Abstract
This study aimed at prospectively compared efficacy of non-contrast-enhanced (non-CE) MRI and MDCT for management of pulmonary nodules. A total of 161 patients with 200 nodules underwent MDCT and non-CE MRI (T1WI, T2WI, and STIR) in conjunction with pathological and/or more than 2 years of follow-up examinations. To compare qualitative detection rates between both modalities, all nodules were visually assessed. To compare quantitative and qualitative diagnostic capabilities of MRI, calculation of contrast ratio and visual assessment of probability for malignancy in each nodule were performed. Then, detection rate and diagnostic capability were statistically compared. Although the overall detection rate of each MR sequence (82.5%) was significantly lower than that of MDCT (97.0%, p < 0.05), that of malignant nodules showed no significant difference (p > 0.05). The diagnostic capability of STIR was significantly higher than those of other MR sequences (p < 0.05). Non-CE MR imaging was found to be as useful as MDCT for management of pulmonary nodules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The challenge of detection, diagnosis and management of pulmonary nodules is one of the most common and most important areas of pulmonary medicine because pulmonary nodules are caused by a variety of conditions, ranging from benign granulomas to operable primary malignant lung nodules [1–3]. Ideally, the goal of screening and management of pulmonary nodules is to promptly detect and bring to surgery all patients with operable malignant nodules while avoiding unnecessary thoracotomy for patients with benign lesions. It is therefore important to detect small pulmonary nodules and differentiate malignant from benign nodules in the least invasive manner and with the least ionizing radiation exposure while making the diagnosis as specific and accurate as possible [2–13]. Many reports on CT findings for this purpose have been published in this connection, although it has been sometimes difficult to distinguish malignant from benign nodules by using only radiological findings. In an attempt to find a solution for this problem, several investigators have assessed the utility of dynamic CT, dynamic magnetic resonance (MR) imaging and positron-emission tomography (PET) or integrated and/or co-registered PET/CT for this purpose [6–13].
In the last decade, continuing technological progress in MR systems and sequencing and reconstruction methods, such as parallel imaging techniques, has led to improvements in soft-tissue contrast enhancement, intrinsic flow sensitivity and image quality of chest MR imaging, and enables obtaining high-resolution MR images of the entire chest [6, 7, 14–22]. In addition, STIR turbo spin-echo MR imaging has been reported as useful for cancer screening and distinguishing metastatic from non-metastatic lesions [19, 20]. Some investigators have suggested that pulmonary nodule detection may be achieved by using state-of-the-art MR systems rather than CT [14, 15, 18, 21, 22].
We hypothesized that non-contrast-enhanced (non-CE) CE pulmonary MR imaging may be at least as efficient as thin-section MDCT for detection and management of pulmonary nodules and may have no significantly negative influence on survival of patients with malignant nodules. The purpose of the study presented here was to prospectively and directly compare the efficacy for management of pulmonary nodules of non-CE pulmonary MR imaging and thin-section MDCT.
Materials and methods
Subjects
The institutional review board of Kobe University Hospital approved this study, and informed consent was obtained from every subject prior to their enrollment in this study.
Between April 2001 and August 2005, 406 consecutive patients (281 males and 125 females; mean age, 67.7 years; age range 29–91 years) with pulmonary abnormalities detected on chest radiographs and/or routine CT at nearby hospitals were referred to our hospital. Then, patients were selected according to the following criteria: (1) presence of pulmonary nodules equal to or less than 30 mm in diameter and with no calcification and (2) ability to cooperate with the procedure. Diameter was defined as the greatest diameter observed on chest radiograph and/or routine MDCT scans that was obtained with a lung window setting. No calcification in pulmonary nodules meant absence of visible calcification on routine or thin-section CT scans (i.e., 1–5-mm section thickness) obtained with a mediastinal window setting.
Of the 406 patients, 161 patients (110 male, 51 female; mean age, 66.5 years; age range 29–84 years) met the criteria and prospectively underwent thin-section MDCT, non-CE pulmonary MR imaging, transbronchial and/or percutaneous biopsies, bronchoalveolar lavage, microbacterial examination, resection during thoracotomy or video-assisted thoracic surgery, as well as pathological examinations of specimens and/or more than 2-year follow-up thin-section CT examinations. These patients were comprised of 73 smokers and 88 non-smokers. Cigarette smoke exposures of smoking patients were assessed as “pack years” and ranged from 120 to 3,600 pack years (mean ± standard deviation: 830 ± 720 pack years). Intervals between the initial thin-section MDCT and non-CE pulmonary MR imaging examinations ranged from 2 to 14 days (mean, 10.3 days).
