Abstract
Background
More than 70% of human immunodeficiency virus (HIV)-positive children sustain respiratory diseases in their lifetime. Imaging plays an important role in establishing early and correct diagnosis.
Objective
To evaluate the diagnostic accuracy of 3-Tesla (T) thorax MRI in HIV-positive children, using chest CT as the gold standard.
Materials and methods
We included 25 children with confirmed HIV-positive status and pulmonary complaints who were referred for chest CT. All children had 3-T thorax MRI using T2-W turbo spin-echo sequence, steady-state free precession gradient echo sequence, T2-W turbo spin-echo MultiVane XD sequence, and T1-weighted modified Dixon sequences. We evaluated the images for various pulmonary and mediastinal findings and calculated the sensitivity and specificity of 3-T thoracic MRI.
Results
Sensitivity of 3-T MRI was 100% for detecting nodules >4 mm (95% confidence interval [CI] 66.3–100%), pleural effusion (CI 29.2–100%) and lymphadenopathy (CI 81.5–100%). It demonstrated a specificity of 100% for nodules >4 mm (CI 79.4–100%), pleural effusion (CI 84.6–100%) and lymphadenopathy (CI 59–100%). For consolidation/collapse, sensitivity and specificity were 93.8% (CI 69.8–99.8%) and 88.9% (CI 51.8–99.7%), respectively. The sensitivity and specificity for detecting bronchiectasis were 75% (CI 42.8–94.5%) and 100% (CI 75.3–100%), respectively, while for ground-glass opacity, sensitivity and specificity were 75% (CI 34.9–96.8%) and 94.1% (CI 71.3–99.9%), respectively. Nodules <4 mm were not well detected on MRI, with sensitivity of 35% (CI 15.4–59.2%).
Conclusion
Thoracic MRI at 3 T demonstrates a high sensitivity and specificity for detecting nodules >4 mm, effusion and lymphadenopathy in HIV-positive children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human immunodeficiency virus (HIV) infection in childhood is one of the leading causes of immunodeficiency and susceptibility factor for infections and malignancies [1, 2]. Lungs are the target organ, and more than 70% of children sustain respiratory diseases in their lifetime [3, 4]. Clinical presentations in HIV-positive children are varied, nonspecific and atypical, posing a diagnostic challenge. Laboratory diagnosis is also difficult in suspected pulmonary and mediastinal disease. Imaging thus plays an important role in establishing early and correct diagnosis. Imaging further helps in establishing the pattern of involvement and the severity of the pulmonary disease. This is crucial in the management and follow-up of HIV-infected children with lung diseases.
The gold standard for imaging HIV-positive children with abnormal chest radiograph and those with pulmonary symptoms who have normal or equivocal chest radiograph is CT chest [5]. However, HIV-positive children, because of their immunocompromised status, are at high risk of developing recurrent pulmonary diseases and permanent lung changes, requiring repeated CT examination, thus leading to high cumulative radiation exposure, which is of concern, especially in children [6,7,8].
Magnetic resonance imaging (MRI) has the advantage of being radiation-free and thus seems to be an attractive noninvasive diagnostic modality in children [9,10,11,12,13,14]. Studies have described the diagnostic accuracy of thorax MRI with respect to CT scan [15,16,17,18,19,20,21,22,23,24,25,26], but to the best of our knowledge, no previous study has focused on thorax MRI for evaluating pulmonary and mediastinal abnormalities in HIV-positive children.
The aim was to evaluate the diagnostic utility of 3-T thorax MRI in HIV-positive children who have pulmonary complaints, using CT chest as the reference standard.
Materials and methods
This prospective study was approved by our institutional ethics committee. The study was conducted over a period of 18 months, from January 2017 to June 2018, and included a total of 25 children with proven HIV-positive status who presented to the pediatric immunology clinic of our institute. Patient confidentiality was maintained in accordance with Health Insurance Portability and Accountability Act guidelines. We obtained informed written consent from the parents or guardians of all pediatric patients included in this study. We also obtained assent from the children aged 7 years and older prior to enrollment.
All children underwent 3-T thorax MRI and chest CT within 72 h of each other to avoid divergence of findings. We recorded detailed records of clinical history and examination, HIV diagnostic test results, current CD4 counts and other relevant investigations of the child.
