Abstract
Tropical aspidochirotid holothuroids are among the largest coral reef invertebrates, but gaps remain in our understanding of their ecological roles in lagoon sediment habitats, a vast component of coral-reef ecosystems. Stichopus herrmanni, listed as vulnerable (IUCN), is currently a major fishery species on the Great Barrier Reef (GBR) and throughout the Indo-Pacific. It is critical to characterise how this species interacts with its environment to understand how its removal may impact ecosystem functionality. We investigated seasonal variation in movement, bioturbation, feeding and gonad development of S. herrmanni over 3 yr at One Tree Reef, which has been a no-take area for decades. We determined the direct influence of the deposit-feeding activity of S. herrmanni on sediment turnover and granulometry, and on the abundance of infauna and benthic productivity in a comprehensive in situ analysis of tropical holothuroid feeding ecology. This species is highly mobile with identifiable individuals exhibiting site fidelity over 3 yr. With the potential to turn over an estimated 64–250 kg individual−1 yr−1, S. herrmanni is a major bioturbator. Stichopus herrmanni is a generalist feeder and influences trophic interactions by altering the abundance of infauna and microalgae. Stichopus herrmanni exhibited decreased feeding activity and gonad development in winter, the first documentation of a seasonal disparity in the bioturbation activity of a tropical holothuroid. Sediment digestion and dissolution by S. herrmanni has the potential to influence seawater chemistry, a particularly important feature in a changing ocean. Our results provide essential baseline data on the functional roles of this ecologically important species to inform development of ecosystem-based bêche-de-mer fisheries management on the GBR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite their prominence in benthic sediment systems, gaps remain in our understanding of the feeding biology and trophic ecology of aspidochirotid sea cucumbers (Holothuroidea). Aspidochirotids are epibenthic deposit feeders that ingest sediment and associated organic matter using specialised feeding tentacles (Roberts 1979; Roberts et al. 2000; Purcell et al. 2016a). They can process large amounts of sediment in association with their feeding activity, and so have been called the earthworms of the sea (Bonham and Held 1963; Purcell et al. 2016a). Active selective foraging by size and/or organic content of sediment particles has been reported for shallow-water (Yingst 1976; Uthicke 1999; Slater and Carton 2010; Slater and Jeffs 2010; MacTavish et al. 2012; Navarro et al. 2013) and deep-sea (Roberts et al. 2000; Hudson et al. 2005) species. Questions remain on the details of the diet of aspidochirotids, how they influence their environment through their feeding and bioturbation, and whether they are selective or non-selective feeders with respect to sediment grain size and/or nutrient profiles (Hammond 1982; Klinger and Johnson 1998; Roberts et al. 2000).
Many aspidochirotids are commercially valuable (Conand 1998, 2001; Purcell et al. 2012, 2014), harvested to generate the dried body wall product called bêche-de-mer (or trepang). This high-value product is traded in the Asian market and provides a source of income and food to millions worldwide (Purcell et al. 2013, 2014; Eriksson and Clarke 2015). Due to their ease of collection, holothuroids are particularly vulnerable to overfishing with many in a perilous state of conservation (Conand 2001; Uthicke et al. 2004; Friedman et al. 2011; Eriksson and Byrne 2015). Increased demand for bêche-de-mer is driving worldwide exploitation (Conand 1998, 2001; Purcell et al. 2013), and 16 species are now listed as threatened to extinction by the IUCN (Conand et al. 2014; Purcell et al. 2014). Many species are now locally extinct (Hasan 2005; Anderson et al. 2011; Branch et al. 2013; Price et al. 2013; Purcell et al. 2014), with at least 70% of the world’s tropical holothuroid fisheries considered exploited, over-exploited or depleted (Purcell et al. 2013).
Understanding the ecological roles of exploited species is critical for ecosystem-based fisheries management (Anderson et al. 2011; Purcell et al. 2016a). Tropical aspidochirotids are among the largest and often most abundant non-coral invertebrates in coral reef carbonate sediment habitats. Reef sediment habitats are vast, sometimes accounting for up to 95% of areal benthic coverage, and constitute the majority of calcium carbonate stored in coral-reef ecosystems (Gattuso et al. 1998). In these habitats, aspidochirotids turn over vast quantities of sediment through their deposit-feeding activity (Yamanouti 1939; Bonham and Held 1963; Hammond 1982; Klinger et al. 1994; Uthicke 1999; Mangion et al. 2004; Shiell and Knott 2010). This bioturbation activity directly increases oxygen levels in the sediment (Hammond 1982), with increased sediment anoxia noted following their removal from reef flat habitats (Lee et al. 2017). The digestion and dissolution of carbonate sands in the holothuroid gut may further alter biogeochemical processes by reducing sediment grain size and increasing local alkalinity in reef environments (Hammond 1981; Schneider et al. 2011, 2013; Purcell et al. 2016a). This compensatory process could contribute to the biogenic buffering of ocean acidification, and thus reef resilience, in a changing ocean (Schneider et al. 2011, 2013).
Tropical aspidochirotids also play critical roles in trophic functionality. They are reported to consume bacteria, diatoms and microalgae, with infauna considered to be a minor food source (Moriarty 1982; Moriarty et al. 1985; Uthicke 1999; Roberts et al. 2000). Nitrogen excretion by aspidochirotids encourages benthic productivity in oligotrophic coral reef systems (Uthicke and Klumpp 1998; Uthicke 2001; Wolkenhauer et al. 2010; Purcell et al. 2016a). In manipulative experiments, seagrass growth and biomass decreased following the removal of the sandfish, Holothuria scabra (Wolkenhauer et al. 2010). Similarly, field-based incubations showed that the nitrogen waste products of H. atra promoted benthic microalgal growth and productivity, suggesting that holothuroids fertilise their own gardens (Uthicke and Klumpp 1998). Overall, the ecological consequences of removing holothuroids from coral reefs are likely to be detrimental to ecosystem functionality (Purcell et al. 2016a).
The distances covered by tropical holothuroids as they feed are not well known, largely because they are difficult to tag or track (Conand 1983, 1989, 1991; Purcell et al. 2016a, b). While they have been considered to be largely sedentary (Conand 1983, 1991; Purcell 2010), a recent study on two large tropical holothuroids, Bohadschia argus and Thelenota ananas, showed that these species moved between 2–8 and 5–9 m d−1, respectively (Purcell et al. 2016b). While this suggests they are highly mobile, these species also display long-term site fidelity (Purcell et al. 2016b). The curryfish, Stichopus herrmanni, has been recorded to move 0.4–0.7 m h−1 (Purcell and Eriksson 2015), with no difference in activity between day and night (Eriksson et al. 2013). Information on the effects of season on holothuroid activity and bioturbation is limited to one study on H. scabra, which exhibited increased activity coinciding with annual peaks in water temperature and gonad development (Shiell and Knott 2010). These features of movement, activity and site fidelity have important implications for conservation of holothuroids and the identification of effective marine protected areas (Purcell and Kirby 2006; Purcell et al. 2016b; Wolfe and Byrne 2017), and are especially important to understand for fished species.