In every patient with pulmonary abnormalities, routine and/or thin-section MDCTs obtained in our hospitals were reviewed with routine CTs obtained at a nearby hospital. If patients had pulmonary nodules that showed clear evidence of growth, we performed tissue diagnosis and microbiological examinations by transbronchial and/or percutaneous biopsies, bronchoalveolar lavage, and/or resection during thoracotomy or video-assisted thoracic surgery. If patients had pulmonary nodules that were stable and less than 10 mm in diameter, we followed these nodules by repeated CT examination every 6 months. When the nodules showed clear evidence of growth on follow-up CT, we performed surgical resections and pathological examinations. On the other hand, if patients had pulmonary nodules that were stable and equal to or more than 10 mm in diameter, we performed tissue diagnosis and microbiological examinations by transbronchial and/or percutaneous biopsies, bronchoalveolar lavage, and/or resection during thoracotomy or video-assisted thoracic surgery. When pulmonary nodules equal to or more than 10 mm in diameter were diagnosed as malignant, these nodules were surgically resected with lymph node samplimg. When pulmonary nodules equal to or more than 10 mm in diameter were diagnosed as benign or could not be diagnosed as malignant, patients having these nodules underwent repeated CT examinations in every 6 months and were followed at least 2 years. During follow-up periods, if the nodules showed clear evidence of growth, we performed surgical resections and pathological examinations. Final diagnoses in all patients were confirmed by microbiological examination (n = 8), cytological or histological examinations of specimens obtained by CT-guided transthoracic needle biopsy (n = 2), video-assisted thoracoscopic surgery (VATS) (n = 3), transbronchial lung biopsy (n = 5) or surgical resection (n = 95) and follow-up MDCT examinations (n = 87).
All nodules with a diameter of 30 mm or less detected on CT within the lung parenchyma, and which had been followed up more than 2 years with thin-section CT examinations, and/or nodules detected within resected specimens, were considered as the gold standard. All nodule diameters were defined as the largest diameter on thin-section MDCT as displayed on a lung window setting (window width, 1,600 H; window level, -550 H). A nodule for which there was no change in size for a minimum 2-year follow-up was defined as benign. According to the results of microbiological, cytological, histological and/or more than 2-year follow-up examinations, all pulmonary nodules were classified into two groups: malignant pulmonary nodules and benign pulmonary nodules. The standard reference for nodules in a given subject was decided by consensus among an experienced pulmonary pathologists with 23 years of experience (C.O) and two experienced chest radiologists with, respectively, 13 and 15 years of experience (Y.O and D.T).
Chest CT examinations
All initial CT examinations were performed with a four-detector row system (Somatom Plus 4 Volume Zoom; Siemens Medical Solution, Forchheim, Germany) and a 16-detector row system (Aquilion 16; Toshiba Medical Systems, Otawara, Japan). The entire lung was examined from the diaphragm to the lung apex during a single breath hold at end-inspiration. The parameters of the four-detector row MDCT examination were: field of view 300−350 mm, 512 × 512 matrix, 4 × 1.0 mm or 16 × 0.5 mm collimation, 0.94–1.5 beam pitch, 0.5 s/rotation, 120 kVp and 110 effective mAs (330 mA). All 16-detector row MDCT examinations were performed on a 16-slice CT scanner with the following parameters: 120 kVp, 150 mAs, 16 × 0.5 collimation, helical pitch 15, 300–350 field of view, 512 × 512 matrix, and 0.5 s/rotation. All CT data were reconstructed to yield a thin-section CT of the entire lung with 1.0 mm contiguous section thickness.
