Abstract
Objectives
To evaluate the diagnostic performance of five MR sequences to detect pulmonary infectious lesions in patients with invasive fungal infection (IFI), using multidetector computed tomography (MDCT) as the reference standard.
Methods
Thirty-four immunocompromised patients with suspected IFI underwent MDCT and MRI. The MR studies were performed using five pulse sequences at 3.0 T: T2-weighted turbo spin echo (TSE), short-tau inversion recovery (STIR), spectrally selective attenuated inversion recovery (SPAIR), T1-weighted high resolution isotropic volume excitation (e-THRIVE) and T1-weighted fast field echo (T1-FFE). The size, lesion-to-lung contrast ratio and the detectability of pulmonary lesions on MR images were assessed. Image quality and artefacts on different sequences were also rated.
Results
A total of 84 lesions including nodules (n = 44) and consolidation (n = 40) were present in 75 lobes. SPAIR and e-THRIVE images achieved high overall lesion-related sensitivities for the detection of pulmonary abnormalities (90.5 % and 86.9 %, respectively). STIR showed the highest lesion-to-lung contrast ratio for nodules (21.8) and consolidation (17.0), whereas TSE had the fewest physiological artefacts.
Conclusions
MRI at 3.0 T can depict clinically significant pulmonary IFI abnormalities with high accuracy compared to MDCT. SPAIR and e-THRIVE are preferred sequences for the detection of infectious lesions of 5 mm and larger.
Key Points
• A radiation-free radiological method is desirable for assessing pulmonary infectious lesions
• MRI at 3 T can depict lung infiltrates with good concordance to MDCT
• SPAIR and e-THRIVE are favourable sequences for the detection of pulmonary lesions
• The greatest benefit is for the diagnosis of lesions larger than 5 mm
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive fungal infection (IFI) in the lung is a frequent serious pulmonary complication in immunocompromised hosts, especially in patients with haematologic malignancies and who have undergone chemotherapy [1, 2]. Untreated IFI often has a rapid and fatal course. Thus, early and accurate diagnosis of IFI is important to improve patient survival [3]. Unfortunately, serologic and culture tests have proved unreliable in many cases [4]. Although chest CT has been generally accepted as an important tool with high utility for this fungal disease [5], high accumulative radiation exposure from repetitive CT examinations may be undesirable. Radiation dose exposure, and the accompanying increased cancer risk, may limit the utilization of CT in patients with severe neutropenia at high risk of IFI, especially in young patients who require frequent follow-up studies.
As a radiation-free method, MRI has been proposed as a promising alternative imaging technique to CT [6]. Nevertheless, the major challenges to pulmonary MR imaging, caused by low proton density, susceptibility effects, and cardiac and respiratory motion artefacts, impair the image quality. Following technical advances of high-performance gradients, phased-array receiver coils and refined imaging sequences, high field MRI systems are becoming clinically available for pulmonary imaging [7]. Recent studies demonstrated its capability for imaging a variety of lung diseases compromising focal lung lesions, diffuse infiltrative lung disease and metastases [8–10].
Several pulse sequences have been evaluated for lung MR and shown to be effective for the detection and characterization of pulmonary diseases, most at field strengths of up to 1.5 T [11–13]. However, to our knowledge, until now few studies have focused on the choice of the optimal MR imaging sequence for the detection of pulmonary IFI lesions at 3.0 T [14]. The purpose of this study was to evaluate the diagnostic performance of five MR sequences, in regard to the detection of pulmonary abnormalities indicative of IFI, in comparison with multidetector computed tomography (MDCT), which was used as the gold standard.
