Abstract
The purpose of this study was to evaluate the feasibility of high-field magnetic resonance imaging (MRI) of the lung using a T2-weighted fast-spin echo (TSE) sequence. Comparison was made with helical computed tomography CT findings in patients with diffuse pulmonary diseases. Prospective segment-wise analysis of high-field MR imaging findings in 15 patients with diffuse pulmonary diseases was made using helical CT and HRCT as the standard of reference. The MR studies were performed on a 3.0-T whole body system (Intera 3T, Philips Medical Systems) using a T2w TSE sequence with respiratory and cardiac gating (TE 80 ms TR 1,500–2,500 ms; turbo factor 17; 22 slices with 7/2-mm slice thickness and gap; 256×192 matrix). MR artifacts were graded on a three-point scale (low, moderate, high). Lung MR studies were prospectively analyzed segment-by-segment and diagnosed as healthy or pathological; results were compared with helical CT findings. In all 15 patients, MR imaging of the lung was successful. All 15 MR studies were compromised by artifacts; however, the severity of these artifacts was classified as low or moderate in 8/15, respectively, 7/15 cases. A total of 143/285 lung segments showed diffuse lung disease in helical CT. With MRI, 133 of these 143 segments (93%) were judged to be diseased. The ten segments that received false negative MR diagnoses displayed non-acute pulmonary lesions with inherently low proton density (scars, granulomas). MRI at 3.0 T can detect diffuse pulmonary disease with a high sensitivity. Based on this experience, further pulmonary studies with high-field systems appear justified and promising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-Tesla high-field magnetic resonance imaging (MRI) scanners have recently become available for clinical use, but have so far been used almost exclusively for neuroimaging studies. However, high-field MRI is now increasingly being applied in the field of whole body imaging.
Even with MR scanners of regular field strength (up to 1.5 T), imaging of the lung parenchyma is difficult because of physical and physiological factors such as low proton density, susceptibility effects, respiratory movements and cardiac and vascular pulsations [1, 2]. With high field scanners these problems become even greater. Susceptibility effects and motion artifacts, for example, should be more pronounced. Nevertheless, the reduced signal from normal lung parenchyma because of susceptibility effects and the theoretically increased signal from solid lung changes may result in a higher contrast between lesions and healthy parenchyma using 3.0 T.
We therefore performed this initial study to assess whether lung MRI is feasible at all under these circumstances. To our knowledge, no data concerning clinical lung MRI at 3.0 T have been published.
Materials and methods
Fifteen patients (4 women and 11 men, age 25–80 years) undergoing computed tomography (CT) as the imaging gold standard for monitoring known diffuse lung disease were additionally examined prospectively in a 3.0-T MR scanner.
The patients were suffering from the following diseases: sarcoidosis (n=4), pulmonary fibrosis (n=6), usual interstitial pneumonia (n=1), diffusely infiltrating bronchial carcinoma (n=1), lymphoma infiltration (n=1), cystic fibrosis (n=1) and idiopathic fibrosing alveolitis (n=1). The symptoms ranged from unremarkable to severe dyspnea requiring oxygene supplementation.
The MRI was performed after the patients had given informed consent to participate in the study in accordance with the guidelines of the local ethics committee.
Examination procedure
All patients were examined in a supine position; patients requiring continuous O2 delivery (two patients) were given the oxygen by nasal cannula during the examination. No contrast medium or sedation was given.
MRI studies
The MRI was performed with a 3.0-T scanner (Intera, Philips Medical Systems, Best, The Netherlands) in combination with a six-channel synergy surface coil. The gradient amplitude was 30 mT/m, the rise time 0.2 ms and the slew rate thus 150 T/m/s. The examinations were performed with diastolic (trigger delay 300–500 ms, depending on the heart rate) vector cardiographic (VCG) gating and expiratory respiratory gating using a respiratory belt.
A T2-weighted turbo spin echo sequence with the following parameters was used as examination sequence:
-
TE (echo time) 80 ms
-
TR (repetition time) between 1,500 and 2,500 ms because of the dependence on heart rate and respiratory rate
-
Slice thickness 7 mm with 2-mm slice gap
-
22 transverse slices
-
Turbo factor 17
-
Field of view 400 mm
-
Matrix size 256×192 reconstructed to 512
-
SENSE (sensitivity encoding) factor 2
The diagnostic sequence was preceded by a single reference scan as required for parallel imaging with SENSE. The sequence was combined with spectrally selective attenuated inversion recovery (SPAIR) fat saturation. The measuring time was 5–7 min depending on the heart and respiratory rates. The optimum window settings were chosen individually for each examination.
