Abstract
A hydroponics experiment was conducted to investigate the effect of salinity (NaCl) and cadmium (Cd) stresses on growth, lipid peroxidation, and antioxidant enzyme activities of three wheat cultivars differing in salt tolerance. Cd and NaCl stresses inhibited plant growth, reduced chlorophyll content, and increased melondialdehyde content and the activities of superoxide dismutase, catalase and peroxidase. The combined effect of NaCl and Cd on these parameters was larger than both NaCl and Cd alone. There was an obvious difference in the response to the both stresses among the three genotypes, with Pir Sabak-85 being less affected.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd) is a toxic metal pollutant for humans, animals and plants and it enters environment mainly from industrial processes and application of Cd-containing phosphorous fertilizer. Excess Cd accumulation in soil may induce various visible symptoms of phytotoxicity, such as chlorosis and growth reduction of root and shoot. Cadmium may also induce oxidative stress in plant cells. Depending on concentration, cadmium can either inhibit or stimulate the activities of several antioxidative enzymes before any visible symptom of toxicity appears (Sandalio et al. 2001; Lozano-Rodriguez et al. 1997).
Salinity is also one of the major abiotic stresses. The problem of salinity becomes even more acute when plants are exposed to Cd stress. On the other hand, plant species differ in salt and Cd tolerance. Salt stress may also results in an oxidative stress (Comba et al. 1998; Hernandez and Alamansa 2002). It has been well documented that NaCl and Cd stress in combination causes higher plasma membrane permeability and enhances the production of oxygen radicals and H2O2 in plants (Muhling and Lauchli 2003).
On the other hand, plants develop the protective system against oxidative stress. One of the protective mechanisms is the enzymatic antioxidant system, which involves the sequential and simultaneous action of a number of enzymes including superoxide dismutase (SOD) and peroxidase (POD). In fact, activities of antioxidant enzymes are inducible by oxidative stress or Cd (Patra and Panda 1998; Wu et al. 2003). In addition, it has been found that Cd uptake was enhanced when plants were grown in soils with high NaCl content (Weggler et al. 2000). The present study was conducted to assess the effect of salinity (NaCl) and Cd on growth and antioxidant enzyme activity of three wheat cultivars differing in salt tolerance.
Materials and Methods
The experiment was conducted in a greenhouse of Huajiachi campus, Zhejiang University, Hangzhou, China during 2007 using three wheat cultivars, namely Bakhtawar-92 (Bak 92), Pir Sabak-85 (Pir 85) and Khyber-87 (Khy 87). Seeds were firstly surface sterilized with 0.1% H2O2 for 20 min, rinsed thoroughly with deionized water, then soaked overnight in sterile water at room temperature, and germinated in sterilized moist quartz sand. At two leaf stage, plants were selected for uniformity and transplanted into plastic trays containing 40 L nutrient solution (Hoagland and Arnon 1950), covered by a polystyrol plate with 48 evenly distributed holes with two seedlings per hole. Cadmium in the form of CdCl2 was added into the solution to obtain three Cd levels; 0, 2 and 4 μM and salinity were added as NaCl to three levels: 0, 75 and 150 mM. The composition of the nutrient solution was as follows (mg L−1): (NH4)2SO4, 48.2; KH2PO4, 24.8; K2SO4, 15.9; KNO3, 18.5; Ca(NO3)2.4 · H2O, 86.17; MgSO4 · 7H2O, 154.88; MnCl2 · 4H2O, 0.90; H3BO3, 2.90; ZnSO4 · 7H2O, 0.11; CuSO4 · 5H2O, 0.04; Fe citrate, 5.0; H2MoO4, 0.01. The experiment was arranged as a split-split plot design with Cd as main plot factor, salinity as sub plot factor and cultivars as sub–sub plot factor. There were three replications for each treatment. The nutrient solution was continuously aerated and renewed after every 7 days.