Globally, tropical bêche-de-mer fisheries follow a predictable pattern of serial exploitation of high-value species, with a transfer of effort to lower value species (Conand 1998, 2001; Branch et al. 2013; Purcell et al. 2013, 2014). These patterns of overfishing are typical of developing countries, but similar trends are also documented for higher-income regions including on Australia’s World Heritage-listed Great Barrier Reef (GBR) (Eriksson and Byrne 2015). On the GBR, catches of the mid-value curryfish, S. herrmanni, increased at a rate of ~200% per year between 2007 and 2011 (Eriksson and Byrne 2015). This species is increasing in value as it becomes rarer in tropical waters worldwide (e.g. Torres Strait: Skewes et al. 2004; Papua New Guinea: Kinch et al. 2008; East Africa: Eriksson et al. 2010; GBR: Eriksson and Byrne 2015). Stichopus herrmanni is now listed as vulnerable to extinction (Conand et al. 2014; Purcell et al. 2014).
We investigated seasonal variation in movement, bioturbation, feeding and gonad development in S. herrmanni at One Tree Reef (OTR), GBR, over 3 yr. Previous studies indicated that spawning in this species occurs during summer with suppressed gonad growth in winter (Conand 1993a, b). We hypothesised that this holothuroid would be highly mobile, as shown recently for S. herrmanni (Purcell and Eriksson 2015), and other large tropical holothuroids (Purcell et al. 2016b). We predicted seasonal activity and bioturbation would increase in summer, coinciding with increased water temperature and gonad development, as shown for H. scabra (Shiell and Knott 2010). It has been suggested that S. herrmanni does not exhibit selective feeding behaviour (Roberts and Bryce 1982; Klinger et al. 1994), and so we hypothesised that this species is a generalist grazer with non-selective feeding traits. We compared the biota in ambient sediment to that in the oesophagus, intestine and faecal casts of S. herrmanni to characterise the available food source and their diet. Since the digestive biology of aspidochirotids is likely to have an important biogeochemical impact due to the dissolution of carbonate sediment in the low-pH sea cucumber gut (Hammond 1981; Schneider et al. 2011, 2013), gut pH was determined at different stages of sediment digestion. Coelomic fluid pH was also measured to assess acid–base regulation in S. herrmanni (Collard et al. 2013, 2014), and the reproductive cycle was assessed through gonad index analysis. The data assimilated here provide new empirical information on the ecological roles of S. herrmanni in a lagoon sediment habitat on the GBR, information critical for the development of ecosystem-based fisheries management (Anderson et al. 2011; Purcell et al. 2016a), and the conservation of this exploited bêche-de-mer species.
Methods
Habitat conditions
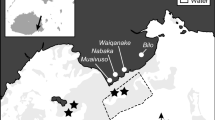
Fieldwork was conducted in the shallow (1–3 m) sandy habitat ‘Shark Alley’ in OTR lagoon (23°30′S, 152°05′E), southern GBR, Australia (Fig. 1a). The feeding biology and movement of S. herrmanni (Fig. 1b) were investigated each season (summer, autumn, winter, spring) between 2013 and 2015, with each season replicated twice. Temperature was intensively monitored in Shark Alley across 2016, using in situ HOBO loggers to determine the conditions directly experienced by S. herrmanni in Shark Alley. Average temperatures in 2013–2015 were also calculated for each season using average daily temperatures, available from the in situ weather station positioned inside OTR lagoon (http://data.aims.gov.au/aimsrtds/station.xhtml?station=131).
Movement and bioturbation
For each observation period, ten S. herrmanni (length 30.8 ± 4.0 cm; width 8.9 ± 1.0 cm; mean ± SD; n = 80) were tracked in situ for 5 h across afternoon low tides (neap low ~1500 hrs) to quantify their movement and bioturbation activity. Each season was repeated twice across 2013–2015, so that a total of 20 individuals were tracked per season. No surveys were conducted at night as S. herrmanni does not behave differently between day and night (Eriksson et al. 2013). Tagging S. herrmanni is notoriously difficult (Conand 1983, 1989, 1991), and so was not attempted. Identifiable individuals (i.e. by colouration, scarring, spot patterns) were noted during each survey.
To determine the distance moved and faecal cast production of S. herrmanni, the initial position of each individual was marked with a stake, and individuals were revisited hourly. Each hour the distance between the initial stake and new position of the holothuroid was measured. The exact route of each individual was measured following the trail of faecal casts produced (Fig. 1b), not a straight line. After measuring the distance travelled, all faecal casts were collected and the holothuroids’ new position was marked. Faecal casts are encased in a thin mucous layer and were collected carefully by hand. This process was repeated hourly for each individual at low tide when OTR lagoon is ponded, removing confounding hydrodynamic interference with the integrity of faecal casts. The total distance moved and amount of sediment released in a 5-h period were then determined.
Daily movement rates were calculated from the average hourly distance moved for each individual. Analyses of the amount of sediment released each hour showed that time had no significant effect on defecation rates across the 5-h sampling periods (RMANOVA: F 4,80 = 1.4, p = 0.23). Thus, average hourly defecation rates per individual were calculated from the total amount of sediment released over 5 h. Maximum average daily and annual defecation rates were extrapolated from this estimate, assuming that S. herrmanni is a continuous feeder (i.e. 24 h) (Klinger and Johnson 1998; Eriksson et al. 2013). Minimum sediment turnover rates were calculated using the 15-h feeding period determined for S. herrmanni by Yamanouti (1939).
Surface sediment cores were also collected adjacent to each S. herrmanni to compare ambient sediment to that digested by the holothuroids. Cores were taken with plastic coring tubes stoppered at 5 mm depth, as this is the estimated feeding depth of deposit-feeding holothuroids (Uthicke 1999).
The sediment grain size profiles of S. herrmanni faecal casts and ambient sediment cores were compared (n = 20 season−1). Sediment samples were partitioned by wet sieving with fresh water into the size fractions 1400, 1000, 500 and <500 µm, and dried in a 60 °C oven for >24 h. The dry weight of each size class was calculated as a percentage of the total sample weight. The total dry weight (g) of each faecal cast was used to extrapolate hourly, daily and yearly bioturbation rates of S. herrmanni over each observation period.
Infauna in ambient, gut and faecal cast sediment
A separate set of faecal cast (n = 10) and ambient sediment (n = 10) samples were collected from Shark Alley each season and fixed in 10% formaldehyde in filtered seawater with a Rose Bengal dye. The presence of infauna (~100–1000 µm length) was determined under a dissecting microscope, identified by their pink stain. The total number of organisms present in 2-mL subsamples of sediment were counted and categorised into predominant groups: Amphipoda, Nematoda, Polychaeta, Platyhelminthes and other Crustacea (e.g. Copepoda, Ostracoda, Isopoda). Biota in the ambient sediment cores and S. herrmanni faecal casts were compared. To identify the infauna ingested by S. herrmanni, sediment samples were also taken from the oesophagus (right behind the mouth) of dissected individuals (n = 8), before digestive processes impaired the ability to identify gut contents.
