Abstract
Epilithic algae are a ubiquitous component of coral reefs. Components of the epilithic algal matrix (EAM) can have a significant influence on coral settlement and benthic feeding by fishes. We employed a herbivore exclusion experiment on a fringing reef in Viti Levu, Fiji, to investigate the functional role of herbivorous fishes in affecting the EAM between different habitat types and levels of community-based fishing restriction. We surveyed the herbivorous fish community and deployed experimental tiles on the reef flat and lagoonal slope and inside and outside of an area where fishing is restricted (tabu). Tiles were deployed for 3 months, half within cages to exclude herbivorous fishes. We then identified algal type and quantified epilithic algal turf height, sediment dry weight, and detritus within the EAM on each tile. EAM that developed under herbivory was remarkably similar, regardless of the differences in habitat or fishing restriction. In contrast, EAM within cages was characterised by longer turf, heavier sediment load, and high variance in turf length and sediment load. Habitat type played a strong role in determining EAM characteristics where herbivores were excluded. Caged EAM on the reef flat was characterised by algal turf and fleshy macroalgae, whereas EAM in cages on the lagoonal slope was overwhelmingly dominated by filamentous and mat-forming cyanobacteria. The results presented here demonstrate the importance of herbivorous fishes in maintaining a benthic environment favourable to coral settlement and survival. Our results suggest that herbivore biomass per se is not a reliable predictor of foraging activity. Moreover, these results show that the absence of herbivore foraging can have different consequences depending on the habitat type, even within the same reef.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The persistence of reef-building corals depends in large part upon the supporting role of herbivorous fishes to maintain a benthic environment conducive to coral recruitment, survival, and growth (Bellwood et al. 2004). As is becoming increasingly recognised within the literature, herbivorous fish species do not contribute equally to the ecological functions of algal and sediment removal (Montgomery et al. 1989; Bellwood and Choat 1990; Kelly et al. 2016). In fact, only members of the families Siganidae, Kyphosidae, and some Acanthuridae are physiologically capable of breaking down and assimilating algal material (Crossman et al. 2005), despite scarine labrids and other acanthurids being classed as “herbivores”. Scarine labrids and some Acanthuridae target and assimilate lipid- and protein-enriched detritus, microbes, and invertebrates abundant between filamentous thalli of epilithic, turf-forming algae (i.e. the epilithic algal matrix or EAM) (Crossman et al. 2001; Clements et al. 2017). Therefore, fish do not have to be targeting algal material per se to be functional herbivores. Irrespective of whether herbivorous fishes are actually herbivorous, their feeding behaviour reduces height of the epilithic algal turf, density, and sediment content within the EAM (Randall 1961; Bardach 1961). Foraging by herbivorous fishes thus maintains short, productive turfs and can also generate bare space beneficial to settling corals (reviewed by McCook 1999; also see Russ 2003). Three feeding modes are particularly important to herbivorous fish functioning on coral reefs in the Indo-Pacific. Scrapers (most members of the tribe Scarini, Labridae) use strong jaws and fused teeth to remove the EAM entirely (Bonaldo et al. 2014). Grazers (Acanthurus and Zebrasoma spp., Acanthuridae) use incisiform teeth to crop algal thalli, typically without removing EAM-bound sediment (Jones 1967; Tebbett et al. 2017a). Rakers (Ctenochaetus spp., Acanthuridae) use long teeth to comb trapped sediment and detritus from within the EAM, typically without removing algal material (Purcell and Bellwood 1993; Tebbett et al. 2017a).
In addition to foraging activity, environmental factors (e.g. light, water flow, temperature, and nutrient availability) strongly influence the growth of benthic algae and sediment accumulation within the EAM (Carpenter and Williams 1993; McCook 1999; Littler et al. 2006). Investigating the relative importance of these factors can provide useful information about the resilience of a particular coral reef ecosystem to disturbance. Nearshore coral reefs are typically subjected to finer sediment and higher organic loads than those further offshore (Smithers and Larcombe 2003). The influx and accumulation of sediments and detritus can differ with depth and location on a reef. Reef flats, for instance, are exposed to intense light levels, higher water temperatures, and oscillatory flow (i.e. wave motion), whereas lagoonal slopes are subject to lower light intensity, cooler temperatures, and laminar flow (i.e. tidal currents) (Vogel 1994; Purcell and Bellwood 2001). Laminar flow or variability in the height of algal turfs can work to increase sediment accumulation within the EAM by extending the diffusive boundary layer above the substratum or turf canopy. An increase in the depth of the diffusive boundary layer will increase the deposition of fine sediments and limit oxygen availability within the EAM (Carpenter and Williams 1993; Vogel 1994). Abnormally low foraging activity or a sudden input of fine sediment can accelerate sediment deposition and oxygen depletion, in turn potentially deterring herbivores from foraging (Goatley et al. 2016). If trapped sediments become consolidated overtime, this could effectively exclude herbivores and prevent coral larvae from settling (Birrell et al. 2005).