Chlorophyll content, expressed as SPAD value, was measured on the second fully expanded leaves using a chlorophyll meter (Minolta SPAD-502) on 28 days after treatment. On the same day, plants were harvested. After being washed thoroughly with sterile distilled water, plants were separated into roots and shoots, and homogenized with a mortar and pestle under chilled conditions in a buffer solution specific for each enzyme. The homogenate was filtered through four layers of muslin cloth and centrifuged at 12,000×g for 10 min at 4°C, and the supernatants were used for enzyme assays. The activities of SOD, POD, catalase (CAT) and melondialdehyde (MDA) content were simultaneously determined according to Zhang (1992).
Superoxide dismutase activity was assayed by using the photochemical NBT method. The samples (0.5 g) were homogenized in 5 mL extraction buffer consisting of 50 mM phosphate, pH 7.8, 0.1% (w/v) BSA, 0.1% (w/v) ascorbate, 0.05% (w/v) ß-mercaptoethanol. The assay mixture in 3 mL contained 50 mM phosphate buffer, pH 7.8, 9.9 mM l-methionine, 57 μM NBT, 0.025% (w/v) Triton X-100, and 0.0044% (w/v) riboflavin. The photo-reduction of NBT (formation of purple formazan) was measured at 560 nm and an inhibition curve was made against different volumes of extract. One unit of SOD is defined as that being present in the volume of extract that causes inhibition of the photo-reduction of NBT by 50%.
Peroxidase activity was measured with guaiacol as the substrate in a total volume of 3 mL. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 6.1), 1% guaiacol, 0.4% H2O2 and enzyme extract. Increase in the absorbance due to oxidation of guaiacol (E = 25.5 mM−1 cm−1) was measured at 470 nm. Enzyme activity was calculated in terms of μmol of guaiacol oxidized min−1 g−1 fresh weight at 25 ± 2°C.
Catalase activity was measured by monitoring the disappearance of H2O2 through measuring the decrease in absorbance at 240 nm (an extinction coefficient of 0.036 mM−1 cm−1) of a reaction mixture consisted of 25 mM potassium phosphate buffer (pH 7.0), 10 mM H2O2 and enzyme extract.
Melondialdehyde content and was determined as 2-thiobarbituric acid (TBA) reactive metabolites. Plant fresh tissues (0.2 g) were homogenized extracted in 10 mL of 0.25% 2-thiobarbituric acid (TBA) made in 10% trichloroacetic acid 10,000×g for 10 min, the absorbance of the supernatant was measured at (TCA). Extract was heated at 95°C for 30 min and then quickly cooled on ice. MAD was expressed as μmol g−1 fresh weight by using an extinction coefficient of 155 mM cm−1.
Data were analyzed for analysis of variance (ANOVA). The significance of difference among means was compared by using Least Significant Difference (LSD) test.
Results and Discussion
The effect of Cd and NaCl on plant growth was evaluated by examining plant height, root length and tillers per plant of wheat seedlings. There was a marked decrease in shoot height, root length and tillers per plant for the plants exposed to Cd and NaCl stresses, when compared with the control (Table 1). The combination of two stresses (NaCl + Cd) led to a further significant (p ≤ 0.05) reduction in these growth traits, when compared with Cd or NaCl stress alone. Significant (p ≤ 0.05) differences in plant height, root length and tillers per plant were observed among cultivars. Pir 85, a salt tolerant cultivar, exposed to NaCl and Cd showed a significantly (p ≤ 0.05) smaller reduction in plant height and root length when compared with Bak 92 and Khy 87, suggesting tolerance to salinity and cadmium toxicity is inherently associated.
Chlorophyll content may be reflected by SPAD value. Analysis of the data showed that both Cd and NaCl stresses caused significant (p ≤ 0.05) decrease of SPAD value (Table 2), and the extent of the decrease became greater with increased Cd level in the culture solution. The results are in agreement with that reported by Larson et al. (1998). The combined stress (Cd + NaCl) had a significantly (p ≤ 0.05) lower SPAD value than either NaCl or Cd stress alone. There was no significant difference among the three cultivars in the response of SPAD value to the both stresses. Ouzounidou et al. (1997) reported that the inhibition of photosynthesis by Cd stress resulted from the indirect effect of either decreased chlorophyll content or decreased stomatal conductance.