Inclusion criteria were children with HIV-positive status, age group 5–15 years, and with symptoms suggestive of pulmonary and mediastinal involvement requiring CT for further evaluation and management. Exclusion criteria were age younger than 5 years or older than 15 years, contraindication for performing thorax MRI (e.g., metallic implants, cochlear implants), contraindication for performing chest CT, a lapse of >72 h between acquisition of MRI and CT, or refusal to give informed consent.
Study protocol
All of the children fulfilling the inclusion criteria underwent chest CT and 3-T thorax MRI within 72 h of each other. A contrast-enhanced chest CT was performed on a 64-slice scanner (Aquilion 64; Toshiba America Medical Systems, Otawara, Japan). All contrast-enhanced CT scans were obtained with the following parameters: 32×0.5-mm collimation, weight-based kilovoltage, low-dose tube current, high-speed mode, and a pitch equivalent of 0.844. High-resolution chest CT was performed in addition, if required. The axial images were reconstructed at a slice thickness of 0.5 mm for review. CT images were evaluated on TeraRecon (Foster City, CA) workstation with standard soft-tissue (mediastinal) settings (level: 70 Hounsfield units [HU]; width: 450 HU) and lung window settings (level: −500 HU; width: 1,500 HU).
Thorax MRI was performed on a 3-T machine (Ingenia; Philips, Best, the Netherlands) equipped with a high-performance gradient system using a body coil. No sedation and no contrast agent were administered in any of the children. MR imaging was performed from the lung apices to the domes of diaphragm with the following sequences: (1) axial and coronal T2-W turbo spin echo (TSE); (2) axial balanced turbo field echo (bTFE), which is a steady-state free precession gradient echo sequence (SSFP GRE); (3) axial T1-W modified Dixon (mDixon); (4) axial T2-W MultiVane XD, a turbo spin-echo sequence where XD denotes a significantly improved method for blade corrections; and (5) axial diffusion-weighted imaging (DWI). A table summarizing the various parameters used for the different MRI sequences has been provided in online supplement.
Image analysis
MRI and CT images were independently reviewed by two pediatric radiologists (K.S.S. and A.K.S, with 10 years and 7 years of experience in thorax MRI, respectively). Both the reviewers were blinded to the clinical profile of the patients. Identification information from images was removed and the order of MRI images and CT images was also randomized.
When there was a discrepancy between reviewers’ radiologic assessments, they obtained consensus in a third reviewing session. Findings recorded in CT and MRI tables were also cross-matched for similarity in the third reviewing session.
The findings were interpreted as consolidation/collapse, cavity, bronchiectasis, nodule, lymphadenopathy, ground-glass opacity, and pleural effusion according to the glossary of terms from the Fleischner Society [27]. Because there were no well-established criteria for defining parenchymal findings on thorax MRI, we used CT criteria described for the various findings in the present study for MRI.
Statistical analysis
We calculated the sensitivity and specificity of MRI on a per-patient basis, keeping CT scan as the gold standard, using a chi-square test. We calculated 95% confidence intervals for sensitivity and specificity using the binomial exact method (Clopper–Pearson interval). Alpha was set to 0.05.
Results
We included 25 children with confirmed HIV-positive status who presented with pulmonary symptoms requiring CT for diagnosis or further evaluation. Mean age of the study population children was 10.1 years (range 5–15 years), with 19 boys (76%) and 6 girls (24%).
Of the 25 children in the study population, 13 (52%) had clinical suspicion of pulmonary tuberculosis; of these, 4 had features of dissemination to other organs. Six (24%) children were clinically suspected of having pneumonia and two (8%) interstitial lung disease. Two (8%) children had empyema with intercostal chest tube in situ. Two (8%) were asymptomatic for pulmonary symptoms but their chest radiograph was suggestive of pulmonary involvement. Seven (28%) children had CD4 count <100 cells/μl, four (16%) had CD4 count of 100–200 cells/μL and 14 (56%) children had CD4 count >200 cells/μL.
Twenty-one children underwent both contrast-enhanced CT and high-resolution CT chest, while four children underwent only contrast-enhanced CT chest. The mean CT dose index (CTDI) in all children was 57.2 mGy (+23.3 standard deviation [SD]).