Non-CE pulmonary MR imaging
For non-CE pulmonary MR imaging, we used a 1.5-T superconducting magnet (Intera T-15; Philips Medical Systems, Best, The Netherlands) with a phased array coil for sensitivity encoding (SENSE body coil, Philips) to obtain axial ECG-gated and respiratory-triggered T1-weighted spin-echo (SE) imaging (TR 1 < R-R > ms/TE 15 ms, number of excitation 2), axial ECG- and respiratory-gated T2-weighted turbo SE imaging (TR 2–3 < R-R > ms/TEeff 90 ms, echo train length 8, number of excitation 4) and respiratory-gated short inversion time (TI) inversion recovery turbo SE imaging (TR 3,600–5,000 ms/TEeff 15 ms/TI 150 ms, echo train length 5, number of excitations 4). Actual TR of T1WI ranged between 500 ms and 1,333 ms, and that of T2WI ranged between 1,333 ms and 5,000 ms. A reduction factor of 2 was used for each sequence. Other parameters were: FOV 350–400 mm, 205–256 × 256 matrix, 410–512 × 512 reconstruction matrix and 5-mm contiguous section thickness. These images were images that used no contrast-media. The mean examination time was 7 min (range: 4–15 min) for T1-weighted SE imaging (T1WI), 7 min (range: 5–15 min) for T2-weighted turbo SE imaging (T2WI) and 9 min (range: 4–15 min) for STIR turbo SE imaging.
Pathological examinations
All resected lung specimens were fixed at end-inspiration volume, which was modified for correlation with findings from CT and histopathologic examinations. The specimens were cut into serial 1-mm-thick sections by referring to the axial CT images, stained with hematoxylin-eosin, and examined by the pulmonary pathologist (C.O.) who was blinded to the initial information about the MDCT-detected abnormal lesions. Each pathologically detected abnormal finding in the sectioned specimens was diagnosed by the same pathologist on the basis of consensus with the two radiologists (Y.O. and D.T.), after which the subsegmental location, position (central or peripheral), large diameter and diagnosis were recorded.
Image analysis
Comparison of nodule detection capability of thin-section MDCT and non-CE pulmonary MR imaging
All MDCT and MR data were interpreted or analyzed with a picture archiving and communication (PACS) system (ShadeQuest; Yokogawa, Tokyo, Japan).
To determine the nodule detection capability of non-CE pulmonary MR imaging and thin-section MDCT, two chest radiologists with, respectively, 6 and 7 years of experience (A.K. and H.K.) without access to any patient information independently assessed all thin-section MDCT and non-CE pulmonary MR images for the presence or absence of pulmonary nodules in random order by means of a five-point visual scoring system with the following ratings: 1, definitely negative; 2, probably negative; 3, equivocal; 4, probably positive; 5, definitely positive. The final score for each nodule was then decided by consensus of the two readers.
Comparison of diagnostic capability of qualitatively and quantitatively assessed non-CE pulmonary MR sequences
To determine the capability of non-CE pulmonary MR imaging for distinguishing malignant from benign nodules, each nodule detected on T1WI, T2WI and STIR was analyzed both qualitatively and quantitatively.
For qualitative assessment of non-CE pulmonary MR imaging, all MR images were independently interpreted by the same two chest radiologists without access to any patient information. Each pulmonary nodule was evaluated with the following five-point visual scoring system: the signal intensity of the pulmonary nodule was (1) markedly lower than that of the right rhomboid muscle or almost equal to that of lung parenchyma; (2) lower than that of the muscle; (3) equal to that of the muscle; (4) slightly higher than that of the muscle; (5) markedly higher than that of the muscle. The final score for each detected nodule was the decided by consensus of the two readers.
For quantitative assessment of non-CE pulmonary MR imaging, regions of interest (ROIs) were placed over each pulmonary nodule detected on each of the sequences and over the rhomboid muscle by one of the chest radiologists (H.K.) with the same PACS system as that used for non-CE pulmonary MR imaging. An ROI was placed over the tumor encompassing the entire cross-sectional area of the nodule, making it as large as possible, while the ROI placed over the muscle was fixed at 120 mm2. The contrast ratio for each nodule and the muscle (CR) was then calculated by using the following formula:
where SIPulmonary nodule is the signal intensity of the pulmonary nodule and SIRhomboid muscle the signal intensity of the muscle.
Statistical analysis
Comparison of nodule detection capability of thin-section MDCT and non-CE pulmonary MR imaging
To compare the pulmonary nodule detection capability of thin-section MDCT and non-CE pulmonary MR imaging, both interobserver agreements were evaluated by means of kappa statistics. Interobserver agreement was considered to be slight when κ was less than 0.21, fair for a κ range from 0.21 to 0.40, moderate for a range from 0.41 to 0.60, substantial for a range from 0.61 to 0.80 and almost perfect for a range from 0.81 to 1.00. This was followed by ROC analyses to compare the detection capability of thin-section MDCT, T1WI, T2WI and STIR. Finally, the overall detection rates of the four modalities based on final scores were compared with the gold standard and with each other by means of McNemar’s test.