Surface sediment photopigments
Faecal cast (n = 6) and ambient sediment (n = 12) samples were also collected each season for analysis of chlorophyll-a. Samples were dried in an oven at 60 °C for ~24 h, and the dry weight was measured. Chlorophyll-a was extracted from the dry sediment samples with 90% acetone. Samples were kept dark and cool (~4 °C) for 18–24 h before analysing the supernatant by spectrophotometry at wavelengths of 630 and 664 nm. Chlorophyll-a levels were calculated using formulae produced by Jeffery and Humphrey (1975), relative to the dry sediment weight. Mean values for chlorophyll-a were taken for each season.
Internal pH
A total of 32 S. herrmanni were collected from Shark Alley and quickly transported to One Tree Island Research Station (OTIRS) for analysis of internal pH. Half of the specimens were used immediately, to ensure their stomachs were filled with sediment (full; n = 16). The remaining S. herrmanni were left for >24 h in flow-through aquaria to empty their digestive tracts (empty; n = 16). The body wall of each individual was cut open, and the coelomic fluid drained. pH of the coelomic fluid was measured immediately. The pH probe was then carefully inserted into a small incision in the foregut of each S. herrmanni, and the pH was recorded. pH was also measured in incisions at the hindgut for several full and empty individuals (n = 6), and of ambient seawater samples (n = 9). All pH measurements were made using a ROSS Sure-flow sensor (Orion, Thermo Scientific) or WTW Multiline probe, calibrated to NIST buffers 7.00 and 10.02 (Hach, Radiometer Analytical).
Gonad index
The gonad index was determined for S. herrmanni collected in summer (n = 29), autumn (n = 8), winter (n = 9) and spring (n = 24). Collections were made over a number of years (2008–2016), due to permit restrictions on harvest numbers. Stichopus herrmanni were collected from Shark Alley and returned to OTIRS for dissection. Gonad index was calculated as the weight-percent of the gonads relative to the combined drained body wall and viscera weights. The weight range (drained) of individuals used for gonad index analyses was 667–2370 g (n = 70). Two of nine specimens completely lacked identifiable gonads in winter and were recorded as zero weight. Data were combined by season of collection.
Statistics
Average hourly movement and sediment turnover rates of S. herrmanni were analysed using analysis of covariance (ANCOVA), with season as the fixed factor and holothuroid size as the covariate. Year was not considered a fixed factor due to the unbalanced sampling of each season twice over 3 yr (2013–2015). Sediment granulometry (grain particle size) was analysed by converting the dry weight-percent of each sieve class into a measure of kurtosis (i.e. skewedness, tailedness, homogeneity) for each sample using the logarithmic method of the GRADISTAT package (Version 8.0, Crowthorne, UK) (Blott and Pye 2001). The value of kurtosis for each sediment sample was then analysed using a two-way ANOVA, with season and sediment type (faecal cast/ambient sediment) as the fixed factors.
Seasonal differences in infauna composition between S. herrmanni faecal casts and ambient sediments were visualised using non-metric multidimensional scaling (MDS) plots. Abundance data of infauna groups were arcsine transformed before visualisation in the two-dimensional MDS space. Within this space, communities that have similar compositions cluster together, while those that are different are spaced further apart. Further determination of differences in the abundance of infauna groups due to our two factors (season, faecal cast/ambient sediment) was done using PERMANOVA, calculating the F-statistic using 9999 permutations for all factors (Anderson et al. 2008). Similarity of percentages (SIMPER) tests were performed to examine combined effects. MDS, PERMANOVA and SIMPER tests were done using Primer V6.
Benthic photopigment data were analysed using a two-way ANOVA, with season and sediment type (faecal cast/ambient sediment) as the fixed factors. Internal pH of S. herrmanni was analysed using a two-way ANOVA, with gut condition (empty, full) and position in the gut (coelomic fluid, foregut, hindgut) as fixed factors. Data on the gonad index of S. herrmanni were analysed using one-way ANOVA, with season as the fixed factor. Homogeneity of variance and normality were checked and confirmed for all data series (Quinn and Keough 2003). Percentage data were arcsine transformed before analysis. Post hoc Tukey’s HSD tests were used to determine where significant differences lay. All ANOVAs were analysed using JMP 501 (Cary, NC, USA).
Results
Habitat conditions
The average water temperature in Shark Alley was 24.5 ± 0.01 °C (mean ± SE) in 2016, with a minimum and maximum temperature of 17.5 and 32.3 °C, respectively (Electronic supplementary material. ESM, Fig. S1). The average daily water temperature in OTR lagoon 2013–2015 was 27.2 °C (±0.07) during summer, 25.1 °C (±0.14) in autumn, 21.5 °C (±0.07) in winter and 23.9 °C (±0.95) in spring (ESM Fig. S1). Based on average daily water temperatures in OTR lagoon, the maximum temperature was 29.4 °C (summer), and the minimum was 19.4 °C (winter) (ESM Fig. S1).
Movement and bioturbation
Stichopus herrmanni moved an average of 47.2 ± 9.2 cm h−1 across all seasons, ranging between 5.8 and 192 cm h−1. The average distance moved was greatest in summer (62.4 ± 13.0 cm h−1), and smallest in winter (37.5 ± 7.5 cm h−1) and spring (38.8 ± 7.9 cm h−1), but these data did not differ significantly (Fig. 2a; ESM Table S1). However, the spread of data indicated that the upper 25th percentile was fastest in summer (Fig. 2a). Stichopus herrmanni moved an average of 9 m d−1 in winter and 15 m d−1 in summer. In total, six recognisable individuals were repeatedly observed within the study area 3–4 times each over 3 yr.
a Distance moved (cm h−1) and b amount of sediment turned over (g h−1) by Stichopus herrmanni for each season (n = 20; ±SE). Boxes represent the interquartile range (25th and 75th percentile), the horizontal line is the median, and the whiskers represent the data range. Different letters indicate significantly different groups (Tukey’s HSD test)
There was a significant seasonal difference in the total amount of sediment released by S. herrmanni over the 5-h monitoring period. The length of S. herrmanni (range 19–44 cm) had no effect on sediment defecation (Fig. 2b; ESM Table S1). Tukey’s HSD test revealed that rates of bioturbation were higher in summer (28.6 g h−1) and autumn (25.2 g h−1), compared to winter (13.7 g h−1) and spring (11.7 g h−1) (Table 1; Fig. 2b; ESM Table S1). Calculated values for minimum and maximum daily and annual defecation rates are displayed in Table 1.
Sediment grain size was dominated by small sediment particles (<500 µm) compared to larger grain sizes (>500 µm) in all seasons (Fig. 3; ESM Table S1). There was a significant difference in sediment grain size distribution (kurtosis) between the faecal casts and ambient sediment, and between seasons, but no interactive effect (Fig. 3; ESM Table S1). Tukey’s HSD tests revealed that there were more fine sediment particles (<1000 µm) and fewer large sediment fragments (>1000 µm) in the faecal casts produced by S. herrmanni than in the surrounding environment (Fig. 3). Between seasons, Tukey’s HSD tests showed that the kurtosis of each sample was greatest in spring and summer (i.e. least homogenous), and lowest in winter (i.e. most homogenous) (Fig. 3; ESM Table S1).