Materials and methods
Patients
The study was approved by the hospital institutional review board. Written informed consent was obtained from all patients. Between May 2013 and April 2014, a total of 34 immunocompromised patients (29 men, 5 women; mean age 34 ± 14 years; range 18–62 years) were included in this prospective study. The patients were suffering from the following diseases: acute myeloid leukaemia (n = 16), acute lymphatic leukaemia (n = 11), chronic myeloid leukaemia (n = 3), multiple myeloma (n = 2), breast cancer (n = 1) and long-term corticosteroids for Crohn’s disease (n = 1). Inclusion criteria were host factors according to the EORTC/MSG 2008 criteria [15], persisting fever under antibacterial treatment, newly developed abnormalities highly suspected of fungal infections such as nodule(s) or consolidation on CT images. Patients with contraindications to MRI, i.e. pacemakers, claustrophobia or metallic implants, were excluded.
Thoracic MRI was performed within 2 days (mean, 1.4 days) of CT. Based on these criteria, the proven diagnosis of IFI was verified histologically in five patients by video-assisted thoracoscopic surgery (n = 3) or percutaneous CT-guided fine needle aspiration (n = 2). The remaining 29 patients were classified as probable diagnosis, defined by positivity for galactomannan antigen (n = 25) or positive culture from bronchoalveolar lavage or sputum (n = 12).
Imaging protocols
MRI was performed on a 3.0-T system (Achieva TX, Philips Medical Systems, Best, the Netherlands) with a 16-channel Torso-XL coil. The gradient system was capable of a maximum gradient strength of 80 mT/m and a peak slew rate of 200 mT/m/ms. The imaging protocol included five pulse sequences: (1) respiratory triggered (RT) T2-weighted two-dimensional (2D) turbo spin echo (TSE), (2) RT T2-weighted spectrally selective attenuated inversion recovery (SPAIR) fat saturation, (3) RT T2-weighted 2D short-tau inversion recovery (STIR), (4) breath-hold T1-weighted three-dimensional (3D) high resolution isotropic volume excitation (e-THRIVE) and (5) breath-hold T1-weighted 2D fast field echo (T1-FFE).
To allow a better comparison, all 2D sequences were applied with equal spatial resolution, whereas the 3D e-THRIVE sequence was applied with its inherent higher spatial resolution. Table 1 summarizes the MR imaging parameters. For breath-hold sequences, the examinations were performed with expiratory gating using a respiratory belt.
All chest CTs were obtained with a 64-detector system [Somatom Definition, Siemens Medical Solutions, Forchheim, Germany, (n = 25)] or a 16-detetor system [Light-Speed 16, GE Health, Milwaukee, USA, (n = 9)] at full inspiration. With the Somatom Definition, the reconstructed slice thickness was 5 mm at 120 kV and 220 mAs with automatic dose modulation, while with Light-Speed at 120 kV and 250 mAs. The lungs were assessed in the lung window (window width, 1,500 HU; window level, −700 HU).
Image analysis
Two chest radiologists with 8 and 5 years of experience in thoracic CT and MRI interpretation, who reached the decisions on radiological findings by consensus, performed evaluation retrospectively in a three-step manner [16].
First, the two chest radiologists assessed the CT images, which served as reference standards. The observers evaluated the presence, number, size and location of pulmonary infiltrations that were highly indicative of IFI, including consolidation and nodules. The size of a lesion was determined by the largest diameter as displayed with lung window settings and lesions were classified into three groups: 1–5 mm, 6–10 mm and more than 10 mm. The lesions were assigned to one of five pulmonary lobes (upper, middle or lower right lobe; upper or lower left lobe). When the diagnosis of pulmonary fungal infection was questionable (i.e. particularly for lesions smaller than 5 mm), the lesion was evaluated on the basis of previous or follow-up CT.
Second, the two chest radiologists evaluated MR images obtained with five MR sequences using the same procedure as for the CT images. To avoid recall bias, the CT and MR images were assessed with an interval of more than 4 weeks. On MRI, the lesion size was measured along the maximal diameter; however, these values were not used for lesion size categorization. Circular operator-defined regions of interest with an average number of 90 mm2 were positioned in the lesions and adjacent lung tissues. The lesion-to-lung contrast ratio (C lesion) was defined by the ratio of the mean signal intensity of the pulmonary lesions and portions of adjacent lung parenchyma away from prominent intraparenchymal blood vessels. To minimize partial volume effects, C lesion was only measured for nodules (n = 24) or consolidation (n = 39) with a diameter of larger than 10 mm. Additionally, qualitative ratings regarding the level of imaging artefacts (physiological motion artefacts and blurring) and overall image quality with each individual MR sequence were recorded.