Comparison of diagnostic capability of qualitatively or quantitatively assessed non-CE pulmonary MR sequences
To compare the capability of qualitatively assessed non-CE pulmonary MR imaging for distinguishing malignant from benign nodules, both interobserver agreements were also evaluated by means of the kappa statistics in a manner similar to that used for the evaluation of detection.
For comparison of the diagnostic capability of qualitatively and quantitatively assessed non-CE pulmonary MR imaging, ROC analyses were performed, and the results were tested by means of McNemar’s test with the feasible threshold values of each sequence adapted for the highest accuracy value of qualitative and quantitative assessments.
Comparison of 5-year survival rates of patients with malignancy, detected on thin-section MDCT and pathologically confirmed, and detected and diagnosed by using best sequences in qualitatively and quantitatively assessed non-CE pulmonary MR imaging
To determine the influence on patients’ survival by thin-section MDCT and non-CE pulmonary MR imaging, the Kaplan-Meier method followed by log-rank test was used to compare 5-year survival rates of patients with malignancy, detected by using the best sequence for detection and/or the most accurate diagnosis based on qualitatively and quantitative assessment of non-CE MR imagings, with overall survival of the patients detected on thin-section CT and pathologically diagnosed as having malignancy. Cause-specific survival was estimated by counting death from disease as an event and censoring death from other cause and survival at the time of the last follow-up examination.
A p value of less than 0.05 was considered to indicate a statistically significant difference for all statistical analyses. All statistical analyses were performed by using the software StatMate III, ATOMS (Oita, Japan).
Results
Result of the classification of nodules
According to the results of pathological and/or more than 2-year follow-up examinations, 200 pulmonary nodules (mean diameter, 15.6 mm; range 1–30 mm) were diagnosed and classified into two groups: malignant pulmonary nodules (n = 103) and benign pulmonary nodules (n = 97).
The malignant nodules comprised 41 adenocarcinomas, 25 squamous cell carcinomas, 18 bronchioloaveolar carcinomas (BACs), 10 pulmonary metastases (including 3 intrapulmonary metastases), 5 malignant lymphomas, 2 large cell neuroendocrine carcinomas, a carcinoid tumor and a large cell carcinoma. In 83 primary lung cancer patients, 63 patients were diagnosed as stage IA, 15 patients as stage IIA and two patients as stage IIIB. In addition, two patients had double primary lung cancers, and one patient had three localized bronchioalveolar carcinomas (BACs). All double lung cancers and localized BACs were diagnosed as stage IA. Carcinoid tumor (n = 1) was localized one lung lobe. On the other hand, three malignant lymphoma patients were diagnosed as stage I (n = 2) and stage II (n = 1). Ten pulmonary metastases were caused by esophagus cancer (n = 2), malignant melanoma (n = 1), colon cancer (n = 1) and renal cancer (n = 1), respectively. Ninety-seven benign nodules comprised 76 nodules, which were diagnosed on the basis of radiological evidence of no growth over a 2-year follow-up period, six tuberculomas, four atypical adenomatous hyperplasias (AAHs), four organizing pneumonias, three minute meningothelioid nodules, two atypical mycobacterioses and two hamartomas.
Comparison of nodule detection capability of thin-section MDCT and non-CE pulmonary MR imaging
Rated scores for both methods by the two readers are shown in Table 1. Interobserver agreement for thin-section MDCT was substantial (κ = 0.77), while those for non-CE MR sequences were almost perfect (T1WI: κ = 0.87; T2WI: κ = 0.91; STIR: κ = 0.95).
Results of ROC analyses and comparison of true-positive rates are shown in Fig. 1 and Table 2. The areas under the curve (Az) of T1WI (Az =0.91), T2WI (Az =0.91) and STIR (Az =0.91) were significantly lower than that of thin-section MDCT (Az=0.99; p < 0.001). The overall detection rate of thin-section MDCT was significantly higher than that of any sequence of non-CE pulmonary MR imaging (p < 0.05). Although the detection rate of malignant nodules on thin-section MDCT (103/103:100%) was not significantly different from that of any qualitatively assessed non-CE pulmonary MR imaging (99/103:96.1%), the detection rate of benign nodules on thin-section CT (91/97:93.8%) was significantly higher than that of any qualitatively assessed non-CE pulmonary MR imaging (66/97: 68.1%; p < 0.05). On thin-section MDCT, six nodules (3%, mean diameter; 1.5 mm), including three AAHs and three minute meningothelioid nodules (MMNs), were missed and detected only by pathological examination. For each sequence of non-CE pulmonary MR imaging, 35 nodules (17.5%, mean diameter; 5.9 mm), including 4 BACs, 3 AAHs, 3 MMNs and 25 benign nodules, which were confirmed by follow-up CT examinations, but not by pathological examinations, were missed.