Numerous caging experiments have revealed the striking effects of herbivory and eutrophication on EAM community dynamics (reviewed by Littler and Littler 2007). Since Littler and Littler’s (2007) review, the role of benthic sediments on coral reefs has received increasing attention. Results from experimentally manipulating the amount of sediment and detritus within the EAM have suggested harmful effects to benthic algae and herbivore deterrence (Clausing et al. 2014; Gordon et al. 2016b; Tebbett et al. 2017b). It is therefore important to jointly consider the different factors that might influence the dynamic interaction between herbivory, algae, and non-algal components of the EAM (e.g. sediment and detritus).
Here, we quantify the effect of herbivorous fish foraging on the development of the algal, detrital, and sediment components of the EAM on a fringing reef in Fiji, using a caging experiment to prevent large herbivores from feeding on texturised tiles simulating bare reef substratum. We ask whether the role of fish-based herbivory varies between two inshore habitat types (i.e. reef flat and lagoonal slope) and is stronger inside than outside locally managed tabu areas, where fishing is restricted by local communities (Gillett 2014). It should be noted here that this study is not intended to provide a comprehensive view of herbivory, as urchins were excluded within this experiment. We expected that (a) foraging would play a strong role in reducing algal turf height and EAM-bound sediment load on all tiles open to herbivory; (b) algal turf height and sediment load would be higher for caged tiles on the lagoonal slope than on the reef flat; (c) fishing restriction would lead to higher herbivore biomass within the tabu area; and d) this higher herbivore biomass would lead to reduced algal turf height and sediment load on open tiles inside versus outside the tabu area.
Materials and methods
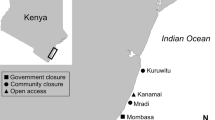
This study was carried out between September 2015 and January 2016 and focused on the fringing reef within the Navakavu traditional fishing grounds (qoliqoli) on the south-east coast of Viti Levu, Fiji (18.13°S 178.38°E). Covering 18.5 km2 of reef, the Navakavu qoliqoli is collectively managed by Waiqanake, Muaivuso, Namakala, and Nabaka villages (Fig. 1; Gillett 2014). Sites were selected in two habitat types: reef flat (1–4 m depth at slack tide) and lagoonal slope (4–7 m depth at slack tide), both within and outside of the Navakavu tabu area. This design yielded four sites, compared orthogonally between reef flat versus lagoonal slope and tabu versus fished. The sites were selected for similarities in percentage of coral cover, wave and wind exposure (facing SE trade winds), and general habitat complexity (see Ford et al. 2018b). Ford et al. (2018b measured nutrient concentrations at the sites during the experiment and concluded that “The proximity of paired sites and insignificant differences between inorganic nutrient concentrations (Appendix B) suggested that differences between paired tabu and [non-tabu sites] were not driven by water quality.” (p. 313). Subsistence and commercial fishing occurs outside of the tabu area, with local fishers using barrier nets, handlines, trolling, and spear guns to target piscivorous and herbivorous reef fish (RSM, pers. obs.). The time period in which the tiles were deployed coincides with the major coral spawning season in the area (Quinn and Kojis 2008). However, the tiles were retrieved prior to a minor coral spawning season occurring from March to April, identified by Mildner (1991).
A 2013 report estimated that the reef within the Navakavu qoliqoli provides protection against coastal flooding for 760 homes, with nearly 99% of people in at least one of the nearby villages reliant on fishing for income as well as subsistence (with many herbivorous fish species targeted, O’Garra 2007). The current tabu area within the Navakavu qoliqoli was established as part of the Locally Managed Marine Areas (LMMA) network and—with the exception of 1 week in 2012—has been closed to fishing since 2003 (Gillett 2014). This tabu area spans 16% of the qoliqoli (of which the majority is coral reef, but which also includes areas of seagrass and mangroves) and has long served as an archetype of successful management, inspiring communities throughout Fiji to establish LMMAs. Nevertheless, poaching within the tabu area remains a problem, with close proximity to a large commercial fish market in the city of Suva and under-equipped local wardens providing the only enforcement (Gillett 2014).