Infauna in ambient, gut and faecal cast sediment
There were clear differences in the abundance of infauna in ambient sediment (54.8 ± 2.7 individuals mL−1) compared to faecal cast samples (4.05 ± 0.2 individuals mL−1; Table 2; Fig. 4). There was no distinct differentiation of infauna among seasons, but infauna found in ambient sediment samples in winter were different to other seasons (Fig. 4). PERMANOVA confirmed these patterns, detecting differences in the abundance of infauna among seasons and between faecal casts and ambient sediment, with an interactive effect (Fig. 5a, b; ESM Table S2). Infauna were more abundant in ambient sediment samples (67%) than in casts produced by S. herrmanni (14%) (Table 2; Fig. 5a, b). There were also more organisms present in ambient sediment during summer and autumn than in winter and spring (Fig. 5a). SIMPER tests suggest that this was driven by low numbers of amphipods in spring (7%) compared to the remaining seasons (14–15%) (Fig. 5a). Sediment in the oesophagus of S. herrmanni (n = 8) included the most common organisms in ambient sediment samples (Table 2).
Non-metric multidimensional scaling ordination plots on Bray–Curtis similarity of meiofauna community structure in Shark Alley for Stichopus herrmanni faecal casts (open shapes) and ambient sediment cores (closed shapes), taken during summer (triangles), autumn (diamonds), winter (squares), and spring (circles)
Benthic community composition: average abundance of infauna (mL−1) in a naturally occurring sediment cores and b casts produced by Stichopus herrmanni (n = 10 season−1; ±SE). c Average level of chlorophyll-a (µg g−1 dry sediment) in S. herrmanni faecal casts (n = 6 season−1) and ambient sediment cores (n = 12 season−1; ±SE). Different letters indicate significantly different groups (Tukey’s HSD test)
Surface sediment photopigments
The average level of benthic chlorophyll-a was significantly higher in ambient sediment samples (3.00–3.28 µg g−1) than in faecal casts (1.87–2.52 µg g−1) (Fig. 5c; ESM Table S1). Chlorophyll-a did not differ among seasons in the environment or in S. herrmanni faecal casts (Fig. 5c).
Internal pH
The pH of the digestive tract of S. herrmanni was significantly different for individuals with their gut empty and full of sediment, and for measurements taken in the foregut, hindgut and coelomic fluid (Fig. 6; ESM Table S1). There was also a significant interaction between these factors (Fig. 6; Table S1). pH was highest in the coelomic fluid, regardless of whether the digestive tract was empty (pH 7.91 ± 0.03) or full (pH 7.98 ± 0.03) (Fig. 6). pH was significantly lower in the foregut than in the hindgut (Fig. 6). In digestive tracts full of sediment, the average pH of the hindgut was 7.65 (±0.07), but was 7.49 (±0.05) in the foregut (Fig. 6). In empty guts, the average pH was 7.37 (±0.06) and 7.05 (±0.09) in the hindgut and foregut, respectively (Fig. 6). The pH of ambient seawater was 8.12 ± 0.03.
Gonad index
The gonad index differed between seasons (Table 3; ESM Table S1). Stichopus herrmanni had a higher gonad index in the summer (4.85%) than in other seasons (Table 3; ESM Table S1). Gonads were notably reduced during autumn (0.41%) and winter (0.19%), and increased in size in spring (0.99%) (Table 3).
Discussion
Movement and bioturbation
This is the longest in situ study tracking the seasonal activity and bioturbation of individual aspidochirotids (Table 4). We showed that S. herrmanni moved considerably, at an average rate of 47.2 cm h−1 throughout the year. This speed is comparable to that documented for S. herrmanni in New Caledonia (Purcell and Eriksson 2015), and for other tropical holothuroids (<100 cm h−1: Conand 1991; Roberts et al. 2000; Uthicke 2001; Shiell and Knott 2010; Purcell et al. 2016b). However, some S. herrmanni reached speeds >300 cm h−1. The average hourly movement of S. herrmanni was greater in summer and autumn (50–62 cm h−1) compared to winter and spring (37–39 cm h−1), but this trend was non-significant and was likely driven by several particularly active individuals. Seasonal trends of decreased holothuroid activity (movement) in winter have been documented for the sandfish, H. scabra, on Ningaloo Reef, Western Australia (Shiell and Knott 2010). More data are needed to identify seasonal activity patterns in S. herrmanni, including for lower-latitude populations that may be less influenced by seasonal change.
Stichopus herrmanni moved an average of 9 and 15 m d−1 during winter and summer, respectively. A recent study of other large tropical holothuroids, B. argus and T. ananas, showed that they moved 2–8 and 5–9 m d−1, respectively (Purcell et al. 2016b). These two species exhibited site fidelity and long-term home ranging behaviour, as indicated by the reoccurrence of recognisable individuals over 2 yr (Purcell et al. 2016b). While we did not tag or track individuals over the years, several recognisable S. herrmanni were repeatedly encountered in Shark Alley. Thus, adult S. herrmanni may also display home range affinity. The apparent site fidelity of S. herrmanni, and patterns of ontogenetic migration within their recruitment reef (Eriksson et al. 2013; Palazzo et al. 2016; Wolfe and Byrne 2017), highlight their vulnerability to overharvesting on reefs open to fishing, and the potential for heavily targeted species to suffer marked population declines, local extinction and poor recovery (Gillanders et al. 2003; Uthicke et al. 2004; Hasan 2005; Purcell 2010; Friedman et al. 2011). This has important implications for fisheries management regarding connectivity between recruitment and nearby adult habitats within the same reef (Wiedemeyer 1994; Gillanders et al. 2003; Grüss et al. 2011; Bourjon and Morcel 2016).
Rates of sediment turnover by S. herrmanni were significantly lower in winter and spring than in summer and autumn, providing the first documentation of a seasonal disparity in bioturbation for a tropical holothuroid (Table 4). This is likely due to low water temperatures and a reduced gonad index (i.e. reduced energetic demands) during winter, as suggested for seasonal effects on the movement of H. scabra (Shiell and Knott 2010). We estimate that sediment turnover by S. herrmanni could range from 64 to 250 kg individual−1 yr−1 (Fig. 7), depending on season and diurnal feeding patterns (Yamanouti 1939; Purcell and Eriksson 2015). Even at the calculated minimum value, the total rates of sediment turnover reported here for S. herrmanni are above those previously documented for this and other aspidochirotids (9–105.1 kg individual−1 yr−1) (Table 4). This reflects our robust sampling technique, collecting all faecal casts produced over 5 h in a hydrodynamically stable environment, compared to previous studies estimating total turnover rates from single faecal casts (e.g. Yamanouti 1939; Bonham and Held 1963; Hammond 1982; Klinger et al. 1994).