Results of ROC analyses of thin-section MDCT and each MR sequence to determine qualitative overall detection capability. Thin-section MDCT (black square) (Az =0.99; p < 0.05) shows significantly better capability for nodule detection than any of the non-CE MR imaging sequences [T1WI (black diamond): Az =0.91, T2WI (black triangle): Az =0.91, and STIR (black circle): Az =0.91]
Comparison of diagnostic capabilities of qualitative or quantitative assessment of non-CE pulmonary MR sequences
Rated scores by the two readers for qualitatively assessed non-CE pulmonary MR imaging are shown in Table 3. Interobserver agreement for T1WI (κ = 0.80), T2WI (κ = 0.72) and STIR (κ = 0.79) was substantial.
The results of ROC analyses of qualitatively assessed non-CE pulmonary MR imaging are shown in Fig. 2 and Table 4. Azs of T1WI (Az =0.65) and of T2WI (Az =0.66) were significantly lower than that of STIR (Az =0.74; p < 0.05). The feasible threshold values of qualitatively assessed T1WI, T2WI and STIR were determined as 2, 3 and 4, respectively. The results for diagnostic ability when these threshold values were applied are also shown in Table 4. The specificity (40/66: 60.6%; p < 0.05) and accuracy (123/165: 74.5%; p < 0.05) of STIR were significantly higher than the corresponding values for T1WI (25/66: 37.9% and 112/165: 67.9%) and those for T2WI (32/66: 48.5%, and 112/165: 67.9%).
Results of ROC analyses for distinguishing malignant nodules from benign nodules by using qualitatively assessed non-CE MR imaging. STIR (black circle) (Az =0.74) shows a better capability for distinguishing malignant nodules from benign nodules than do T1WI (black diamond) (Az =0.65; p < 0.05) and T2WI (black triangle) (Az =0.66; p < 0.05)
The results of ROC analyses of quantitatively assessed non-CE pulmonary MR imaging are shown in Fig. 3 and Table 5. Azs of T1WI (Az =0.66) and T2WI (Az =0.65) were significantly lower than that of STIR (Az =0.76; p < 0.05). The feasible threshold values of quantitatively assessed T1WI, T2WI and STIR were determined as 0.8, 1.8 and 1.2, respectively. The results for diagnostic ability when these threshold values were applied are also shown in Table 5. The specificity (36/66: 54.5%) of STIR was significantly higher than that of T1WI (26/66: 39.4%; p < 0.05) and T2WI (25/66: 37.9%; p < 0.005).
Results of ROC analyses for distinguishing malignant nodules from benign nodules by using quantitatively assessed non-CE MR imaging. STIR (black circle) (Az =0.76) shows a better capability for distinguishing malignant nodules from benign nodules than do T1WI (black diamond) (Az =0.66; p < 0.05) and T2WI (black triangle) (Az =0.65; p < 0.05)
Representative cases are shown in Figs. 4 and 5.