Cd stress resulted in significantly higher MDA content when compared with the control (Table 2). It was also observed that MDA content increased with increasing level of NaCl in the nutrient solution. There was a significant difference (p ≤ 0.05) among the three cultivars in response of MDA content to the stress, with Bak 92 being more increased under the both stresses than Pir 85 and Khy 87. The interactive effect of Cd and NaCl was also significant (p ≤ 0.05) on MDA content.
The extent of damage to cell by free radical species may be characterized by lipid peroxidation, which may be implicated in increased MDA content (Chaoui et al. 1997). The current results suggest that Cd toxicity lead to production of superoxide radicals, resulting in increased lipid peroxidation. Hegedus et al. (2001) reported an increase in MDA content with elevated Cd level in nutrient solution and proposed that the enhanced level of MDA could be attributed to the presence of poisoning AOS. In this study, we also observed an increase in MDA content under salt stress, which is agreement with the findings reported by Hernandez and Alamansa (2002).
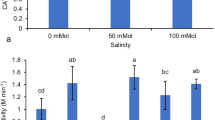
Addition of Cd in nutrient solution enhanced CAT activity of all three cultivars (Table 3). Moreover, the increase of CAT activity was closely related to salinity level in the culture solution. However, no significant difference among three wheat cultivars was found in the response of CAT activity to both Cd and salinity stresses. The combined effect of Cd and NaCl on CAT activity was significant (p ≤ 0.05). Cd stress (4 μM) did not result in significant increase in CAT activities for all three cultivars, which is agreement with those reported by Hegedus et al. (2001). On the other hand, Shah et al. (2001) observed significant decline in CAT activity when wheat plants were exposed to Cd stress. Variation in CAT activity due to Cd stress may be partly attributed to possible variation among species, growth stage, stress intensity, and duration of exposure. Differential responses of CAT activities due to Cd stress might reveal different targets of oxidative damage. Salt stress increased the activities of CAT in all the three genotypes, suggesting that accumulation of ROS might occur in response to salt stress. These findings are in agreement with some previous reports (Noctor and Foyer 1998). More recently, in rice roots exposed to salt stress, decreased CAT activity was reported by Khan et al. (2002).
Significant (p ≤ 0.05) increase in POD activity was observed in all cultivars when exposed to Cd and NaCl treatments (Table 3). There was a distinct difference in POD activity among the three cultivars in the response to the both stresses. It was observed that Pir 85 had lower POD activity than Bak 94 and Khy 87. The combined stress of Cd and NaCl caused a significant increase of POD activity for the three wheat cultivars in comparison with that under Cd or NaCl stress alone. In general, higher activity of POD in plants subjected to harsh environment is favorable for their tolerance and survival. In the present study, both Na and Cd stresses led to a significant increase of POD activity in all cultivars except for Pir 85. In the combined (NaCl + Cd) treatment, POD activity further increased compared to NaCl or Cd treatment alone. An increase in POD activity is frequently an indication of unfavorable growth conditions (Dietz 1996). However, the effect of increased POD activity on plant growth is still unclear. According to the results that Pir 85 showed less growth inhibition and lower POD activity under both Cd and salinity stresses, we may assume that the cultivar experiences is relatively less oxidative stress than other two cultivars.
Wheat plants exposed to 4 μM Cd or 150 mM NaCl showed a significant (p ≤ 0.05) increase in SOD activity (Table 3) in comparison with the control (without addition of Cd or NaCl), respectively. However, there was not significant difference in SOD activity between 0 and 75 mM NaCl treatments, and between 0 and 2 μM Cd treatments. The additional effect of Cd and NaCl in terms of increasing SOD activity was significant when compared with Cd or NaCl alone. In addition, no significant difference was detected among the three cultivars in response of SOD activity to both stresses. In general, SOD activity is enhanced when plants are subjected to environmental stress (Dixit et al. 2001). However, the extent and duration of the enhancement varied with stress intensity, species, age of the plants and repeated stress periods (Piquery et al. 2000). Previous research on barley found that SOD activity increased shortly after Cd treatment and then fell with longer exposure (Chaoui et al. 1997). The current results showed SOD activity would increase in response to Cd or NaCl stress.