Computed tomography and magnetic resonance imaging findings
Of the 25 children, 20 (80%) had a pulmonary nodule <4 mm on CT chest, picked up in only 7 children (28%) on MRI. In one child with extensive bronchiectasis, we falsely diagnosed a nodule <4 mm on MRI because of partial volume averaging. There was perfect correlation between the modalities to detect nodules >4 mm (n=9, 36%; Fig. 1). Sixteen (64%) children showed consolidation/collapse on CT scan. Fifteen (60%) children had consolidation/collapse on MRI correlating with CT, while one child was falsely diagnosed to have consolidation/collapse on MRI, which was interpreted as ground-glass opacity on CT scan. Eight (32%) children showed ground-glass opacities on CT, and six of these cases were picked up on MRI. In one child with multiple nodules <4 mm, ground-glass opacities were falsely diagnosed on MRI. Twelve (48%) children had bronchiectasis on chest CT, picked up in only nine children (36%) on MRI. There was 100% correlation between modalities to detect pleural effusion (n=3, 12%; Fig. 2) and lymphadenopathy (n=18, 72%; Fig. 3). Statistical analysis of radiologic findings with CT as a gold standard is summarized in Table 1.
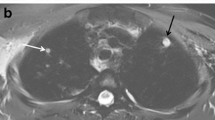
A 10-year-old boy with human immunodeficiency virus (HIV) with chronic cough, fever and CD4 count of 44 cells/μL. a Axial lung window CT image demonstrates few pulmonary nodules (>4 mm) in left lower lobe (arrow). b, c Corresponding axial T2-weighted (repetition time/echo time [TR/TE] = 1,250/80 ms) turbo spin-echo (b) and MultiVane T2-weighted (TR/TE = 2,000/76.7 ms) (c) MR images show a nodule (arrow) in the left lower lobe
Hydropneumothorax in a 5-year-old boy with human immunodeficiency virus (HIV) and chronic cough, fever and CD4 count of 47 cells/μL. a Axial contrast-enhanced CT lung window image demonstrates left hydropneumothorax (arrow). b Axial MultiVane T2-weighted (repetition time/echo time [TR/TE] = 2,000/76.7 ms) MR image shows left-side hydropneumothorax (arrow)
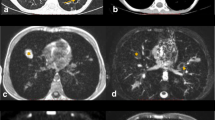
Lymphadenopathy in a 15-year-old boy with human immunodeficiency virus (HIV), with clinical and immunological failure (CD4 39 cells/μL) and respiratory distress. a, b Axial contrast-enhanced CT mediastinal window image (a) and lung window image (b) demonstrate necrotic mediastinal lymph node (short arrow in a) and subsegmental consolidation in the left upper lobe (long arrow in a and arrow in b). c Axial turbo spin-echo T2-weighted MR image (repetition time/echo time [TR/TE] = 1,250/80 ms) demonstrates the mediastinal lymph node and consolidation in the left upper lobe (arrows). d Axial steady-state free precession MR image (TR/TE = 2.6/1.3 ms) shows the mediastinal lymph node and consolidation in the left upper lobe (arrows)
Chest CT also revealed air-filled lung cysts in three children, patchy mosaic parenchymal attenuation in five, calcified pulmonary nodule in one, focal pleural thickening in one and interlobular septal thickening in one. On MRI, focal pleural thickening and interlobular septal thickening were noted in one child each, as in CT scan. However, lung cyst was noted in only one of the three cases seen on CT chest. Calcified pulmonary nodule and mosaic attenuation were not depicted on MRI. In one child, azygous lobe/fissure was noted in both CT and MRI.
Extrapulmonary findings were also detected in some children, as described in Table 2. Eighteen children had mediastinal/hilar lymphadenopathy on both CT and MRI, of whom six had diffusion restriction within the lymph nodes. Four of six children with axillary lymph nodes had diffusion restriction within. There was no statistically significant correlation of CD4 and diffusion restriction in lymph nodes (P=0.077). Two children with cavitary consolidation on CT and MRI had diffusion restriction in lung parenchyma corresponding to areas of consolidation.