In order to track the development of the EAM and its ability to trap sediment and detritus, 15 × 15 cm sandstone tiles were deployed at each site to simulate bare reef substratum (e.g. recently dead coral). While limestone tiles would have been an ideal proxy for natural reef substratum, they were unavailable at the time of the study. Sandstone was the material available that best approximated the appearance and texture of natural substratum. Individual grains of silicate-based sandstone are indeed more abrasive than carbonate-based limestone, but at least one experimental study has shown parrotfish do not appear to avoid silicates in favour of carbonates (Gordon et al. 2016a). At each site, eight pairs of tiles were positioned horizontally, with each tile pair 10 cm apart and 10 cm above the substratum. The tiles were deployed directly into the study area and not preconditioned. Depressions were drilled in the upper surface of each tile (196 tile−1, 1 mm in depth and diameter and 1 cm apart) to replicate micro-complexity (Edmunds et al. 2014). Each tile was secured to a larger foundation tile using stainless steel bolts, which was then secured to the substratum with rebar. The tile assemblies were haphazardly placed at least 2 m apart from each other throughout each site. In order to exclude herbivorous fishes, four of the eight tile pairs were enclosed by modified crab traps with transparent 15 mm plastic mesh. Our attempts to measure flow and light intensity within the cages did not prove successful, but similar mesh has been found to cause minimal reductions in both intensities in a similar reef environment (Clausing et al. 2014). The cages excluded larger herbivorous fishes (the largest parrotfish observed within the cage mesh was less than 10 cm by fork length, FL), and the elevated placement excluded echinoderms (RSM, pers. obs.). The remaining four pairs at each site were not enclosed by mesh. Experimental sites were visited biweekly to clean the cages’ mesh and check for damage. Minimal damage of the cage structure occurred throughout the experiment, and no signs of intruding fishes were apparent. The tiles were deployed for 12 weeks between October 2015 and January 2016.
Six and 12 weeks after installing the tiles, photographs of the upper surface of each tile were taken in situ (Electronic Supplementary Material), and the dominant macroalgal functional groups (either filamentous or corticated foliose algae—hereafter referred to as foliose, following Steneck and Dethier 1994) were noted. 12 weeks into the experiment, turf height was measured to the nearest millimetre at three randomly chosen points on each tile using a modified ruler. When interpreting the results, it is important to consider that the three measurements have not produced as accurate results as would a high number of measurements per tile. After 12 weeks, tiles were carefully placed into sealed plastic bags in situ to retain all materials within the EAM. To remove attached or accumulated material, all surfaces were scrubbed with a toothbrush (taking approximately 1 min tile−1). The loose material was washed with freshwater and passed through a 125-µm sieve to separate algal thalli from detritus and inorganic sediment. Hereafter, organic material in the > 125-µm sample is classified as algal material and < 125 µm is classified as detritus. Inorganic sediment amounts involve both coarse and fine fractions, only coarse organic material was removed for sediment analysis. Each sample was allowed to settle in a refrigerator (at 4 °C) for 24 h before carefully decanting the supernatant to avoid discarding visible material. The remaining material was dried under a fume hood (30 to 40 °C) for 48 h, sealed in plastic bags, and shipped to the Leibniz Centre for Tropical Marine Research (ZMT) in Bremen, Germany, for further analysis.
Upon arrival at the ZMT, samples were placed in a drying oven at 105 °C for 12 h and weighed to the nearest milligram to provide total dry weight for each tile. The samples were then combusted at 450 °C for 3 h (Luczak et al. 1997). After cooling to room temperature, the resulting weight difference provided the mass of detritus, validated by a known standard (Fichtennadeln HE3401520, HEKAtech GmbH, Wegberg, Germany). The remaining material was nearly entirely carbonate and silicate sediment. This was confirmed by further combustion at 950 °C for 3 h (following Heiri et al. 2001) and validation against two known standards (≥ 99% calcium carbonate, Merck, Darmstadt, Germany and 1–4 mm quartz chips, IVA Analysentechnik GmbH & Co. KG, Meerbusch, Germany).
To determine differences in the herbivorous fish assemblages between sites, the biomass and community composition (by feeding mode, Table 1) were quantified. Eight replicate 30 × 5 m belt transects were conducted using SCUBA by a consistent observer (RSM) counting and identifying fish to species level and assigning these to 5-cm-size class (FL). Large (> 10 cm FL), relatively vagile fish were counted on the first pass while a second diver followed with the transect tape (sensu Dickens et al. 2011). On a second pass, the same observer noted small, site-attached fish 5–10 cm FL. Fish transects were conducted in September 2015 at a similar time of day, tide, and weather in order to maximise comparability of fish assemblages. A second set of transects was conducted at the reef flat sites in December 2015 at a similar time of day, tide, and weather to the initial transects in order to detect differences in the herbivorous fish community during the course of the experiment. Herbivore biomass was calculated as \( {\text{biomass}} = as^{b} \), where s is the median of the size class (e.g. 12.5 cm for 10–15 cm) and coefficients a and b were taken from the published literature (compiled by Green and Bellwood 2009).
All statistical analyses and graphics were conducted using R (R Core Team 2016), and Figs. 2 through 5 are generated using the graphics package ggplot2 (Wickham 2009).