Considering the amount of sediment turned over by S. herrmanni in a year, this species is likely to be ecologically important in its lagoon habitat, influencing benthic processes and trophic interactions (Fig. 7). This is especially true for unfished reefs, such as OTR and Heron Reef, where S. herrmanni is abundant (31.25–1100 individuals ha−1; Eriksson et al. 2013; Wolfe and Byrne 2017), and prompts the question of the ecological impact of removing this bêche-de-mer species from tropical sediment habitats.
Our understanding of the feeding mechanisms and preferences of tropical holothuroids with respect to sediment grain size, nutrient profiles and associated biota is poor. Very little new data are available since the Roberts et al. (2000) review on holothuroid feeding strategies. The ability of holothuroids to select particular sediment grain sizes appears to be species specific and associated with feeding tentacle morphology (Roberts and Bryce 1982; Roberts et al. 2000). The size range of particles ingested by shallow-water tropical species is very broad, from a median of 100 µm in B. argus to ~6000 µm in H. atra (Yamanouti 1939; Roberts et al. 2000). As shown in the profile of ambient sediment from Shark Alley, this area of OTR lagoon has a relatively uniform sediment granulometry, dominated by fine particles (~73% < 1400 µm). Early research suggests S. herrmanni does not reduce sediment grain size through its digestion (Yamanouti 1939). In contrast, we found the faecal casts produced by S. herrmanni had a comparatively finer mean grain size (~81% < 1400 µm) than the ambient environment. Whether S. herrmanni selects smaller sediment particles in its feeding (e.g. Roberts 1979; Roberts et al. 2000), or reduces grain size through the partial dissolution of sediment in the low-pH gut (e.g. Hammond 1981; Schneider et al. 2013) could not be determined in our study. The feeding niche and distribution of tropical aspidochirotids in lagoon systems is likely also influenced by other factors such as reef shelter (Klinger et al. 1994; Klinger and Johnson 1998), and hydrodynamic displacement (Wolfe and Byrne 2017).
Generalist consumer diet
Through an assessment of three stages of sediment processing by S. herrmanni (ambient sediment, oesophagus, faecal casts) we provide new insights into the trophic role of S. herrmanni in its tropical lagoon habitat. This is the first study to examine sediment in the oesophagus, where ingested biota can still be identified. Faecal casts had lower levels of infauna and chlorophyll-a than the ambient sediment, and infauna was found in the oesophagus of S. herrmanni, indicating that this species ingests and consumes benthic infauna and microalgae (Fig. 7). In contrast, previous studies concluded that infauna is not consumed by tropical aspidochirotids (Moriarty et al. 1985), and that infauna may not play a large role in the overall nutrition of some holothuroids (Uthicke 1999). However, these studies examined sediment in the stomach and intestines, which was likely too advanced in the digestive process to be able to identify organisms. Stichopus herrmanni processes large quantities of sediment, and we suggest that this species is a generalist grazer that ingests what is available in the environment. Our data suggest that S. herrmanni does not select for specific infauna or benthic microalgal food, as suggested previously for this species (Roberts and Bryce 1982). However, since infauna abundance was lower in the oesophagus than in ambient sediment, it is likely that some mobile species are able to escape predation by S. herrmanni.
Infauna were more abundant in the sediment during summer and autumn, coinciding with higher rates of bioturbation and movement by S. herrmanni. There was significantly less infauna during winter and spring, likely driven by low water temperatures on this southern reef, with a ~15 °C difference between summer maximum and winter minimum temperatures. Seasonal changes in the composition of benthic habitats, including infauna and algae, are common in coral reef systems (Uthicke and Klumpp 1998; Diaz-Pulido et al. 2009; de Oliveira et al. 2016; van Hoytema et al. 2016). We did not find significant seasonal changes in benthic productivity (chlorophyll-a) at our study site, as shown previously for OTR (Johnstone et al. 1990). However, benthic productivity can be very patchy in lagoon sediment (Koop and Larkum 1987; Johnstone et al. 1990). More research is needed to understand the seasonality of benthic algae in tropical lagoon systems, and the influence of bioturbators like S. herrmanni on the production or disruption of benthic algal mats and associated infauna.
Aspidochirotids can increase the productivity of benthic microalgae and seagrass systems through their bioturbation activity and increasing local nutrients (especially ammonium) through their excretory physiology (Uthicke and Klumpp 1998; Uthicke 2001; Wolkenhauer et al. 2010; Costa et al. 2014). Local enhancement of productivity by aspidochirotids has also been used to benefit integrated tropical mariculture systems (Namukose et al. 2016). This is important for the health of benthic lagoon systems in the presence or absence (i.e. fishing) of holothuroids. Information on the ecological consequences of removing bêche-de-mer species from coral-reef ecosystems is limited (e.g. Uthicke and Klumpp 1998; Wolkenhauer et al. 2010; Lee et al. 2017) and warrants greater attention (Purcell et al. 2016a). This could be examined using inclusion–exclusion experiments (e.g. Wolkenhauer et al. 2010) and/or stable isotope analyses (e.g. Slater and Carton 2010) to understand community-level responses, trophic cascades and potential phase shifts in sediment ecosystems resulting from the overharvest of large bêche-de-mer species.
Internal pH and seawater chemistry
The gut pH of S. herrmanni was higher when full of sediment (pH 7.04–7.81) compared to empty digestive tracts (pH 6.42–7.77), as shown previously for H. atra, H. mexicana and Isostichopus badionotus (Hammond 1981; Schneider et al. 2013). Tropical deposit-feeding holothuroids can play a pivotal role in the carbonate chemistry of coral-reef ecosystems, at least at the local scale (Hammond 1981; Schneider et al. 2011, 2013; Purcell et al. 2016a; Vidal-Ramirez and Dove 2016). It has been hypothesised that the digestion and dissolution of carbonate sediment in the holothuroid gut may buffer external changes in local carbonate chemistry (Fig. 7), and that this may be particularly important under scenarios of ocean acidification (Schneider et al. 2011, 2013). Our results show that the digestion of carbonate sediment in the gut of S. herrmanni drives an increase in gut pH, likely due to dissolution of carbonate sediment. This may influence external seawater chemistry when redeposited into the environment, potentially benefiting local reef calcifiers. This feature of their feeding biology warrants greater attention in the face of continued global change.
In contrast to changing pH in the gut, the coelomic fluid pH of S. herrmanni (7.91–7.98) was stable and similar to that recorded for the external environment (pH 8.12 ± 0.03; n = 9). Thus, coelomic fluid pH conforms to environmental pH (Fig. 7), as shown for tropical and temperate holothuroids (Collard et al. 2013, 2014). Holothuroids have a reduced ability to regulate their internal acid–base levels compared to other echinoderms (e.g. sea urchins), which may impact their physiological and metabolic processes as seawater chemistry changes due to ocean acidification (Collard et al. 2013, 2014). Conversely, as poorly calcified organisms, holothuroids may be more resilient to changing ocean chemistry compared to more heavily calcified reef species (e.g. corals, sea urchins, molluscs) (Dupont et al. 2010; Byrne 2011; Collard et al. 2013).