Third, the side-by-side comparisons between pulmonary lesions defined on MDCT and those detected on MR were performed simultaneously by two radiologists in consensus. This assessment was followed by similar comparisons of the visibility, number and size of lesions. The numbers of true-positive and false-positive diagnoses were recorded for each MR sequence.
Statistical analysis
The Friedman nonparametric test for related samples was performed to assess for statistically significant differences among the five MR sequences with respect to size, C lesion and level of physiological motion artefacts and overall image quality. When a statistically significant difference (p < 0.05) was observed, differences were tested by means of post hoc analysis using the pairwise Wilcoxon test. The lesion-, lobe- and patient-based diagnostic performance of each MR sequence for lesion detection were calculated. All tests were performed using SPSS version 13.0. Significance was accepted at p < 0.05 for all tests.
Results
All MR examinations were successfully completed despite the fact that the examinations were complicated by irregular breathing and coughing. With MDCT, 84 pulmonary lesions indicative of IFI were detected in 75 lobes in 34 patients. On the basis of their appearance, the lesions were classed as nodules (n = 44) and consolidation (n = 40).
Overall image quality and imaging artefacts
The TSE sequence achieved the best rating of all sequences with the fewest motion artefacts, closely followed by the T1-FFE and SPAIR sequences (Table 2). In comparison with these sequences, breath-hold e-THRIVE was considered to have slightly lower image quality. But the e-THRIVE sequence showed a relatively high variability with respect to the physiological motion artefacts, ranging from “negligible” to “distinct”. STIR images showed the lowest overall image quality, with a moderate level of physiological motion artefacts.
Assessment of size and lung-to-lesion contrast ratio
The sizes of pulmonary lesions measured on MR images and MDCT are reported in Table 3. Mean size of nodules and consolidation differed significantly between MDCT and MRI sequences (p = 0.019 and 0.005, respectively, Friedman test). For the visualization of nodules, STIR images showed a significantly larger extent of lesions than CT images (p = 0.020), but the size difference did not exceed 20 %. No significant difference in lesion size was found between MDCT and any other sequence.
The signal intensity measurements of nodules and consolidation showed similar rating on different MR sequences (Table 4). The highest mean C lesion values were demonstrated for the STIR sequence (21.8 and 17.0 for nodules and consolidation, respectively), followed by the T1-FFE sequence. SPAIR provided slightly lower results, with mean C lesion of 19.6 and 16.2 for nodules and consolidation. The lowest C lesion for nodules (16.1 ± 8.0) and consolidation (14.8 ± 8.0) was on e-THRIVE and TSE, respectively.
Lesion detection
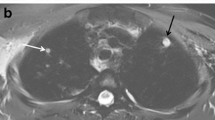
The sensitivities of all five MRI sequences for the detection of pulmonary lesions, according to the lesion type and size, as compared to MDCT are listed in Table 5. For nodules detection, regardless of lesion size, the highest sensitivity (88.6 %) was with the SPAIR sequence. These detection rates increased as nodule diameter increased on MR images for five different sequences. Taking all nodules independently of their size, mean sensitivities of 84.1 %, 68.2 % and 68.2 % were obtained with e-THRIVE, TSE and STIR images. The lowest sensitivity (54.5 %) was achieved using the breath-hold T1-FFE. For visualization of consolidation, SPAIR, TSE, STIR and e-THRIVE images provided favourable results, with mean sensitivities ranging from 90.0 % to 92.5 %, whereas T1-FFE sequence showed much lower sensitivity of 60 %. Because no true-negative nodules could exist in this evaluation, no specificity could be calculated. Examples of the correlation between the findings in MDCT and different MR sequences are given in Figs. 1 and 2.