Analysis of magnetic resonance sequences
For nodules <4 mm, T2-W TSE and T2-W MultiVane XD sequences had the highest detection rate among all sequences, while T1-W mDixon sequence did not pick up nodules <4 mm. There was 100% sensitivity (66.4–100%) and specificity (79.4–100%) to detect nodules >4 mm by T2-W TSE sequence. Sensitivity of SSFP and T2-W MultiVane XD was 44.4% (13.7–78.8%) and 33.3% (7.5–70.1%), respectively. T1-weighted mDixon sequence had the lowest detection rate among all sequences (sensitivity 22.2% [2.8–60%]). Consolidation/collapse was well detected on all sequences, with sensitivity >80% except by TI-W mDixon sequence, which had a sensitivity of 75% (47.6–92.7%). T2-weighted TSE had the highest sensitivity, at 93.8% (69.8–99.8%).
Ground-glass opacity was best picked up on T2-W TSE sequence, with a sensitivity and specificity of 75% (34.9–96.8%) and 94.1% (71.3–99.9%), respectively. Steady-state free precession and T1-W mDixon sequences had low sensitivities of 25% (3.2–65.1%) and 12.5% (0.3–52.7%), respectively. Ground-glass opacity was falsely diagnosed in one child on T2-W TSE, T2-W MultiVane XD and T1-W mDixon sequences and in two children on SSFP sequence.
The sensitivities to detect bronchiectasis were 75% (42.8–94.5%), 81.8% (48.2–97.7%) and 27.3% (6.0–60.9%) for T2-W TSE, T2-W MultiVane XD and SSFP sequences, respectively. Bronchiectasis was not detected on T1-W mDixon in any child. T2-weighted TSE and SSFP sequences showed 100% (29.2–100%) sensitivity and 100% specificity (84.6–100%) for detecting pleural effusion.
Lymphadenopathy was best detected on T2-W TSE sequence, with a sensitivity of 100% (81.5–100%) and specificity of 100% (59–100%). It was least picked up on T1-W mDixon sequence, with a sensitivity of 11.1% (1.4–34.7%).
Interobserver agreement
For CT, both observers (K.S.S. and A.K.S.) had perfect agreement to detect nodules <4 mm, nodules >4 mm, consolidation/collapse, ground-glass opacities, pleural effusion and lymph nodes in 80%, 36%, 64%, 32%, 12% and 72% of children, respectively. Observer 1 (K.S.S.) detected bronchiectasis in 48% of children and Observer 2 (A.K.S.) detected it in 44% of children.
For MRI there was perfect agreement between the observers to detect nodules >4 mm, ground-glass opacities, pleural effusion and lymphadenopathy, in 36%, 28%, 12% and 72% of children, respectively. Consolidation was detected in 64% of children by Observer 1 (K.S.S.) and in 56% of children by Observer 2 (A.K.S.). Bronchiectasis was detected in 40% of children by Observer 1 and in 32% of children by Observer 2. Nodules <4 mm were detected in 32% of children by Observer 1 and 36% by Observer 2.
Discussion
We used a higher-strength magnetic field (3 T) in our study with different MR sequences to evaluate pulmonary and mediastinal abnormalities. Few authors have used 3-T magnetic field strength to evaluate pulmonary findings in specific conditions [28,29,30,31,32]. We optimized and standardized these sequences to obtain the maximum possible information with minimum possible scan duration. Scan times for T2-W TSE (coronal), T2-W TSE (axial), bTFE, MultiVane XD and DWI sequences were 1.45, 0.45, 0.40, 0.30 and 2.12 min, respectively. Total MR room time was 20–30 min. Although we did not specifically use breath triggering, few authors have advocated its utility, especially in children.
In our study, thorax MRI had 100% sensitivity (66.4–100%) and specificity (79.4–100%) for detecting nodules >4 mm. MRI detected nodules <4 mm in only 7 children as compared to 20 children on CT. In one child with extensive bronchiectasis, nodules were falsely diagnosed because of partial volume averaging. The sensitivity and specificity for nodules <4 mm were 35% (15.4–59.2%) and 80% (28.4–99.5%), respectively. There were different detection rates of nodules <4 mm and nodules >4 mm because of different thicknesses of the sections used for CT (0.5 mm) and MRI (4 mm); this result was similar to previous studies published by Sodhi et al. [33], Eibel et al. [19] and Ozcan et al. [24]. Two children had multiple nodules <4 mm on CT scan that were falsely diagnosed as consolidation and ground-glass opacities in one child each on MRI scan.