Reproduction
The gonad index data indicated that the reproductive activity of S. herrmanni on OTR peaked in summer (Dec–Feb) and that gonad growth was suppressed during winter (Jun–Aug) (Fig. 7), with two of nine individuals lacking identifiable gonads. This pattern is similar to that reported for another high-latitude population of this species in New Caledonia (Conand 1993a) and has been observed in temperate and tropical stichopodid species (Cameron and Fankboner 1989; Conand 1993b; Hu et al. 2010). Considering the time of spawning and likely planktonic duration, summer spawning of S. herrmanni agrees with observations of annual recruitment of juveniles in 2015 and 2016 on the GBR (Wolfe and Byrne 2017). These data on reproduction and recruitment are an important consideration for fisheries management, especially considering the likely trajectory of declining stock of this species on the GBR and elsewhere (Conand et al. 2014; Eriksson and Byrne 2015).
Implications for bêche-de-mer management on the GBR
Ecosystem resilience is strengthened by ecological functionality, which depends on species across trophic levels (Kroeker et al. 2011; Connell and Ghedini 2015). Emerging research on the contribution of holothuroids to ecosystem functionality indicates that they enhance local productivity and sediment health (Uthicke and Klumpp 1998; Uthicke 1999; Wolkenhauer et al. 2010; Purcell et al. 2016a; Lee et al. 2017) and may help buffer the effects of ocean acidification (Schneider et al. 2011, 2013). Fundamental gaps in information on holothuroid biology and ecology have not only limited our ability to gauge stock productivity, sustainable extraction rates and the ecological impacts of bêche-de-mer fisheries, it has hampered management dialogues (Wiedemeyer 1994; Anderson et al. 2011; Purcell et al. 2013; Eriksson and Byrne 2015). Improving our understanding about how these organisms interact with their environment and influence ecosystem processes is critical to understanding how bêche-de-mer fisheries impact coral reefs. This information is needed to support decision-making on conservation and ecosystem-based management (Anderson et al. 2011; Purcell et al. 2016a). Current approaches to bêche-de-mer fisheries are not viable for holothuroids (Conand 2001; Purcell et al. 2012, 2013, 2014), prompting the suggestion that a paradigm shift in fisheries management is needed.
As global patterns of exploitation continue it is likely that ecosystem function will be compromised in the absence of the ecosystem services holothuroids provide (e.g. Moriarty et al. 1985; Uthicke and Klumpp 1998; Uthicke 1999; Michio et al. 2003; Wolkenhauer et al. 2010; Costa et al. 2014; Purcell et al. 2016a; Lee et al. 2017). Biological and ecological information, as determined here, are important to inform effective ecosystem-based management strategies for target bêche-de-mer species. While we now have good information on the biology and ecology for high-latitude tropical populations of S. herrmanni (New Caledonia, Heron Island, One Tree Island), data are needed to determine the feeding ecology, population dynamics and spawning cycles of this species in its warmer, more tropical northern range across the Indo-Pacific (Purcell et al. 2012). It is critical that empirical data are collected for reefs both open and closed to harvest across the geographical range of bêche-de-mer targets. We urge the need for increased locally relevant information for targeted bêche-de-mer species to reduce the risk of continued global exploitation, threatened species and localised extinctions.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: Guide to software and statistical methods. Primer-E Ltd, Plymouth
Anderson SC, Flemming JM, Watson R, Lotze HK (2011) Rapid global expansion of invertebrate fisheries: trends, drivers, and ecosystem effects. PLoS One 6:e14735
Blott SJ, Pye K (2001) GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surface Processes and Landforms 26:1237–1248
Bonham K, Held E (1963) Ecological observations on the sea cucumber Holothuria atra and H. leucospilota at Rongelap Atoll, Marshall Islands. Pacific Science 17:305–314
Bourjon P, Morcel E (2016) Observations of juvenile Actinopyga echinites and Actinopyga mauritiana (Echinodermata: Holothuroidea) near the reef crest in a lagoon of Réunion. SPC Bêche-de-mer Information Bulletin 36:84–86
Branch TA, Lobo AS, Purcell SW (2013) Opportunistic exploitation: an overlooked pathway to extinction. Trends Ecol Evol 28:409–413
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Cameron JL, Fankboner PV (1989) Reproductive biology of the commercial sea cucumber Parastichopus californicus (Stimpson) (Echinodermata: Holothuroidea) II. Observations on the ecology of development, recruitment, and the juvenile life stage. J Exp Mar Bio Ecol 127:43–67
Collard M, Eeckhaut I, Dehairs F, Dubois P (2014) Acid-base physiology response to ocean acidification of two ecologically and economically important holothuroids from contrasting habitats, Holothuria scabra and Holothuria parva. Environ Sci Pollut Res Int 21:13602–13614
Collard M, Laitat K, Moulin L, Catarino AI, Grosjean P, Dubois P (2013) Buffer capacity of the coelomic fluids in echinoderms. Comp Biochem Physiol 166:199–206
Conand C (1983) Methods of studying growth in holothurians and preliminary results from a bêche-de-mer tagging experiment in New Caledonia. SPC Fisheries Newsletter 26:31–38
Conand C (1989) Les Holothuries Aspidochirotes du lagon de Nouvelle-Calédonie: biologie, écologie et exploitation. Etudes et Thèses. O.R.S.T.O.M., Paris, p 393
Conand C (1991) Long-term movements and mortality of some tropical sea cucumbers monitored by tagging and recapture. In: Yanagisawa T, Yasumasu I, Oguro C, Suzuki N, Motokawa T (eds) Biology of Echinodermata. Balkema, Rotterdam, pp 169–175
Conand C (1993a) Ecology and reproductive biology of Stichopus variegatus an Indo-Pacific coral reef sea cucumber (Echinodermata: Holothuroidea). Bull Mar Sci 52:970–981
Conand C (1993b) Reproductive biology of the holothurians from the major communities of the New Caledonian Lagoon. Mar Biol 116:439–450
Conand C (1998) Overexploitation in the present world sea cucumber fisheries and perspectives in mariculture. Echinoderms 1998: Proceedings of the 9th International Echinoderm Conference, San Francisco, CA, USA. A.A. Balkema, Rotterdam, pp 449–454
Conand C (2001) Overview of sea cucumber fisheries over the last decade—what possibilities for a durable management? Echinoderms 2000: Proceedings of the 10th International Conference, Dunedin, New Zealand. Swets and Zeitlinger, Lisse, pp 339–344