A 20-year-old man with acute myeloid leukaemia and neutropenia. MDCT (a) shows a segmental consolidation with surrounding glass-ground opacities in the left upper lobe and an ill-defined nodule in the right upper lobe. Both findings are also detected with five different MR sequences: e-THRIVE (b), T1-FFE (c), SPAIR (d), STIR (e) and TSE (f). Additionally, pleural effusion on the left side can be clearly detected by three T2-weighted MR sequences
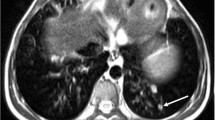
The sensitivities, specificities and positive and negative predictive values for the detection of all pulmonary lesions are given in Table 6, separately analysed on a lobe and patient basis. A retrospective review indicated that four false-positive diagnoses were caused by blurring and cardiac pulsation artefacts. Additionally, vessels seemed to have caused the remaining two misinterpretations. Of three false-positive findings in SPAIR, one occurred in a non-affected lobe in a patient with two nodules in the other ipsilateral lobe, and two 3-mm-sized false-positive findings occurred in a pulmonary lobe which was already involved with infectious lesions. Figure 3 shows two small pulmonary nodules detected by MDCT and all MR sequences but not on T1-FFE.
A 20-year-old man with acute myeloid leukaemia and neutropenia after intensive chemotherapy. Two pulmonary nodules less than 5 mm (arrow), surrounding a patchy consolidation, were detected by MDCT (a), e-THRIVE (b), SPAIR (d), STIR (e) and TSE (f). However, the small nodules were missed on T1-FFE (c) images
Discussion
Although challenges for MRI of lung parenchyma diseases remain, MRI is an attractive tool for evaluating pulmonary lesions with high anatomic resolution and lesion contrast without ionizing radiation. Previous studies [14, 17] have shown the sufficient diagnostic accuracy of MRI for the detection of pneumonia/lung infiltrates in neutropenic patients.
On the basis of a sequence-by-sequence assessment, the MR protocol in this work showed a relatively high variability with respect to the diagnostic performance of different MR sequences. The highest sensitivity for the detection of IFI lesions was observed for SPAIR. Both nodules and consolidation larger than 5 mm were detected accurately by SPAIR images with good concordance to MDCT. This can be explained by the spectral fat saturation used in this study that provided high image quality and increased signal in tissue oedema related to active inflammation [18]. Furthermore, in accordance with previous studies [19], the mean rate of observed artefacts on SPAIR was generally low. The attenuated fat signal and respiratory trigger (RT) technique may considerably reduce the ghost and motion artefacts.
The protocol includes a T1-weighted 3D gradient echo with an ultra-short TE (<1 ms)—e-THRIVE sequence, which was rated as the second best sequence for the visualization of IFI pulmonary lesions. Similar to a previous study using 3D volumetric interpolated breath-hold examination (VIBE) [12], pulmonary abnormalities in cancer patients were detected with a sensitivity of 87.3 %. The superiority of e-THRIVE for the assessment of lung lesions can be attributed to the superior spatial resolution and its high lesion contrast. In our study, the e-THRIVE sequence with an interpolated section thickness of 1.6 mm provided an overall sensitivity of 86.9 % for the detection of all pulmonary lesions. However, their image quality showed a high variability concerning the physiological motion artefacts, ranging from “negligible” to “distinct”, which largely depends on the breath-hold capability and compliance of the patient.
The STIR sequence achieved less favourable sensitivities than the SPAIR sequence with different rates for different lesion types and size: 90.0 % for consolidation, 38.5 % for nodules less than 5 mm in diameter, 71.4 % for nodules measuring 6–10 mm and 83.3 % for nodules larger than 10 mm. However, the poor image quality of the STIR sequence, hampered by severe pulsation artefacts adjacent to pericardial in our study, may limit its utility in pulmonary MRI. Interestingly, the STIR images provided the highest lesion contrast, with mean C lesion of 21.8 and 17.0 for nodules and consolidation, respectively. This advantage can be due to its high sensitivity to changes in T2 of the sequence and fat suppression which enabled better delineation of lesions adjacent to the mediastinum [20]. In addition, the extent of nodules on STIR images (2.2 ± 1.0) was significantly larger than that on MDCT (1.9 ± 0.9), owing to areas of ground-glass opacities surrounding the lesions.