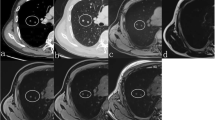
A 67-year-old male with lung cancer in left lower lobe. a: Thin-section MDCT shows partly solid nodule with a diameter of 25.5 mm in the left lower lobe. b: T1WI shows low signal intensity for the nodule. Visual score and CR for the nodule are 2 and 0.4, respectively. c: T2WI shows high signal intensity for the nodule. Visual score and CR of the nodule are 5 and 2.0. d: STIR shows high signal intensity for the nodule. Visual score and CR of the nodule are 5 and 1.8. Both qualitative and quantitative assessments of STIR identified this case as true-positive
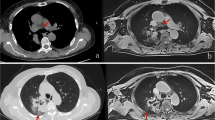
A 50-year-old male with pathologically unidentified benign nodule in left upper lobe. a: Thin-section MDCT shows a solid nodule with a diameter of 9.8 mm in the left upper lobe. b: T1WI shows low signal intensity for the nodule. Visual score and CR of the nodule are 2 and 0.3, respectively. c: T2WI shows high signal intensity for the nodule. Visual score and CR of the nodule are 5 and 3.8. d: STIR shows low signal intensity for the nodule. Visual score and CR of the nodule are 2 and 0.4. Both qualitative and quantitative assessment of STIR identified this case as true-negative
Comparison of 5-year survival rates with overall survival for patients with malignancy detected by means of non-CE pulmonary MR imaging and diagnosed by using best sequences of qualitatively and quantitatively assessed non-CE pulmonary MR imaging
Ninety-two patients had malignant nodules, and ten patients (10.9%) had died of the disease (ten primary lung cancers); one patient had died of intercurrent disease (colon cancer).
Overall survival curves for patients with malignant nodules detected on thin-section MDCT and qualitatively assessed non-CE pulmonary MR imaging, and diagnosed by using the best sequence of qualitatively and quantitatively assessed non-CE pulmonary MR imaging after detection by non-CE pulmonary MR imaging are shown in Fig. 6 and Table 6. The 5-year survival rates for patients with malignant nodules detected on thin-section MDCT (83.5%) and qualitatively assessed non-CE pulmonary MR imaging (80.8%) showed no significant differences compared to those for patients with malignant nodules diagnosed by using qualitatively (78.1%) and quantitatively (78.5%) diagnosed STIR (p > 0.05).
Results of survival curves The graph shows overall survival curves for patients with malignant nodules detected on thin-section MDCT, qualitatively assessed with non-CE pulmonary MR imaging, or detected by non-CE pulmonary MR imaging and diagnosed by using the best sequence of qualitatively and quantitatively assessed non-CE pulmonary MR imaging. There were no significant differences among the overall survival curves for any of the methods (p > 0.05)
Discussion
Our results indicate that non-CE pulmonary MR imaging can detect malignant nodules as capably as thin-section MDCT, although the overall detection rate was significantly reduced due to a major decrease in the detection rate for benign nodules as compared with that of thin-section MDCT. In addition, when compared with thin-section MDCT, non-CE pulmonary MR imaging can qualitatively and quantitatively distinguish malignant from benign nodules without a significant reduction in the 5-year survival rate. To the best of our knowledge, ours is the first published report on a prospective comparison of detection rate, diagnostic capability and influence on patients’ outcome of non-CE pulmonary MR imaging with those of thin-section MDCT in a large prospective cohort.
When comparing interobserver agreement for nodule detection of non-CE pulmonary MR imaging with that of thin-section MDCT, interobserver agreements of all MR sequences were almost perfect (κ > 0.81) and better than that of thin-section MDCT. This fact suggested that non-CE pulmonary MR imaging is considered as having less interobserver variance for nodule detection than thin-section CT, even if the overall detection rate of non-CE pulmonary MR imaging was inferior to that of thin-section MDCT.
In comparison with the nodule detection capabilities of thin or routine CT examinations, the overall detection rate of non-CE pulmonary MR imaging has been reported in the past literature to be between 45.5 and 96.0% for 1.5-T scanners and between 56.0 and 57.0% for 3.0-T scanners [14, 15, 18, 21, 22]. This means that CT has been recognized as the best screening tool for lung cancer detection and surveillance of pulmonary metastases in routine clinical practice or lung cancer screening trials. However, after detection of pulmonary nodules, several problematic factors, such as difficulty of distinguishing benign from malignant nodules to avoid surgery for removal of a benign nodules, the cost per operation, which ranges between $20,000 and $25,000 [5], and the morbidity and the 2.0-3.8% mortality associated with wedge resection of lung nodules in community hospitals in the USA [23–25]. It would therefore be preferable to accept a decrease in the detection rate of benign nodules without significantly missing malignant nodules, even if the overall detection rate may decrease if the detection of benign nodules is significantly reduced. With these points in mind, our results suggest that any sequence of non-CE pulmonary MR imaging may be potentially a better screening tool than thin-section MDCT. In addition, our overall detection rate for each of the sequences is compatible with that reported in the past literature [14, 15, 18, 21, 22].