Conand C, Polidoro BA, Mercier A, Gamboa R, Hamel J-F, Purcell SW (2014) The IUCN Red List assessment of aspidochirotid sea cucumbers and its implications. SPC Bêche-de-mer Information Bulletin 34:3–7
Connell SD, Ghedini G (2015) Resisting regime-shifts: the stabilising effect of compensatory processes. Trends Ecol Evol 30:513–515
Costa V, Mazzola A, Vizzini S (2014) Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J Exp Mar Bio Ecol 461:226–232
de Oliveira DSA, Derycke S, Da Rocha CMC, Barbosa DF, Decraemer W, Dos Santos GAP (2016) Spatiotemporal variation and sediment retention effects on nematode communities associated with Halimeda opuntia (Linnaeus) Lamouroux (1816) and Sargassum polyceratium Montagne (1837) seaweeds in a tropical phytal ecosystem. Mar Biol 163:102
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Weeks S, Evans RD, Williamson DH, Hoegh-Guldberg O (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS One 4:e5239
Dupont S, Ortega-Martinez O, Thorndyke M (2010) Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19:449–462
Eriksson H, Byrne M (2015) The sea cucumber fishery in Australia’s Great Barrier Reef Marine Park follows global patterns of serial exploitation. Fish Fish 16:329–341
Eriksson H, Clarke S (2015) Chinese market responses to overexploitation of sharks and sea cucumbers. Biol Conserv 184:163–173
Eriksson H, Thorne BV, Byrne M (2013) Population metrics in protected commercial sea cucumber populations (curryfish: Stichopus herrmanni) on One Tree Reef, Great Barrier Reef. Mar Ecol Prog Ser 473:225–234
Eriksson H, Torre-Castro M, Eklof J, Jiddawi N (2010) Resource degradation of the sea cucumber fishery in Zanzibar, Tanzania: a need for management reform. Aquatic Living Resources 23:387–398
Friedman K, Eriksson H, Tardy E, Pakoa K (2011) Management of sea cucumber stocks: patterns of vulnerability and recovery of sea cucumber stocks impacted by fishing. Fish Fish 12:75–93
Gattuso J, Frankignoulle M, Wollast R (1998) Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu Rev Ecol Syst 29:405–434
Gillanders BM, Able KW, Brown JA, Eggleston DB, Sheridan PF (2003) Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Mar Ecol Prog Ser 247:281–295
Grüss A, Kaplan DM, Guénette S, Roberts CM, Botsford LW (2011) Consequences of adult and juvenile movement for marine protected areas. Biol Conserv 144:692–702
Hammond LS (1981) An analysis of grain size modification in biogenic carbonate sediments by deposit-feeding holothurians and echinoids (Echinodermata). Limnol Oceanogr 26:898–906
Hammond LS (1982) Patterns of feeding and activity in deposit-feeding holothurians and echinoids (Echinodermata) from a shallow back-reef lagoon, Discovery Bay, Jamaica. Bull Mar Sci 32:549–571
Hasan MH (2005) Destruction of a Holothuria scabra population by overfishing at Abu Rhamada Island in the Red Sea. Mar Environ Res 60:489–511
Hu C, Xu Y, Wen J, Zhang L, Fan S, Su T (2010) Larval development and juvenile growth of the sea cucumber Stichopus sp. (curryfish). Aquaculture 300:73–79
Hudson LR, Wigham BD, Solan M, Rosenberg R (2005) Feeding behaviour of deep-sea dwelling holothurians: inferences from a laboratory investigation of shallow fjordic species. J Mar Syst 57:201–218
Jeffery SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophyll a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Johnstone RW, Koop K, Larkum AWD (1990) Physical aspects of coral reef lagoon sediments in relation to detritus processing and primary production. Mar Ecol Prog Ser 66:273–283
Kinch J, Purcell S, Uthicke S, Friedman F (2008) Papua New Guinea: a hotspot for sea cucumber fisheries in the western Central Pacific. In: Toral-Granda V, Lovatelli A Vasconcellos M (eds) Sea cucumbers. A global review of fisheries and trade. FAO Fisheries and Aquaculture Technical Paper. No. 516. FAO, Rome, pp 57–77
Klinger TS, Johnson CR (1998) Spatial and temporal distribution of feeding of Aspidochirotida (Holothuroidea) on Heron Island, Great Barrier Reef. Echinoderms 1998: Proceedings of the 9th International Echinoderm Conference, San Francisco, CA, USA. A.A. Balkema, Rotterdam
Klinger TS, Johnson CR, Jell J (1994) Sediment utilization, feeding-niche breadth, and feeding-niche overlap of Aspidochirotida (Echinodermata: Holothuroidea) at Heron Island, Great Barrier Reef. Echinoderms through time. Proceedings of the 8th International Echinoderm Conference, Dijon, France. A.A. Balkema, Rotterdam
Koop K, Larkum AWD (1987) Deposition of organic material in a coral reef lagoon, One Tree Island, Great Barrier Reef. Estuar Coast Shelf Sci 25:1–9
Kroeker KJ, Micheli F, Gambi MC, Martz TR (2011) Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc Natl Acad Sci U S A 108:14515–14520
Lee S, Ferse S, Ford A, Wild C, Mangubhai S (2017) Effect of sea cucumber density on the health of reef-flat sediments. In: Mangubhai S, W. Lalavanua W, Purcell SW (eds.) Fiji’s sea cucumber fishery: advances in science for improved management. Report No. 01/17, Wildlife Conservation Society, Suva, Fiji, pp 54–61
MacTavish T, Stenton-Dozey J, Vopel K, Savage C (2012) Deposit feeding sea cucumbers enhance mineralization and nutrient cycling in organically-enriched coastal sediments. PLoS One 7:e50031
Mangion P, Taddei D, Frouin P, Conand C (2004) Feeding rate and impact of sediment reworking by two deposit feeders Holothuria leucospilota and Holothuria atra on a fringing reef (Reunion Island, Indian Ocean). In: Heinzler T, Nebelsick J (eds) Echinoderms: Munchen, Proceedings of the 11th International Echinoderm Conference, Munich, Germany. Balkema, Leiden, the Netherlands, pp 311-–317
Michio K, Kengo K, Yasunori K, Hitoshi M, Takayuki Y, Hideaki Y, Hiroshi S (2003) Effects of deposit feeder Stichopus japonicus on algal bloom and organic matter contents of bottom sediments of the enclosed sea. Mar Pollut Bull 47:118–125
Moriarty DJW (1982) Feeding of Holothuria atra and Stichopus chloronotus on bacteria, organic carbon and organic nitrogen in sediments of the Great Barrier Reef. Aust J Mar Freshw Res 33:255–263
Moriarty DJW, Pollard PC, Hunt WG, Moriarty CM, Wassenberg TJ (1985) Productivity of bacteria and microalgae and the effect of grazing by holothurians in sediments on a coral reef flat. Mar Biol 85:293–300
Namukose M, Msuya FE, Ferse SCA, Slater MJ, Kunzmann A (2016) Growth performance of the sea cucumber Holothuria scabra and the seaweed Eucheuma denticulatum: integrated mariculture and effects on sediment organic characteristics. Aquac Environ Interact 8:179–189