Turf canopy height on tiles between habitat types and levels of fishing restriction. Note that the replication in this figure (n = 16) differs from that used in analyses (n = 8). Letters above error bars (± SE) indicate relative statistical significance (e.g. points marked with an A are significantly different from D)
The three turf height measurements per tile were averaged to produce a single replicate measurement per tile. The effects of herbivory, habitat type, and level of fishing restriction on algal turf height were analysed with a generalised linear mixed model (nlme, Pinheiro et al. 2014) incorporating a fixed variance structure. For sediment load and detritus, the influence of habitat type and herbivory was tested with generalised linear mixed models (lme4, Bates et al. 2015). ID of the foundation tile (each harbouring two experimental tiles) was treated as a random factor to account for the pairing and non-independence of tiles. All other variables were treated as fixed factors. Eight replicates per treatment combination were used. Due to the loss of some sediment samples during processing, the influence of fishing restriction on sediment load and detritus could not be tested. To test differences in the herbivorous fish biomass between habitat types, levels of fishing restriction, and between time points, a linear model was applied. Linear models describing herbivore fish biomass were analysed using ANOVA (car, Fox and Weisberg 2011).
All data were visually checked for normality of residuals and homoscedasticity, and if needed, a Box-Cox transformation (using package MASS, Venables and Ripley 2002) and a variance structure was applied. Each full model (all interactions) was reduced by removing the least significant term if a likelihood ratio test between the reduced and unreduced models revealed no significant difference (Zuur et al. 2007). The minimum adequate model of turf height and sediment data was fitted using restricted maximum likelihood estimation and tested as type III ANOVA with Satterthwaite approximation for degrees of freedom with package lmerTest (Kuznetsova et al. 2016). Post hoc tests were performed with the package emmeans (Lenth 2018) applying Tukey adjustments (α = 0.05).
Results
The exclusion of herbivorous fishes resulted in 14.8 times longer turf length across both habitats and levels of fishing restriction and 8.8 times more sediment within cages across both habitats. Turf height on 75% of all caged tiles exceeded 5 mm by the end of the experiment, while turfs on 97% of all open tiles remained shorter than 5 mm. Turf algae grew longer on caged tiles than open tiles (ANOVA F1,58 = 109.60, p < 0.001). Habitat type also played a role in turf length, where turfs on caged and open tiles were longer on the lagoonal slope than the reef flat (ANOVA F1,58 = 10.66, p < 0.005). Fishing restriction also played a significant role in turf length as turfs were shorter inside the tabu area (ANOVA F1,58 = 6.65, p < 0.05). Additionally, caging produced an interactive effect with habitat type and fishing restriction (ANOVA F1,58 = 10.82, p < 0.005 and ANOVA F1,58 = 43.46 p < 0.0001, respectively, Fig. 2), since both habitat and fishing restriction significantly affected turf height. Algal turf dominated tiles and was common on natural substratum at both habitats (see Ford et al. 2018b for benthic data taken during the course of this study). Caged tiles on the lagoonal slope were characterised by a dense growth of filamentous cyanobacteria and some mat-forming cyanobacteria. 37.5% of caged tiles on the lagoonal slope were found stained black under the EAM after retrieval at the end of the experiment. Fleshy macroalgae (Padina, Phaeophyceae) were common on open tiles and natural substratum at the non-tabu reef flat site.
The sediment loads on open tiles in both habitats were lower than sediment loads on caged tiles (ANOVA F1,20.58 = 51.99, p < 0.0001). Both open and caged tiles on the lagoonal slope accumulated more sediment than those on the reef flat (ANOVA F1,20.58 = 18.29, p < 0.001). An interaction between habitat and caging factors (ANOVA F1,20.58 = 5.64, p < 0.05) was evidenced by more sediment on caged tiles on the lagoonal slope than on the reef flat and a similarity between sediment load on open tiles on the lagoonal slope and caged tiles on the reef flat (Fig. 3).
The amount of detritus accumulated on tiles did not differ with caging, but did differ with habitat type (Fig. 4). The sediment on the reef flat contained a higher proportion of detritus than sediment on the lagoonal slope (ANOVA, F1,13.93 = 23.28, p < 0.001).
Organic content (i.e. detritus) as a per cent of the dry weight of sediment accumulated on tiles between habitat types (n = 16). Caging did not produce a significant effect on detritus composition. Letters above error bars (± SE) indicate statistical significance. Black indicates open tiles, and grey indicates caged tiles
Herbivorous fish biomass did not differ significantly between the reef flat and lagoonal slope (27.3 ± 3.9 and 24.9 ± 3.4 g m−2, respectively), but did differ between levels of fishing restriction (19.4 ± 3.3 g m−2 within the tabu area and 32.8 ± 3.1 g m−2 outside, ANOVA F1,30 = 9.66, p < 0.005). Furthermore, no significant differences were detected in herbivore biomass between September and December (ANOVA F1,29 = 0.02, p = 0.899), nor between levels of fishing restriction in December (ANOVA F1,29 = 2.03, p = 0.165). Scrapers were the most represented feeding mode among herbivorous fishes and exhibited a significantly lower biomass inside the tabu area compared with outside (ANOVA, F1,30 = 10.495, p < 0.005). Scraper size distribution within the tabu area on the reef flat was right-skewed (Fig. 5, ESM); the modal size class within the tabu area was < 10 cm, whereas the modal size class outside the tabu area was 11–15 cm. Biomass of the grazer and raker feeding modes did not differ significantly between habitat type or levels of fishing restriction.