CT studies
The CT scans used as the reference standard were obtained in deep inspiration with single-slice helical computed tomography scanners [Somatom PLUS4, Siemens, Erlangen, Germany (n=5 patients) and Tomoscan AVE1, Philips Medical Systems, Best, The Netherlands (n=10 patients)]. With the Somatom PLUS4, the slice thickness was 8 mm at 120 kV and 240 mA, with the Tomoscan AVE1 7 mm at 120 kV and 225 mA. The images were reconstructed in a lung algorithm and immediately windowed (window width 1,500, window level −500); the matrix size was 512×512. In seven cases additional high resolution CT (HRCT) was performed. In our institution, we routinely acquire five representative HRCT slices [1-mm single slices, 140 kV, 175 mA, a high-resolution algorithm, window settings as mentioned above (1,500/−500)] with patients in a prone position.
Evaluation
The studies were evaluated for each modality separately by a radiologist experienced in pulmonary MRI and CT with an interval of 2 weeks between the assessments; the MRI scans were seen first. The procedure of evaluation was as follows.
MRI
The MRI scans were first evaluated for the presence of artifacts; the overall artifact level was classed as low (barely visible), moderate (clearly visible, but not interfering with evaluation) or high (compromising evaluation).
Each of the 19 lung segments per patient was examined for parenchymal pathology (according to anatomical convention, in the left lung the apico-posterior segment 1/2 was considered one segment, the left medio-basal and antero-basal segment as separated). In problematic cases an anatomical cross-sectional atlas was used to define the segments on MRI films.
A lung segment was classified as pathological if it showed high-signal-intensity structures apart from blood vessels and artifacts.
Computed tomography
The evaluation of the lung parenchyma was also performed per segment (see above). Thickened septa, nodules, infiltrates, bullae or “ground glass opacification” (as visible without HRCT) were regarded as signs of pathological pulmonary processes.
Results
All CT and MRI examinations could be completed. Although two patients required supplemental oxygen, none of the examinations had to be aborted.
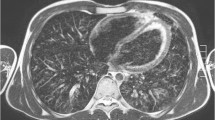
All MR scans were of diagnostic quality despite the fact that the examinations were complicated by coughing and irregular breathing (Fig. 1). Motion artifacts were present on all MRI scans; these, however, were only barely visible (i.e., low) in eight and moderate in seven cases. The artifacts were mainly ghosting artifacts of the chest wall. In case folding artifacts as a result of the parallel imaging, pulsation artifacts and inhomogeneous fat saturation occurred, they did not interfere with the assessment.
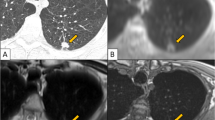
A total of 133/285 lung segments were judged as pathological on the basis of the MRI scans. On the CT examinations—serving as reference standard—pathological changes were detected in 143 /285 lung segments. Thus, 133/143 (93%) of the segmental changes diagnosed by computed tomography were also detected by MRI (Figs. 2, 3).
MRI failed to diagnose changes shown by CT in ten segments in three patients: The segment-based rate of false negative MR studies was thus 7%. In detail:
-
One patient suffered from fibrosis due to chronic obstructive pulmonary disease (COPD) without acute exacerbation and had emphysematous changes and scarring, which were not fully visible on MRI.
-
The second patient had multiple granulomas of up to 5 mm, which were only just identifiable retrospectively with knowledge of the CT findings.
-
The third patient with fibrotic sarcoidosis showed scars and subpleural nodules, not clearly visible on MRI (Fig. 4).
Discussion
Compared with other body regions, relatively little has been published about diagnostic MRI of the lung. There have been individual studies on the detection of pulmonary metastases, on the evaluation of the activity of inflammatory parenchymal diseases of the lung, on MR angiography of pulmonary vessels and on pulmonary perfusion and ventilation using a wide range of different MRI techniques, including gradient echo sequences, successfully [3–11]. However, all of these examinations were performed at field strengths of 1.5 T or lower.
The high field MR scanners that have recently become available are now increasingly being used for whole body examinations. This raises the question of the practicability of lung examinations by high field MRI and the efficiency of the procedure for routine clinical use.