Consolidation/collapse was detected in 15 children on MRI compared to 16 on CT, with high sensitivity and specificity of 93.8% (69.8–99.8%) and 88.9% (51.8–99.7%), respectively. Studies by Sodhi et al. [15, 33] and Ozcan et al. [24] have shown 100% sensitivity of MRI in diagnosing consolidation. A small patch of sub-segmental consolidation in a sub-diaphragmatic location was missed on MRI scan in our study.
Six children had extensive ground-glass opacities, which was picked up on both MRI and CT scan. Two children had mild ground-glass opacity on CT scan that was missed on MRI. The sensitivity and specificity to diagnose ground-glass opacities were 75% (34.9–96.8%) and 94.1% (71.3–99.9%), respectively. The detection rate for ground-glass opacities was higher in our study compared to previous studies [21, 22].
Mild bronchiectasis detected on CT scan in three children was missed on MRI; however extensive bronchiectasis in nine children was picked up on MRI scan. MRI had 100% sensitivity (29.2–100%) and 100% specificity (84.6–100%) for detecting pleural effusion. Similarly, MRI had 100% sensitivity (81.5–100%) and 100% specificity (59–100%) for detecting lymphadenopathy. Similar results were found in previous studies [15, 24, 33].
The MRI protocol used in this study demonstrated that the radiologic findings detected with MRI were in perfect agreement to substantial agreement with those of CT except for nodules <4 mm, where there was only slight agreement between the modalities. There was perfect agreement between CT and MRI findings between the two observers.
T2-weighted TSE sequence was the first sequence performed in all children and had highest diagnostic output out of all sequences with least motion artifacts. T2-weighted TSE sequences were interpretable in all children; however bTFE, mDixon and MultiVane XD were not interpretable and were labeled as negative in two children because of motion artifact. In our study, T2-W TSE and MultiVane XD sequences had the highest detection rate for nodules <4 mm among all sequences, while for nodules >4 mm, T2-W TSE had the highest sensitivity. A recent study with newer sequences (3-D-UTE stack-of-spirals volumetric interpolated breath-hold examination [VIBE] and Pointwise Encoding Time Reduction with Radial Acquisition [PETRA]) performed in free breathing without triggering produced unenhanced CT-like images and isotropic voxels with a size of 0.9 mm [34]. There was a perfect agreement (k=1) between CT and MRI for detecting nodules [34]. However, these newer sequences need larger evaluation in multicenter cohorts.
No significant intersequence difference was seen for detection of consolidation/collapse in our study, although it was better perceived on T2-W TSE and MultiVane XD sequences. Sensitivity to detect pleural effusion was 100% (29.2–100%) for T2-W TSE and bTFE sequences, while in other sequences it was missed in one child with mild bilateral pleural effusion. Ground-glass opacities and bronchiectasis were well detected on T2-W TSE and MultiVane XD sequences, while they were least detected on mDixon sequence. Lymphadenopathy was best detected on T2-W TSE sequence, with no false-positive finding, while in other sequences it was falsely diagnosed in a few children because of poor resolution from surrounding mediastinal structures caused by motion artifacts.
Serra et al. [17], in their study of 21 adults with common variable immunodeficiency, compared chest CT scan and MRI using a T2 rotating blade-like k-space filling sequence and found CT and MRI results to be comparable for moderate to severe degrees of bronchial alterations and parenchymal abnormalities. MRI was weaker for mild abnormalities [17], which was similar to our results of bronchiectasis.
Eibel et al. [19] compared parallel acquisition MR imaging with thin-section helical CT for depicting suspected pneumonia in immunocompromised HIV-negative people and found a sensitivity >95% (ill-defined nodules, consolidation and ground-glass opacities). One false-negative finding was an ill-defined nodule <1 cm and one false-positive finding was ground-glass opacity, which was caused by motion artifact on MRI. The sensitivity for nodules >4 mm and consolidation was similar to findings in our study. In our study, falsely diagnosed ground-glass opacity in one child was a result of confluent nodules on CT.