Navarro PG, Garcia-Sanz S, Barrio JM, Tuya F (2013) Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar Biol 160:2957–2966
Palazzo L, Wolfe K, Byrne M (2016) Discovery and description of Stichopus herrmanni juvenile nursery sites on Heron Reef, Great Barrier Reef. SPC Bêche-de-mer Information Bulletin 36:36–40
Price ARG, Evan LE, Rowlands N, Hawkins JP (2013) Negligible recovery in Chagos holothurians (sea cucumbers). Aquat Conserv 23:811–819
Purcell SW (2010) Managing sea cucumber fisheries with an ecosystem approach. FAO Fisheries and aquaculture technical paper No. 520. FAO, Rome
Purcell SW, Kirby DS (2006) Restocking the sea cucumber Holothuria scabra: sizing no-take zones through individual-based movement modelling. Fish Res 80:53–61
Purcell SW, Eriksson H (2015) Echinoderms piggybacking on sea cucumbers: benign effects on sediment turnover and movement of hosts. Marine Biology Research 11:666–670
Purcell SW, Samyn Y, Conand C (2012) Commercially important sea cucumbers of the world. FAO Species catalogue for fisheries purposes No. 6. FAO, Rome, p 150
Purcell SW, Conand C, Uthicke S, Byrne M (2016a) Ecological roles of exploited sea cucumbers. Oceanogr Mar Biol Annu Rev 54:367–386
Purcell SW, Piddocke TP, Dalton SJ, Wang Y-G (2016b) Movement and growth of the coral reef holothuroids Bohadschia argus and Thelenota ananas. Mar Ecol Prog Ser 551:201–214
Purcell SW, Polidoro BA, Hamel J-F, Gamboa RU, Mercier A (2014) The cost of being valuable: predictors of extinction risk in marine invertebrates exploited as luxury seafood. Proc R Soc Lond B Biol Sci 281:20133296
Purcell SW, Mercier A, Conand C, Hamel J-F, Toral-Granda MV, Lovatelli A, Uthicke S (2013) Sea cucumber fisheries: global analysis of stocks, management measures and drivers of overfishing. Fish Fish 14:34–59
Quinn G, Keough M (2003) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Roberts D (1979) Deposit-feeding mechanisms and resource partitioning in tropical holothurians. J Exp Mar Bio Ecol 37:43–56
Roberts D, Bryce C (1982) Further observations on tentacular feeding mechanisms in holothurians. J Exp Mar Bio Ecol 59:151–163
Roberts D, Gebruk A, Levin V, Manship BAD (2000) Feeding and digestive strategies in deposit-feeding holothurians. Oceanogr Mar Biol Annu Rev 38:257–310
Schneider K, Silverman J, Woolsey E, Eriksson H, Byrne M, Caldeira K (2011) Potential influence of aspidochirotid sea cucumbers on coral reef CaCO3 budget: a case study at One Tree Reef. J Geophys Res 116:G04032
Schneider K, Silverman J, Kravitz B, Rivlin T, Schneider-Mor A, Barbosa S, Byrne M, Caldeira K (2013) Inorganic carbon turnover caused by digestion of carbonate sands and metabolic activity of holothurians. Estuar Coast Shelf Sci 133:217–223
Shiell GR, Knott B (2010) Aggregations and temporal changes in the activity and bioturbation contribution of the sea cucumber Holothuria whitmaei (Echinodermata: Holothuroidea). Mar Ecol Prog Ser 415:127–139
Skewes TD, Dennis DM, Koutsoukos A, Haywood M, Wassenberg T, Austin M (2004) Stock survey and sustainable harvest for Torres Strait bêche-de-mer. Torres Strait Research Program Final Report, AFMA Project Number R01/1345. Australian Fisheries Management Authority, Canberra
Slater MJ, Jeffs AG (2010) Do benthic sediment characteristics explain the distribution of juveniles of the deposit-feeding sea cucumber Australostichopus mollis? Journal of Sea Research 64:241–249
Slater MJ, Carton AG (2010) Sea cucumber habitat differentiation and site retention as determined by intraspecific stable isotope variation. Aquaculture Research 41:e695–e702
Uthicke S (1999) Sediment bioturbation and impact of feeding activity of Holothuria (Halodeima) atra and Stichopus chloronotus, two sediment feeding holothurians, at Lizard Island, Great Barrier Reef. Bull Mar Sci 64:129–141
Uthicke S (2001) Nutrient regeneration by abundant coral reef holothurians. J Exp Mar Bio Ecol 265:153–170
Uthicke S, Klumpp DW (1998) Microphytobenthos community production at a near-shore coral reef: seasonal variation and response to ammonium recycled by holothurians. Mar Ecol Prog Ser 169:1–11
Uthicke S, Welch D, Benzie JAH (2004) Slow growth and lack of recovery in overfished holothurians on the Great Barrier Reef: evidence from DNA fingerprints and repeated large-scale surveys. Conserv Biol 18:1395–1404
van Hoytema N, Bednarz VN, Cardini U, Naumann MS, Al-Horani FA, Wild C (2016) The influence of seasonality on benthic primary production in a Red Sea coral reef. Mar Biol 163:52
Vidal-Ramirez F, Dove S (2016) Diurnal effects of Holothuria atra on seawater carbonate chemistry in a sedimentary environment. J Exp Mar Bio Ecol 474:156–163
Wiedemeyer WL (1994) Biology of small juveniles of the tropical holothurian Actinopyga echinites: growth, mortality, and habitat preferences. Mar Biol 120:81–93
Wolfe K, Byrne M (2017) Population biology and recruitment of the vulnerable sea cucumber, Stichopus herrmanni, on a protected reef. Mar Ecol. doi:10.1111/maec.12389
Wolkenhauer SM, Uthicke S, Burridge C, Skewes T, Pitcher R (2010) The ecological role of Holothuria scabra (Echinodermata: Holothuroidea) within subtropical seagrass beds. J Mar Biol Assoc UK 90:215–223
Yamanouti T (1939) Ecological and physiological studies on the holothurians in the coral reef of Palao Islands. Palao Tropical Biological Station Studies 25:603–635
Yingst JY (1976) The utilization of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian. J Exp Mar Bio Ecol 23:55–69
Acknowledgements
This research was supported by a Ph.D. scholarship from the University of Sydney and grants from the Mohamed bin Zayed Species Conservation Fund, the Great Barrier Reef Foundation, the Great Barrier Reef Marine Park Authority, the Paddy Palin Foundation and the Holsworth Wildlife Research Endowment. We thank Dr. Hampus Eriksson, Alexia Graba-Landry, Camila Ayroza, Steve Doo, Dione Deaker, Milly Raven, Ben Thorne and the staff of One Tree Island Research Station, a facility of the University of Sydney. This project operated under the GBRMPA Permit No. G13/36027.1. We thank the reviewers for comments that improved this manuscript. This is Sydney Institute of Marine Science Contribution No. 210.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Mark R. Patterson
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wolfe, K., Byrne, M. Biology and ecology of the vulnerable holothuroid, Stichopus herrmanni, on a high-latitude coral reef on the Great Barrier Reef. Coral Reefs 36, 1143–1156 (2017). https://doi.org/10.1007/s00338-017-1606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-017-1606-5