As a diagnostic challenge we decided to evaluate patients with diffuse lung disease, using our earlier experience with pulmonary MRI [12].
While protocols for CT examinations of the lung are nowadays largely standardized, this is not the case for MRI. Based on our experience at 1.5 T, we used a cardiac and respiratory-gated T2-weighted turbo spin echo sequence (T2w-TSE) for the study: This technique is very robust towards the susceptibility artifacts, which are expected to be more pronounced at high-field MR [13, 14]. In addition, this sequence provides high contrast between active pathological and healthy lung parenchyma (Fig. 2). We used fat suppression, as we employ it in our whole body screening protocols.
Other sequences (e.g., gradient echo sequences) have been described in the literature for pulmonary imaging at field strengths of up to 1.5 T; however, they have not been employed in pulmonary imaging in a high-field environment.
In order to examine the entire lung at higher resolution, usually multiple breath holds are required. This may be difficult for patients with lung disease. Instead, we recommend respiratory gating, which permits continuous breathing. By respiratory gating, motion artifacts of the chest wall and shifts of the lung parenchyma relative to the slice level are reduced.
As the expiratory phase is better to reproduce and lasts longer than the inspiration phase, we recommend that slice profiles should be measured in expiration.
In line with our experience with 1.5-T lung examinations, fat saturation proved to be useful. The attenuated fat signal of the thoracic subcutis considerably reduces the ghosting artifacts of the ventral chest wall. The automatic shift of the gray scale towards the water protons as the brightest tissue (with suppression of the fat protons, which otherwise have a higher signal intensity) increases the contrast between pathological lesions (which almost always have a high water content) and lung parenchyma. The spectral fat saturation used by us (SPAIR) was very effective; only slight inhomogeneities distant from the isocenter occurred; however, considering the large field of view of 400 mm, this is neither surprising nor scanner specific.
With regard to the cardiac gating, it is important—as at 1.5 T—to place the data acquisition in the diastole when pulsation is less vigorous.
Theoretically, the use of a 3.0-T system should double the signal yield compared with the use of a 1.5-T scanner. However, this is not synonymous with doubling the spatial resolution or halving the examination time. A number of effects specific to high-field imaging play an important role; these include SAR limits (specific absorption rate), which are reached faster, the prolonged T1 times, shortened T2 times, and also increased sensitivity to artifacts. Imaging of the lung parenchyma is especially likely to suffer from the increase in susceptibility effects, and subtle lung changes—which have not yet led to complete tissue consolidation—are therefore more difficult to detect. It is impossible to achieve the same spatial resolution of HRCT with MRI using the currently available techniques. Consequently, we did not target morphologic lung changes in our study.
Considering all these expected problems, the diagnostic yield of the high-field MRI of the lung in our study was surprisingly good: Of the 143 pathological segments detected by CT, 133 segments were also identified by MRI. The reason for the discrepancy in ten lung segments was not the quality of the MR examination or the presence of artifacts interfering with the evaluation. Rather, the false-negative findings compared with CT were caused by the pathological substrate itself: the lesions missed by MRI were parenchyma scars or small granulomas. Irrespective of the field strength, these are difficult or impossible to detect by MRI because of their low proton density and short relaxation times.
On the other hand, this can also be a diagnostic advantage as this permits differentiation between florid/acute lesions and chronic lesions/scars (Fig. 3) [15–17].
Shortcomings of our study are the small patient number and the mode of assessment, differentiating only between affected and non-affected lung parenchyma. The number of patients, however, was sufficient to demonstrate the clinical feasibility of lung MRI at 3.0 T.
If and how further differentiation and categorization of lung lesions is possible with high-field MR, however, will encourage further evaluation based upon the present study.
In the evaluation of diffuse lung disease, HRCT represents the standard procedure for narrowing the differential diagnosis. In our institution, we do not perform whole lung HRCT, but helical CT with five additional HRCT slices in a prone position. The application of HRCT in only seven cases in our study is no major drawback, because helical CT is highly valuable in the detection of interstitial lung disease [18].
In summary, our initial study in a small patient sample shows for the first time that diagnostically useful MRI studies of diffuse pulmonary parenchymal disease can be performed at field strengths of 3.0 T. Of course, lung MRI cannot replace CT (especially HRCT) in the routine evaluation of lung diseases, but the results of our study are promising, and we may be able to integrate diagnostic evaluation of the lung in whole body high-field MRI examinations in the future.