In comparison, for interobserver agreement for differentiation of malignant nodules from benign nodules, all κ values of non-CE pulmonary MR sequences (T1WI: κ = 0.80, T2WI: κ = 0.72, STIR: κ = 0.79) were substantial and had no significant differences. Therefore, this fact suggests that interobserver variance caused by different MR sequences might not have influence on determination of the best sequence for differentiation of nodules.
With STIR turbo SE imaging, TI values are approximately 80 to 150http://devel.springer.de/A++/V2.4/DTD/ms. At these rates, the longitudinal magnetization for virtually all tissues is negative when a 90º pulse is applied, and recovery is just beginning for most tissues. After the second 90º pulse, the T1 and the T2 contrast are additive; that is, increasing the T1 of a tissue increases the tissue’s relative signal intensity and so does increasing its T2. Because many pathological lesions demonstrate an increase in both T1 and T2, the addition of these two types of contrasts to the STIR sequence produces a higher net tissue contrast [19, 20, 26–28]. It has therefore been suggested that the STIR sequence is very sensitive to changes in T1 and T2 and also makes it possible for some T1-dependent decay to be submitted for T2-dependent decay in an equivalent spin-echo sequence. These findings may be of value where the T1 and T2 tissue decays differ and also explain why the contrast or signal values of various tissues in the STIR sequence are greater than those of the equivalent spin-echo sequence. In short, there is some convincing evidence for the utility of the STIR sequence for clinical stage assessment of non-small-cell lung cancer and other malignancies and for screening of malignant tumors [19, 20, 29]. STIR, in comparison with T1WI and T2WI, has therefore been proposed as the best sequence for distinguishing malignant from benign nodules on qualitatively and quantitatively assessed non-CE pulmonary MR imaging. These notions are compatible with the above-mentioned pathological and physical considerations.
Our results demonstrate that 5-year survival rates of patients with malignant nodules, which were qualitatively detected, qualitatively detected and diagnosed, or qualitatively detected and quantitatively diagnosed by the STIR sequence were not significantly different from those of patients with malignant nodules detected by thin-section MDCT. This fact suggests that the STIR sequence might have potential for influencing the prognosis or survival in patients with malignant nodules similar to thin-section MDCT. In addition, adaptation of non-CE pulmonary MR imaging avoids the side effects associated with contrast media and ionized radiation exposure and therefore makes it suitable for patients of any age or gender, especially children, younger adults and pregnant women, although the acquisition time of STIR sequence is longer than that of thin-section MDCT. In general, the prognosis and survival of patients are influenced by entirely different factors, predominantly the tissue type and the stage of the tumor, after the one-time detection of malignant lung lesions at imaging and subsequent resection. Moreover, the capability of non-CE pulmonary MR imaging for prediction of the incidence of pulmonary malignancy during the follow-up period, including all detected lung lesions, could not be compared with that of thin-section MDCT in this study. Therefore, comparative study between non-CE pulmonary MR imaging and thin-section MDCT in large prospective cohort is warranted to discuss the real possibility of non-CE pulmonary MR imaging for adaptation to lung cancer screening and/or pulmonary metastasis surveillance as a substitution for thin-section MDCT.
There are several limitations to this study. First, we could not include pulmonary nodules that could not be detected on thin-section MDCT but might have been detected with pathological examinations, although the gold standards used in this study were based on initial and follow-up radiological examinations and pathological examinations of resected specimens. In addition, the inclusion of pulmonary nodules that could not be detected on thin-section MDCT but might have been detected with pathological examinations in resected specimens may also be problematic when considering section thickness of both methods. Therefore, our overall detection rate and detection rates of malignant and benign nodules with both imaging methods were biased. Second, although we used techniques for correction of cardiac and/or respiratory motion artifacts for each sequence, the black-blood inversion-pulse and half-Fourier single-shot turbo spin-echo (HASTE) techniques were not used for suppression of pulsation artifacts, even though this technique is currently considered useful for nodule detection and assessment of morphology of pulmonary lesions [14]. However, when this study’s protocol was decided, our MR scanner could not obtain ECG- and respiratory-gated T1WI, T2WI and STIR in combination with the black-blood inversion-pulse and HASTE techniques. In addition, we could not compare our non-CE pulmonary MR sequences with recently available gradient-echo sequences, such as 3D volumetric interpolated breath-hold examination (VIBE), true fast imaging with steady-state precession (True FISP) and T1 high-resolution