References
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani EE (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzymes activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147. doi:10.1016/S0168-9452(97)00115-5
Comba ME, Benavides MP, Tomaro ML (1998) Effect of salt stress on antioxidant defense system in soybean root nodules. Aust J Plant Physiol 25:665–671
Dietz KJ (1996) Functions and responses of the leaf apoplast under stress. Prog Bot 58:221–254
Dixit V, Pandy V, Shyam R (2001) Differential responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109. doi:10.1093/jexbot/52.358.1101
Hegedus A, Erdei S, Horvath G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093. doi:10.1016/S0168-9452(01)00330-2
Hernandez JA, Alamansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257. doi:10.1034/j.1399-3054.2002.1150211.x
Hoagland DR, Arnon DI (1950) The water culture method for growing plant without soil. Calif Agric Exp Sta Univ Calif Cir 347:1–32
Khan MH, Singa KLB, Panda SK (2002) Changes in antioxidant levels in Oryza sativa L. roots subjected to NaCl salinity stress. Acta Physiol Plant 24:145–148. doi:10.1007/s11738-002-0004-x
Larson EH, Bornman JF, Asp H (1998) Influence of UV-B radiation and Cd on chlorophyll fluorescence, growth and neutrient content in Brassica napus. J Exp Bot 49:1031–1039. doi:10.1093/jexbot/49.323.1031
Lozano-Rodriguez E, Hernandez LE, Bonay P (1997) Distribution of Cd in shoot and root tissue of maize and pea plants: physiological distribution. J Exp Bot 48:123–128. doi:10.1093/jxb/48.1.123
Muhling KH, Lauchli A (2003) Interaction of NaCl and Cd stress on compartmentation pattern of cations, antioxidant enzymes and proteins in leaves of two wheat genotypes differing in salt tolerance. Plant Soil 253:219–231. doi:10.1023/A:1024517919764
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279. doi:10.1146/annurev.arplant.49.1.249
Ouzounidou G, Moustakes M, Eleftheriou EP (1997) Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Arch Environ Contam Toxicol 32:154–160. doi:10.1007/s002449900168
Patra J, Panda BB (1998) A comparison of biochemical responses to oxidative and metal stress in seedlings of barley (Hordeum vulgare L.). Environ Pollut 101:99–105. doi:10.1016/S0269-7491(98)00009-8
Piquery L, Davoine C, Huault C, Billard JP (2000) Senescence of leaf sheaths of ryegrass stubble: changes in enzyme activities related to H2O2 metabolism. Plant Growth Regul 30:71–77. doi:10.1023/A:1006308928018
Sandalio LM, Dalurzo HC, Gomez MC, Romero P, Del Rio LA (2001) Cadmium induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2212
Shah KH, Ritambhara GK, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144. doi:10.1016/S0168-9452(01)00517-9
Weggler BK, McLaughlin MJ, Graham RD (2000) Salinity increase cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Aust J Soil Res 38:37–45. doi:10.1071/SR99028
Wu FB, Zhang G, Dominy P (2003) Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot 50:67–78. doi:10.1016/S0098-8472(02)00113-2
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation, SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Acknowledgments
We thank for the Higher Education Commission (HEC) of Pakistan for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shafi, M., Bakht, J., Hassan, M.J. et al. Effect of Cadmium and Salinity Stresses on Growth and Antioxidant Enzyme Activities of Wheat (Triticum aestivum L.). Bull Environ Contam Toxicol 82, 772–776 (2009). https://doi.org/10.1007/s00128-009-9707-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9707-7