We also used an RT T2-weighted TSE for the study: this technique is reported to be robust towards the susceptibility artefacts, which are expected to be more pronounced in high-field MR [9]. In this study, the TSE sequence provided the best image quality with the lowest artefacts. However, in concordance with a previous study [21], TSE was rated unsatisfactory for the visualization of nodules in our study (overall sensitivity, 68.2 %), especially for lesions less than 5 mm (23.1 %, 3/13), which can be attributed to the low lesion contrast on partial volume effects.
Additionally, T1-FFE, a fast imaging method with short acquisition time [22], was rated as the worst technique for the visualization of pulmonary lesions. Its overall rate of detection of pulmonary lesions was only 57.1 % (48/84), which might be due to the low signal intensity of pulmonary lesions on T1-weighted images.
There were a total of six false-positive pulmonary lesions in our study, using MR sequences to measure lesions 5 mm or less in diameter. Four false-positive findings were retrospectively identified as artefacts from pulsations of the heart and two were identified as bright blood signals from vessels. Although the diagnostic capabilities of different MR pulse sequences were variable, most pulmonary abnormalities were disseminated; thus the false-positive or false-negative findings did not ultimately alter the decision to administer antifungal medication.
There were limitations to our study. One major limitation is that most cases in our series were diagnosed according to the EORTC/ESG 2008 criteria [15] without a causative pathogen; only five patients were proven histologically. Furthermore, we did not analyse nonspecific findings of IFI like ground-glass areas, which have been demonstrated to be inaccurately visible on MRI [14].
In conclusion, respiratory-triggered SPAIR and breath-hold e-THRIVE pulmonary MRI are sensitive for depicting pulmonary IFI lesions of 5 mm or larger with good concordance to the gold standard of MDCT. This may eventually allow one to use lung MRI as a harmless imaging alternative to MDCT, in particular for frequent follow-up studies in young patients.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- e-THRIVE:
-
T1-weighted high resolution isotropic volume excitation
- HU:
-
Hounsfield unit
- IFI:
-
Invasive fungal infection
- MDCT:
-
Multidetector computed tomography
- RT:
-
Respiratory triggered
- SPAIR:
-
Spectrally selective attenuated inversion recovery
- STIR:
-
Short-tau inversion recovery
- T1-FFE:
-
T1-weighted fast field echo
- TSE:
-
Turbo spin echo
References
Maschmeyer G, Haas A, Cornely OA (2007) Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs 67:1567–1601
Li Y, Xu W, Jiang Z et al (2014) Neutropenia and invasive fungal infection in patients with hematological malignancies treated with chemotherapy: a multicenter, prospective, non-interventional study in China. Tumour Biol 35:5869–5876
Karthaus M, Buchheidt D (2013) Invasive aspergillosis: new insights into disease, diagnostic and treatment. Curr Pharm Des 19:3569–3594
Walsh TJ, Anaissie EJ, Denning DW et al (2008) Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360
Kuhlman JE, Fishman EK, Burch PA, Karp JE, Zerhouni EA, Siegelman SS (1987) Invasive pulmonary aspergillosis in acute leukemia. The contribution of CT to early diagnosis and aggressive management. Chest 92:95–99
Puderbach M, Kauczor HU (2008) Can lung MR replace lung CT? Pediatr Radiol 38:S439–S451
Sieren JC, Ohno Y, Koyama H, Sugimura K, McLennan G (2010) Recent technological and application developments in computed tomography and magnetic resonance imaging for improved pulmonary nodule detection and lung cancer staging. J Magn Reson Imaging 32:1353–1369
Koyama H, Ohno Y, Kono A et al (2008) Quantitative and qualitative assessment of non-contrast-enhanced pulmonary MR imaging for management of pulmonary nodules in 161 subjects. Eur Radiol 18:2120–2131