Size distribution of scraping parrotfishes between the tabu area (light bars) and non-tabu area (dark bars). Bars indicate mean individuals per transect (150 m2) (± SE). Numbers on the x-axis indicate the median of each size class (e.g. 7.5 for 5–10 cm). Note the large number of 5–10 cm (FL) fish within the tabu area
Discussion
Even among open tiles, algal turfs in this experiment grew longer on the lagoonal slope than on the flat, which suggests possible differences in herbivore feeding pressure between the two habitats. There were no significant differences in herbivore biomass between the two habitats (ANOVA F1,29 = 0.17, p = 0.681). The data from this study do not allow for the estimation of a biomass threshold whereby herbivore functions might be lost, but they do suggest that the significant differences in biomass have not translated to major differences in function (i.e. scraping, grazing, and raking). Indeed, using herbivorous fish biomass as a proxy for herbivory can lead to overestimation of the spatial distribution or intensity of foraging (Fox and Bellwood 2007; Goatley et al. 2016). Individual fish may modulate their foraging rates or roving distance depending on environmental factors that may vary through time (Welsh and Bellwood 2015; Bejarano et al. 2017). Additionally, variation in morphological and behavioural traits among genera and species implies that not all herbivores are functionally equivalent (Bellwood and Choat 1990). Furthermore, a fish may not provide the same magnitude of function as it grows (Bonaldo and Bellwood 2008). It is also possible feeding pressure on the lagoonal slope is less intense than on the flat because of the high amounts of inorganic sediment and lower composition of EAM-bound detritus (Gordon et al. 2016a; Tebbett et al. 2017b). Turfs on open tiles were of similar length to natural substratum at the same sites (Ford et al. 2018b). It should be noted that while statistically significant, EAM on open tiles was remarkably similar across habitats and turf length was still maintained under 5 mm, a critical height for coral recruitment identified by Ford et al. (2018b) threshold.
Foliose macroalgae (Padina, Phaeophyceae) were common on caged tiles and natural substratum on the reef flat (R. McAndrews pers. obs.). Interestingly, foliose macroalgae were absent from tiles open to herbivory. The inherently more extensive micro-complexity of natural substratum could have provided refugia for algal propagules to settle and develop herbivore-resistant holdfasts before being exposed to herbivory (Loffler et al. 2018). The 1 × 1 mm depressions drilled into the experimental tiles would have provided refugia for settling algal propagules, but probably not deep enough to protect them from parrotfish bites (Brandl and Bellwood 2016). Parrotfish scraping could therefore have prevented Padina from establishing on open tiles, while allowing a bloom to occur on natural substratum assumed to be under similar grazing pressure.
Caged tiles on the lagoonal slope nearly universally featured dense filamentous cyanobacteria of extremely variable canopy height not seen on the reef flat or open tiles. Additionally, mat-forming cyanobacteria were also observed in the canopy of the cyanobacterial turf. The cyanobacteria-dominated EAM contained high amounts of sediment. Filamentous cyanobacteria were not observed on open tiles or natural substratum, while mat-forming cyanobacteria were observed (R. McAndrews pers. obs.). Thus, it is plausible that the dense filamentous cyanobacteria observed on caged tiles are vulnerable to herbivore foraging, but able to outcompete the algal turfs observed on natural substratum. Indeed, some forms of filamentous cyanobacteria are palatable to herbivore species abundant in the experimental area (Scott and Russ 1987). It has even been theorised that the high lipid and protein contents of some forms of cyanobacteria are a preferred target of parrotfishes (Clements et al. 2017). The prevalence of mat-forming cyanobacteria observed on the natural substratum and on the caged tiles is probably linked to their low palatability to herbivores (Ford et al. 2018a). The densely packed filaments and variability in canopy height on tiles would have expanded the diffusive boundary layer and increased the accumulation of sediment (Carpenter and Williams 1993; Vogel 1994), as was found in this study. The increased thickness of the diffusive boundary layer and high amounts of sediment would have made it difficult for coral larvae to settle on the substratum (Birrell et al. 2005).
Less sediment accumulated on open tiles than on those in cages, suggesting herbivorous fishes were able to remove sediment. This was expected with abundant scraping and raking herbivorous fishes and the maintenance of shorter algal turfs by scraping and grazing herbivores. Foremost, shorter and more uniform turfs maintained by scrapers and grazers are less effective at trapping sediment (Purcell 2000). In addition, scraping and raking herbivores abundant at the sites remove sediment from the EAM when feeding, often depositing it in deeper water (Bellwood 1995; Goatley and Bellwood 2010; Krone et al. 2011). Raking surgeonfishes use long, bristle-like teeth to extract material from between algal thalli (Tebbett et al. 2017a). With herbivorous fishes unable to access caged EAM to remove sediment and shorten turf, sediment accumulated and remained within the EAM. It must be noted that the mesh on the cage structure could have increased the deposition of sediment on caged tiles by reducing flow speeds enough to cause more sediment to settle onto the tiles. Since measurements of flow within the cages were not available, we are unable to determine the magnitude of this effect. Nonetheless, excessive sediment trapped by turfs under-maintained by herbivorous fishes can be inhibitive to coral settlement and health, as well as further deter herbivory (Birrell et al. 2005; Goatley et al. 2016).