References
Leutner C, Schild H (2001) MRI of the lung parenchyma. RoFo 173:168–175
Bergin CJ, Noll DC, Pauly JM, Glover GH, Macovski A (1992) MR imaging of lung parenchyma: a solution to susceptibility. Radiology 183:673–676
Muller NL, Mayo JR, Zwirewich CV (1992) Value of MR imaging in the evaluation of chronic infiltrative lung diseases comparison with CT. Am J Roentgenol 158:1205–1209
Leutner C, Gieseke J, Lutterbey G, Kuhl C, Glasmacher A, Wardelmann E, Theisen A, Schild H (2000) MR imaging of pneumonia in immuncompromised patients. Comparison with helical CT. Am J Roentgenol 175:391–397
Kauczor HU, Hanke A, Van Beek EJ (2002) Assessment of lung ventilation by MR imaging: current status and future perspectives. Eur Radiol 12(8):1962–1970
Knopp M, Hess T, Schad L, Bischoff H, Weisser G, Blüml S, Van Kaick G (1994) MR tomography of lung metastases with rapid gradient echo sequences. Initial results in diagnostic applications. Radiologe 34:581–587
Mc Fadden RG, Carr TJ, Wood TE (1987) Proton magnetic resonance imaging to stage activity of interstitial lung disease. Chest 92:31–39
Haage P, Karaagac S, Adam G, Spuntrup E, Pfeffer J, Gunther RW (2002) Gadolinium containing contrast agents for pulmonary ventilation magnetic resonance imaging: preliminary results. Invest Radiol 37:120–125
Berthezene Y, Vexler V, Kuwatsuru R, Rosenau W, Muhler A, Clement O, Price DC, Brasch RC (1992) Differentiation of alveolitis and pulmonary fibrosis with a macromolecular MR imaging contrast agent. Radiology 185:97–103
Bader TR, Semelka RC, Pedro MS, Armao DM, Brown MA, Molina PL (2002) Magnetic resonance imaging of pulmonary parenchymal disease using a modified breath-hold 3D gradient-echo technique: initial observations. J Magn Reson Imaging 15:31–38
Kersjes W, Mayer, E, Buchenroth M, Schunk K, Fouda N, Cagil H (1997) Diagnosis of pulmonary metastases with turbo-SE MR imaging. Eur Radiol 7:1190–1194
Lutterbey G, Gieseke J, Sommer T Keller E, Kuhl C, Schild H (1996) A new application of MR tomography of the lung using ultra-short turbo spin echo sequences RoFo 164:388–393
Lutterbey G, Leutner C, Gieseke J, Rodenburg J, Elevelt A, Sommer T, Schild H (1998) Detection of focal lung lesions with magnetic resonance tomography using T2-weighted ultrashort turbo-spin-echo-sequence in comparison with spiral computerized tomography. RoFo 169:365–369
Gieseke J, Lutterbey G, Schild HH, Elevelt A, Keller E, Sommer T, Kuhl C (1995) Magnetic resonance imaging of lung parenchyma with cardiac triggering and respiratory compensated ultra fast turbo spin echo. Abstract SMR/ESMRMB Joint Meeting 3:1613
Matsumoto S, Miyake H, Oga M, Takaki H, Mori H (1998) Diagnosis of lung cancer in a patient with pneumoconiosis and progressive massive fibrosis using MRI. Eur Radiol 8:615–617
Primack SL, Mayo JR, Hartmann TE, Miller, RR, Müller NL (1994) MRI of infiltrative lung disease: comparison with pathologic findings. J Comput Assist Tomogr 18:233–238
Matsumoto S, Mori H, Miyake H, Yamada Y, Ueda S, Oga M, Takeo H, Anan K (1998) MRI signal characteristics of progressive massive fibrosis in silicosis. Clin Radiol 53:510
Remy-Jardin M, Remy J, Deffontaines C, Duhamel A (1991) Assessment of diffuse infiltrative lung disease: comparison of conventional CT and high-resolution CT. Radiology 181:157–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lutterbey, G., Gieseke, J., von Falkenhausen, M. et al. Lung MRI at 3.0 T: a comparison of helical CT and high-field MRI in the detection of diffuse lung disease. Eur Radiol 15, 324–328 (2005). https://doi.org/10.1007/s00330-004-2548-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2548-1