Lutterbey G, Gieseke J, von Falkenhausen M, Morakkabati N, Schild H (2005) Lung MRI at 3.0 T: a comparison of helical CT and high-field MRI in the detection of diffuse lung disease. Eur Radiol 15:324–328
Bruegel M, Gaa J, Woertler K et al (2007) MRI of the lung: value of different turbo spin-echo, single-shot turbo spin-echo, and 3D gradient-echo pulse sequences for the detection of pulmonary metastases. J Magn Reson Imaging 25:73–81
Fink C, Puderbach M, Biederer J et al (2007) Lung MRI at 1.5 and 3 Tesla: observer preference study and lesion contrast using five different pulse sequences. Invest Radiol 42:377–383
Frericks BB, Meyer BC, Martus P, Wendt M, Wolf KJ, Wacker F (2008) MRI of the thorax during whole-body MRI: evaluation of different MR sequences and comparison to thoracic multidetector computed tomography (MDCT). J Magn Reson Imaging 27:538–545
Puderbach M, Hintze C, Ley S, Eichinger M, Kauczor HU, Biederer J (2007) MR imaging of the chest: a practical approach at 1.5 T. Eur J Radiol 64:345–355
Attenberger UI, Morelli JN, Henzler T et al (2013) 3Tesla proton MRI for the diagnosis of pneumonia/lung infiltrates in neutropenic patients with acute myeloid leukemia: initial results in comparison to HRCT. Eur J Radiol 83:e61–e66
De Pauw B, Walsh TJ, Donnelly JP et al (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821
Schroeder T, Ruehm SG, Debatin JF, Ladd ME, Barkhausen J, Goehde SC (2005) Detection of pulmonary nodules using a 2D HASTE MR sequence: comparison with MDCT. AJR Am J Roentgenol 185:979–984
Eibel R, Herzog P, Dietrich O et al (2006) Pulmonary abnormalities in immunocompromised patients: comparative detection with parallel acquisition MR imaging and thin-section helical CT. Radiology 241:880–891
Udayasankar UK, Martin D, Lauenstein T et al (2008) Role of spectral presaturation attenuated inversion-recovery fat-suppressed T2-weighted MR imaging in active inflammatory bowel disease. J Magn Reson Imaging 28:1133–1140
Lauenstein TC, Sharma P, Hughes T, Heberlein K, Tudorascu D, Martin DR (2008) Evaluation of optimized inversion-recovery fat-suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging 27:1448–1454
Koyama H, Ohno Y, Aoyama N et al (2010) Comparison of STIR turbo SE imaging and diffusion-weighted imaging of the lung: capability for detection and subtype classification of pulmonary adenocarcinomas. Eur Radiol 20:790–800
Yi CA, Jeon TY, Lee KS et al (2007) 3-T MRI: usefulness for evaluating primary lung cancer and small nodules in lobes not containing primary tumors. AJR Am J Roentgenol 189:386–392
Fischbach F, Lohfink K, Gaffke G et al (2013) Magnetic resonance-guided freehand radiofrequency ablation of malignant liver lesions: a new simplified and time-efficient approach using an interactive open magnetic resonance scan platform and hepatocyte-specific contrast agent. Invest Radiol 48:422–428
Acknowledgements
The scientific guarantor of this publication is Prof. Yikai Xu. The authors of this manuscript declare relationships with the following companies: Philips Electronics Ltd. One co-author (Queenie Chan) is an employee of Philips Electronics Hong Kong Ltd. Dr. Chan contributed to designing the study, the establishment of the radiology project, and editing and revising the manuscript. The authors state that this work has not received any funding. Prof. Xuhui Tan kindly provided statistical advice for this manuscript. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. None of the study subjects or cohorts have been previously reported. Methodology: prospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, C., Tan, X., Wei, Q. et al. Lung MRI of invasive fungal infection at 3 Tesla: evaluation of five different pulse sequences and comparison with multidetector computed tomography (MDCT). Eur Radiol 25, 550–557 (2015). https://doi.org/10.1007/s00330-014-3432-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3432-2