At the end of the experiment, an unexpected observation was made at the base of the EAM. More than one-third of the caged tiles on the lagoonal slope were stained black where the cyanobacteria-dominated EAM had been removed. The original tile colour then returned within seconds. The disappearance of the black colour once the tile was exposed to air suggests that at least part of the sediment and detritus layer at the base of the EAM was anoxic. Anoxic sulphate reduction can occur in low-energy (i.e. low oxygen mixing) environments in the presence of relatively high amounts of detritus (Chapman and Fletcher 2002). Working on similar reefs, Chapman and Fletcher (2002) and Clausing et al. (2014) observed an anoxic layer that developed when sediment depth exceeded 3 mm. In addition to increasing the sediment load, the thicker diffusive boundary layer above cyanobacteria-dominated EAM would have reduced the influx of oxygen (Vogel 1994). Reduced oxygen availability to the deeper areas within the EAM and a high amount of detritus likely contributed to the black colour of tiles here. In contrast, the shorter turfs, lighter deposited sediment loads, and greater influence of wave activity (more turbulent flow leads to better oxygen mixing near the substratum) on the reef flat would have resulted in a thinner diffusive boundary layer and prevented the appearance of an anoxic layer (Vogel 1994). The proportion of EAM-bound detritus in this study was within the upper range of other island-associated reefs in the Pacific (Clausing et al. 2016; Gordon et al. 2016b; Tebbett et al. 2017b), raising the possibility that the anoxia found here may be more widespread. Based on these results, we speculate that if the EAM found on lagoonal slope caged tiles were to be found naturally, scraping and raking herbivores would (a) be deterred by the high amounts of inorganic sediment and anoxia, or (b) increase feeding rates in response to the high amounts of detritus.
Contrary to our expectations, we observed lower herbivorous fish biomass within the tabu area than outside. This was largely the result of differences in parrotfish size distribution on the reef flat. A right-skewed size distribution of parrotfishes in the tabu area meant that the average parrotfish inside is smaller than those outside. Mumby et al. (2006) have suggested that increased predation within areas where fishing is prohibited can result in smaller individuals of some parrotfish species (smaller species that rarely exceed 23 cm in maximum length) but larger individuals of species known to attain larger maximum lengths. This trend was observed to an extent here (ESM) and might explain the size-skewed distribution of parrotfish. It is likely that despite the lower scraper biomass, increased foraging intensity by more numerous smaller parrotfishes is contributing to our observations of shorter algal turf in the tabu area. Although we do not know the difference in sediment load between levels of fishing restriction, the rate of sedimentation on the lagoonal slope did not differ with levels of fishing restriction (Electronic Supplementary Material). Interestingly, despite the reduced biomass and apparent reliance on smaller parrotfishes, a study conducted during the same time period and at the same sites as the current study found dramatically higher densities of coral larval spat on open tiles within the tabu area (Ford et al. 2018b).
This study focused on a single locally managed marine area (LMMA) over a 3-month period. Caution must therefore be taken before applying the results or conclusions presented here to LMMAs elsewhere. Furthermore, this experiment was conducted during the onset of an El Niño summer and the impact of annual variation in environmental parameters should be taken into account (Glynn and Colgan 1992).
Several studies to date have highlighted the influence of EAM-bound sediments and detritus to herbivorous fishes and coral larvae (Birrell et al. 2005; Goatley et al. 2016; Tebbett et al. 2017b), but few have observed the role of natural accumulation of sediment and detritus with and without herbivorous fishes. Our results demonstrate the differential functional role of herbivores between habitats on the same reef. High amounts of sediment and detritus trapped by elongated turfs on the relatively low-energy lagoonal slope created a benthic environment unlikely to facilitate coral settlement and survival. In this study, herbivorous fish foraging maintained EAM consisting of short algal turfs with relatively low sediment loads despite the significant differences in herbivore biomass. Our study adds to the body of literature contending that herbivorous fish community biomass can be an unreliable predictor of foraging activity. We found that the importance of herbivory to maintain an EAM suitable for coral settlement can differ between habitats within the same reef. We urge future studies investigating the impact of herbivory and/or fisheries restriction on epilithic algal communities to consider the role of sediment and detritus and how they might differ between areas of the same reef when making predictions about coral settlement and survival.