A pilot study by Sodhi et al. [22] compared MRI of lung with high-resolution chest CT in children with leukemia who were suspected to have febrile neutropenia. They noted 100% sensitivity, specificity, positive predictive value and negative predictive value for detecting nodules and consolidation [22]. For ground-glass opacities, sensitivity and specificity were 66.7% and 100%, respectively [22]. The sensitivity for detecting ground-glass opacities was higher in our study (75%), probably a result of our more florid cases.
We also evaluated diffusion-weighted imaging in our study. Of the 18 children with lymphadenopathy, only 6 had diffusion restriction. There was no statistically significant correlation of CD4 and diffusion restriction in lymph nodes (P=0.077). None of the lymph nodes <7 mm showed diffusion restriction in the present study. Two children with cavitary consolidation showed strong diffusion restriction, which could represent necrosis; however, few children with consolidation or ground-glass opacities showed variable diffusion restriction in lung parenchyma.
The present study has a few limitations. The sample size was relatively small, which could have altered the statistical results. We performed a non-contrast MRI study. Therefore, we could not analyze the role of contrast enhancement. Children were not sedated for MRI, which resulted in motion artifacts in a few of the sequences; however, all studies were diagnostically acceptable.
Conclusion
Thoracic MRI at 3 T demonstrated a high sensitivity and specificity for detecting nodules >4 mm, effusion and lymphadenopathy in HIV-positive children.
References
(2019) Global HIV/AIDS overview. AIDS.gov. https://www.aids.gov/federal-resources/around.../global-aids-overview. Accessed 29 Aug 2019
Norton KI, Kattan M, Rao JS et al (2001) Chronic radiographic lung changes in children with vertically transmitted HIV-1 infection. AJR Am J Roentgenol 176:1553–1558
Akinbami AA, Adegboyega AO, Oshinaike OO et al (2011) Chest X-ray findings in HIV patients in relation to the CD4 count. Nig Q J Hosp Med 21:306–311
Welte T (2014) Imaging in the diagnosis of lung disease: more sophisticated methods require greater interdisciplinary collaboration. Dtsch Arztebl Int 111:179–180
American College of Radiology (ACR) (2019) ACR appropriateness criteria: acute respiratory illness in HIV-positive patients. www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonThoracicImaging/AcuteRespiratoryIllnessinHIVPositivePatients-Doc2.aspx. Accessed 29 Aug 2019
Brenner DJ, Hall EJ (2007) Computed tomography — an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Sodhi KS, Krishna S, Saxena AK et al (2015) Clinical application of 'justification' and 'optimization' principle of ALARA in pediatric CT imaging: "how many children can be protected from unnecessary radiation?". Eur J Radiol 84:1752–1757
Brody AS, Frush DP, Huda W, Brent RL (2007) Radiation risk to children from computed tomography. Pediatrics 120:677–682
Yikilmaz A, Koc A, Coskun A et al (2011) Evaluation of pneumonia in children: comparison of MRI with fast imaging sequences at 1.5 T with chest radiographs. Acta Radiol 52:914–919
Biederer J, Mirsadraee S, Beer M et al (2012) MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging 3:373–386
Peltola V, Ruuskanen O, Svedstrom E (2008) Magnetic resonance imaging of lung infections in children. Pediatr Radiol 38:1225–1231
Zar HJ, Andronikou S, Nicol MP (2017) Advances in the diagnosis of pneumonia in children. BMJ 358:j2739
Hirsch W, Sorge I, Krohmer S et al (2008) MRI of the lungs in children. Eur J Radiol 68:278–288
Manson DE (2013) MR imaging of the chest in children. Acta Radiol 54:1075–1085
Sodhi KS, Sharma M, Saxena AK et al (2017) MRI in thoracic tuberculosis of children. Indian J Pediatr 84:670–676
Rizzi EB, Schinina V, Cristofaro M et al (2011) Detection of pulmonary tuberculosis: comparing MR imaging with HRCT. BMC Infect Dis 11:243
