defence against cadmium toxicity. FASEB J 1:220–223
Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere A, Vangronsveld J (2005) Induction of oxidative stress and antioxidant mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol Biochem 43:437–444
Szalai G, Kellos T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80
Ting YP, Lawson F, Prince IG (1991) Uptake of cadmium and zinc by alga Chlorella vulgaris: multiion situation. Biotechnol Bioeng 37:445–455
Trivedi S, Erdei L (1992) Effects of cadmium and lead on the accumulation of Ca2+ and K+ and on the influx and translocation of K+ in wheat of low and high K+ status. Physiol Plant 84:94–100
Tudoreanu L, Phillips CJC (2004) Modeling cadmium uptake and accumulation in plants. Adv Agron 84:121–157
Turnau K, Kottke I, Oberwinkler F (1993) Element localization in mycorrhizal roots of Pteridium aquinilium L. Kuhn collected from experimental plots treated with cadmium dust. New Phytol 123:313–324
Uveges JL, Corbett AL, Mal TK (2002) Effects of Pb contamination on the growth of Lythrum salicaria. Environ Pollut 120:319–323
Van der Maesen LJG (1980) India is the native home of the pigeonpea. In: Arends JC, Boelama G, de Grant CT, Leeuwenberg A (eds) Libergratulatorious in Honerem HCD de Wit. Agricultural University Miscellaneous Paper 19. Wageningen, The Netherlands, pp 257–262
Vange V, Hevchand I, Vandvik V (2004) Does seed mass and family affect germination and juvenile performance in Knautia arvensis? A study using failure time methods. Acta Oecol 25(3):169–178
Vassilev A (2002) Physiological and agroecological aspects of cadmium interactions with barley plants: an overview. J Central Eur Agric 4:65–75
Vivas A, Azcon R, Biro B, Barea JM, Ruiz-Lozano JM (2003) Influence of bacterial strains isolated from lead-polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pretense L. under lead toxicity. Can J Microbiol 49:577–588
Vogel-Mikus K, Drobne D, Regvar M (2005) Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonization of pennycress Thalaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ Pollut 133:233–242
Vosatka M, Rydlova J, Sudova R, Vohnik M (2006) Mycorrhizal fungi as helping agents in phytoremediation of degraded and contaminated soils. In: Mackova M, Dowling DN, Maeck T (eds) Phytoremediation Rhizoremediation, 1st ed. Springer-Verlag, Berlin, pp 237–257
Walker WM, Miller JE, Hassett JJ (1977) Effect of lead and cadmium upon the calcium, magnesium, potassium and phosphorus concentration in young corn plants. Soil Sci 124:145–151
Wang X, Zhou QX (2003) Distribution of forms for cadmium, lead, copper and zinc in soil land its influences by modifier. J Agr Environ Sci 22:541–545
Wang FY, Lin XG, Yin R (2007) Effect of arbuscular mycorrhizal fungal inoculation on heavy metal accumulation of maize grown in a naturally contaminated soil. Int J Phytorem 9(4):345–353
Wei SH, Zhou QX (2004) Identification of weed species with hyperaccumulative characteristics of heavy metals. Prog Nat Sci 14:495–503
Weissenhorn I, Leyval C (1995) Root colonization of maize by a Cd-sensitive and a Cd-tolerant Glomus mosseae and cadmium uptake in sand culture. Plant Soil 175:233–238
Wierzbicka M (1994) Resumption of mitotic activity in Allium cepa root tips during treatment with lead salts. Environ Exp Bot 34:173–180
Yen CL, Mar MH, Zeisel SH (1999) Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and activation of a caspase. FASEB J 13:135–142
Younis M (2007) Responses of Lablab purpureus-Rhizobium symbiosis to heavy metals in pot and field experiments. World J Agric Sci 3(1):111–122
Yu X, Cheng J, Wong MH (2005) Earthworm-mycorrhiza interaction on Cd uptake and growth of ryegrass. Soil Biol Biochem 37:195–201
Zenk MH (1996) Heavy metal detoxification in higher plants: a review. Gene 179:21–30
Zornoza P, Vazquez S, Esteban E, Fernandez-Pascual M, Carpena R (2002) Cadmium-stress in nodulated white lupin: strategies to avoid toxicity. Plant Physiol Biochem 40:1003–1009
Acknowledgment
The financial support provided by the University Grant Commission, New Delhi, India, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, N., Aggarwal, N. Effects of Interactions Between Cadmium and Lead on Growth, Nitrogen Fixation, Phytochelatin, and Glutathione Production in Mycorrhizal Cajanus cajan (L.) Millsp.. J Plant Growth Regul 30, 286–300 (2011). https://doi.org/10.1007/s00344-010-9191-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-010-9191-7