References
Bardach JE (1961) Transport of calcareous fragments by reef fishes. Science 133:98–99
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bellwood DR (1995) Direct estimate of bioerosion by two parrotfish species, Chlorurus gibbus and C. sordidus, on the Great Barrier Reef, Australia. Mar Biol 121:419–429
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ Biol Fishes 28:189–214
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bejarano S, Jouffray JB, Chollett I, Allen R, Roff G, Marshell A, Steneck R, Ferse SCA, Mumby PJ (2017) The shape of success in a turbulent world: wave exposure filtering of coral reef herbivory. Funct Ecol 31:1312–1324
Birrell CL, McCook LJ, Willis BJ (2005) Effects of algal turfs and sediment on coral settlement. Mar Pollut Bull 51:408–414
Bonaldo RM, Bellwood DR (2008) Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar Ecol Prog Ser 360:237–244
Bonaldo RM, Hoey AS, Bellwood DR (2014) The ecosystem roles of parrotfishes on tropical reefs. Oceanogr Mar Biol Ann Rev 52:81–132
Brandl SJ, Bellwood DR (2016) Microtopographic refuges shape consumer-producer dynamics by mediating consumer functional diversity. Oecologia 182:203–217
Carpenter RC, Williams SL (1993) Effects of algal turf canopy height and microscale substratum topography on profiles of flow speed in a coral forereef environment. Limnol Oceanogr 38:687–694
Chapman AS, Fletcher RL (2002) Differential effects of sediments on survival and growth of Fucus serratus embryos (Fucales, Phaeophyceae). J Phycol 38:894–903
Clausing RJ, Bittick SJ, Fong CR, Fong P (2016) Sediments influence accumulation of two macroalgal species through novel but differing interactions with nutrients and herbivory. Coral Reefs 35:1297–1309
Clausing RJ, Annunziata C, Baker G, Lee C, Bittick SJ, Fong P (2014) Effects of sediment depth on algal turf height are mediated by interactions with fish herbivory on a fringing reef. Mar Ecol Prog Ser 517:121–129
Clements KD, German DP, Piche J, Tribollet A, Choat JH (2017) Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc 120:729–751
Crossman DJ, Choat JH, Clements KD (2005) Nutritional ecology of nominally herbivorous fishes on coral reefs 1: Dietary analyses. Mar Biol 140:613–623
Crossman DJ, Choat JH, Clements KD, Hardy T, McConochie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605
Dickens LC, Goatley CHR, Tanner JK, Bellwood DR (2011) Quantifying relative diver effects in underwater visual censuses. PLoS ONE 6:e18965
Edmunds PJ, Nozawa Y, Villanueva RD (2014) Refuges modulate coral recruitment in the Caribbean and the Pacific. J Exp Mar Bio Ecol 454:78–84
Ford AK, Bejarano S, Nugues MM, Visser PM, Albert S, Ferse SCA (2018a) Reefs under siege – the rise, putative drivers, and consequences of benthic cyanobacterial mats. Front Mar Sci 5:18
Ford AK, Eich A, McAndrews RS, Mangubhai S, Nugues MM, Bejarano S, Moore BR, Rico C, Wild C, Ferse SCA (2018b) Evaluation of coral reef management effectiveness using conventional versus resilience-based metrics. Ecol Indic 85:308–317
Fox RJ, Bellwood DR (2007) Quantifyingherbivory across a coral reef depth gradient. Mar Ecol Prog Ser 339:49–59
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Gillett R (2014) The Navakavu community fishery in Fiji. FAO, Rome
Glynn PW, Colgan MW (1992) Sporadic disturbances in fluctuating coral reef environments: El Niño and coral reef development in the Eastern Pacific. Integr Comp Biol 32:707–718
Goatley CHR, Bellwood DR (2010) Biologically mediated sediment fluxes on coral reefs: sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar Ecol Prog Ser 415:237–245
Goatley CHR, Bonaldo RM, Fox RJ, Bellwood DR (2016) Sediments and herbivory as sensitive indicators of coral reef degradation. Ecol Soc 21:29–40
Gordon SE, Goatley CHR, Bellwood DR (2016a) Low-quality sediments deter grazing by the parrotfish, Scarus rivulatus on inner-shelf reefs. Coral Reefs 35:285–291
Gordon SE, Goatley CHR, Bellwood DR (2016b) Composition and temporal stability of turf sediments on inner-shelf coral reefs. Mar Pollut Bull 111:178–183
Green AL, Bellwood DR (2009) Monitoring functional groups of herbivorous reef fishes as indicators of coral reef resilience – a practical guide for coral reef managers in the Asia Pacific Region. IUCN, Gland
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Jones RS (1967) Ecological relationships in Hawaiian and Johnston Island Acanthuridae (surgeonfishes) with emphasis on food and feeding habits. Ph.D. thesis, Univerisity of Hawaii
Kelly ELA, Eynaud Y, Clements SM, Gleason M, Sparks RT, Williams ID, Smith JE (2016) Investigating functional redundancy versus complementarity in Hawaiian herbivorous coral reef fishes. Oecol 182:1151–1163
Krone R, Paster M, Schuhmacher H (2011) Effect of the surgeonfish Ctenochaetus striatus (Acanthuridae) on the processes of sediment transport and deposition on a coral reef in the Red Sea. Facies 57:215–221
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) lmerTest: tests in linear mixed effects models. R package version 2.0-33