Serra G, Milito C, Mitrevski M et al (2011) Lung MRI as a possible alternative to CT scan for patients with primary immune deficiencies and increased radiosensitivity. Chest 140:1581–1589
Arslan S, Poyraz N, Ucar R et al (2016) Magnetic resonance imaging may be a valuable radiation-free technique for lung pathologies in patients with primary immunodeficiency. J Clin Immunol 36:66–72
Eibel R, Herzog P, Dietrich O et al (2006) Pulmonary abnormalities in immunocompromised patients: comparative detection with parallel acquisition MR imaging and thin-section helical CT. Radiology 241:880–891
Leutner CC, Gieseke J, Lutterbey G et al (2000) MR imaging of pneumonia in immunocompromised patients: comparison with helical CT. AJR Am J Roentgenol 175:391–397
Montella S, Maglione M, Bruzzese D et al (2012) Magnetic resonance imaging is an accurate and reliable method to evaluate non-cystic fibrosis paediatric lung disease. Respirology 17:87–91
Sodhi KS, Khandelwal N, Saxena A et al (2016) Rapid lung MRI: paradigm shift in evaluation of febrile neutropenia in children with leukemia: a pilot study. Leuk Lymphoma 57:70–75
Gorkem SB, Coskun A, Yikilmaz A et al (2013) Evaluation of pediatric thoracic disorders: comparison of unenhanced fast-imaging-sequence 1.5-T MRI and contrast-enhanced MDCT. AJR Am J Roentgenol 200:1352–1357
Ozcan HN, Gormez A, Ozsurekci Y et al (2017) Magnetic resonance imaging of pulmonary infection in immunocompromised children: comparison with multidetector computed tomography. Pediatr Radiol 47:146–153
Yan C, Tan X, Wei Q et al (2015) Lung MRI of invasive fungal infection at 3 tesla: evaluation of five different pulse sequences and comparison with multidetector computed tomography (MDCT). Eur J Radiol 25:550–557
Attenberger UI, Morelli JN, Henzler T et al (2014) 3 tesla proton MRI for the diagnosis of pneumonia/lung infiltrates in neutropenic patients with acute myeloid leukemia: initial results in comparison to HRCT. Eur J Radiol 83:e61–e66
Hansell DM, Bankier AA, MacMahon H et al (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722
Montella S, Santamaria F, Salvatore M (2009) Assessment of chest high-field magnetic resonance imaging in children and young adults with noncystic fibrosis chronic lung disease: comparison to high-resolution computed tomography and correlation with pulmonary function. Investig Radiol 44:532–538
Sodhi KS, Sharma M, Lee EY et al (2018) Diagnostic utility of 3T lung MRI in children with interstitial lung disease: a prospective pilot study. Acad Radiol 25:380–386
Lutterbey G, Grohé C, Gieseke J et al (2007) Initial experience with lung-MRI at 3.0 T: comparison with CT and clinical data in the evaluation of interstitial lung disease activity. Eur J Radiol 61:256–261
Montella S, Santamaria F, Salvatore M et al (2009) Lung disease assessment in primary ciliary dyskinesia: a comparison between chest high-field magnetic resonance imaging and high-resolution computed tomography findings. Ital J Pediatr 35:24
Fink C, Puderbach M, Biederer J et al (2007) Lung MRI at 1.5 and 3 tesla: observer preference study and lesion contrast using five different pulse sequences. Investig Radiol 42:377–383
Sodhi KS, Khandelwal N, Saxena AK et al (2016) Rapid lung MRI in children with pulmonary infections: time to change our diagnostic algorithms. J Magn Reson Imaging 43:1196–1206
Dournes G, Yazbek J, Benhassen W et al (2018) 3D ultrashort echo time MRI of the lung using stack-of-spirals and spherical k-space coverages: evaluation in healthy volunteers and parenchymal diseases. J Magn Reson Imaging 48:1489–1497
Acknowledgments
The authors acknowledge Ms. Kusum Chopra and Mr. Ramesh Goel for statistical analysis of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 9 kb)
Rights and permissions
About this article
Cite this article
Rana, P., Sodhi, K.S., Bhatia, A. et al. Diagnostic accuracy of 3-T lung magnetic resonance imaging in human immunodeficiency virus-positive children. Pediatr Radiol 50, 38–45 (2020). https://doi.org/10.1007/s00247-019-04523-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04523-0