Lenth RV (2018) emmeans: estimated marginal means, aka least-squares means. R package version 1.1
Littler MM, Littler DS (2007) Assessment of coral reefs using herbivory/nutrient assays and indicator groups of benthic primary producers: a critical synthesis, proposed protocols, and critique of management strategies. Aquat Conserv 17:195–215
Littler MM, Littler DS, Brooks BL (2006) Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 5:565–585
Loffler Z, Graba-Landry A, Kidgell JT, McClure EC, Pratchett MS, Hoey AS (2018) Holdfasts of Sargassum swartzii are resistant to herbivory and resilient to damage. Coral Reefs https://doi.org/10.1007/s00338-018-01745-w
Luczak C, Janquin M-A, Kupka A (1997) Simple standard procedure for the routine determination of organic matter in marine sediment. Hydrobiologia 345:87–94
McCook LJ (1999) Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18:357–367
Mildner SJ (1991) Aspects of the reproductive biology of selected scleractinian corals on western Samoan and Fijian reefs. M.Sc. thesis, James Cook University
Montgomery WL, Myberg AA Jr, Fishelson L (1989) Feeding ecology of surgeonfishes (Acanthuridae) in the northern Red Sea, with particular reference to Acanthurus nigrofuscus (Forsskål). J Exp Mar Biol Ecol 132:179–207
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Box S, Stoffle RW, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101
O’Garra T (2007) Estimating the total economic value of the Navakavu LMMA in Viti Levu island (Fiji). CRISP, Noumea
Pinheiro J, Bates D, DebRoy S, Sarkar D (2014) Linear and nonlinear mixed effects models. R package version 3.1-117
Purcell SW (2000) Association of epilithic algae with sediment distribution on a windward reef in the northern Great Barrier Reef, Australia. Bull Mar Sci 66:199–214
Purcell SW, Bellwood DR (1993) A functional analysis of food procurement in two surgeonfish species, Acanthurus nigrofuscus and Ctenochaetus striatus (Acanthuridae). Environ Biol Fish 37:139–159
Purcell SW, Bellwood DR (2001) Spatial patterns of epilithic algal and detrital resources on a windward coral reef. Coral Reefs 20:117–125
Quinn NJ, Kojis BL (2008) Variation in coral recruitment on Fijian reefs. Proc 11th Int Coral Reef Symp 459–463
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org
Randall JE (1961) Overgrazing of algae by herbivorous marine fishes. Ecology 42:812
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67
Scott FJ, Russ GR (1987) Effects of grazing on species composition of the epilithic algal community on coral reefs of the central Great Barrier Reef. Mar Ecol Prog Ser 39:293–304
Smithers S, Larcombe P (2003) Late Holocene initiation and growth of a nearshore turbid-zone coral reef: Paluma Shoals, central Great Barrier Reef, Australia. Coral Reefs 22:499–505
Steneck RS, Dethier MN (1994) A functional group approach to the structure of algal-dominated communities. Oikos 69:476–498
Tebbett SB, Goatley CHR, Bellwood DR (2017a) Clarifying functional roles: algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs 36:803–813
Tebbett SB, Goatley CHR, Bellwood DR (2017b) The effects of algal turf sediments and organic loads on feeding by coral reef surgeonfishes. PLoS ONE 12:e0169479
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vogel S (1994) Life in moving fluids: the physical biology of flow. Princeton University Press, Princeton
Welsh JQ, Bellwood DR (2015) Simulated macro-algal outbreak triggers a large-scale response on coral reefs. PLoS ONE 10:e0132895
Wickham H (2009) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York
Acknowledgements
We are grateful to the communities of Waiqanake, Muaivuso, and the rest of the Navakavu for their assistance, logistical support, and access to their reefs. The study also benefitted help from Akuila Cakacaka, Wildlife Conservation Society Fiji, Fiji Locally Managed Marine Areas (FLMMA) Network, Michael Schmid, Ulrich Pint, Stephanie Helber, the Fijian Ministry of Forestry and Fisheries Central Division, the University of the South Pacific Chemistry Department, and Matthias Birkicht. We also thank the editor and three anonymous reviewers for their time and comments on a previous draft of this manuscript. This study was financially supported by the German Federal Ministry of Education and Research (BMBF) within the REPICORE project (grant 01LN1303A), the Rufford Foundation (Grant 18231-1), and additional support from the Kellner & Stoll Stiftung. The research reported in this paper contributes to the Programme on Ecosystem Change and Society (www.pecs-science.org).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Morgan S. Pratchett
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McAndrews, R.S., Eich, A., Ford, A.K. et al. Algae sediment dynamics are mediated by herbivorous fishes on a nearshore coral reef. Coral Reefs 38, 431–441 (2019). https://doi.org/10.1007/s00338-019-01780-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01780-1