isotropic volume examination (THRIVE) sequences. Fink et al. suggested that VIBE, true FISP and HASTE sequences were more useful for depiction of lung parenchyma than STIR sequence with long TE in phantom and normal volunteer studies [30]. Therefore, although we demonstrated respiratory-gated STIR sequences with short TE had potential for significant improvement of diagnostic capability as compared with ECG-gated and respiratory-triggered T1-weighted SE and ECG- and respiratory-gated T2-weighted turbo SE sequences, direct comparison among our non-CE pulmonary MR sequences, newly available gradient-echo sequences, STIR sequences with long TE and HASTE sequences in patients with pulmonary nodules may be warranted to determine the best sequence for nodule detection and diagnosis in the near future. Therefore, we will employ these techniques for the MR sequences at 1.5 or 3.0 T currently used by us as well as for MDCT at similar section thickness and try to determine the actual detection rate of non-CE pulmonary MR imaging and directly compare thin-section MDCT with the same section thickness. Third, although STIR has been established as the best sequence of non-CE pulmonary MR imaging for detection and diagnosis of pulmonary nodules, the specificity of quantitatively and qualitatively assessed STIR (54.5% and 60.6%, respectively) was equal to or lower than that of dynamic CT and FDG-PET or PET/CT reported in the past literatures [6–13]. Therefore, non-CE pulmonary MR imaging may be better used for pulmonary nodule detection rather than for diagnosis. In addition, new studies are needed to demonstrate how diagnostic capability can be improved by using dynamic MR imaging for pulmonary nodules detected by non-CE pulmonary MR imaging to make this modality truly useful for management of pulmonary nodules. Fourth, our results should be interpreted while keeping in mind the four types of bias, lead-time, length-time, overdiagnosis bias and inclusion bias, inherent in this study, although clinical outcome in terms of 5-year survival rates for thin-section MDCT could be compared with that for standard-dose protocol and non-CE pulmonary MR imaging. Lead-time bias results from the failure to control for the timing of diagnosis. Cases detected by radiological examination are diagnosed earlier, and the patients live longer from the time of diagnosis, even if death is ultimately not delayed as compared with time of death in a population not examined radiologically [31, 32]. Length-time bias results from the failure to control for the rate of disease progression. The probability that a case will be detected through radiological examination is directly proportional to the length of the asymptomatic interval (the detectable preclinical phase) [31, 32]. Overdiagnosis bias results from the failure to control for the detection of pseudo-diseases or preclinical diseases that would not produce any signs or symptoms before an individual had died of other causes. Pseudo-diseases dilute the subset of radiological examination-detected cases with cases that are effectively disease free, and this can have a major effect on survival and cure rates [31, 33]. Attempts should therefore be made to use non-CE pulmonary MR imaging for lung cancer screening or pulmonary metastases surveillance in patients with malignancy in a large prospective cohort in order to determine the real utility of this modality for these purposes as compared with that of thin-section MDCT with standard and low-dose protocols. Finally, since no randomization was performed, the different survival rates might be the result of inclusion bias, with smaller and thus less advanced nodules included in the group with better survival. However, in terms of smaller and thus less advanced nodules, the population of lung cancer screening is the same. At least, the results of our study suggested one potential with non-CE pulmonary MR imaging for lung cancer screening.
In conclusion, non-CE pulmonary MR imaging was found to be as useful as thin-section MDCT for management of pulmonary nodules. Although the overall detection rate of non-CE pulmonary MR imaging is lower than that of thin-section MDCT, the detection rate for malignant nodules showed no significant differences between the two modalities. In addition, STIR was the best sequence among non-CE pulmonary MR imaging, and there was no significant influence on survival time compared with overall patients.
References
Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, Smith JP (1999) Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354:99–105
Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, Sykes AM, Aughenbaugh GL, Bungum AO, Allen KL (2005) CT screening for lung cancer: 5-year prospective experience. Radiology 235:259–265
Garg K, Keith RL, Byers T, Kelly K, Kerzner AL, Lynch DA, Miller YE (2002) Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 225:506–510
MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF Jr, Swensen SJ, Fleischner Society (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 23:395–400
Swensen SJ (2002) CT screening for lung cancer. AJR Am J Roentgenol 179:833–836
Kim JH, Kim HJ, Lee KH, Kim KH, Lee HL (2004) Solitary pulmonary nodules: a comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. J Comput Assist Tomogr 28:766–775
Ohno Y, Hatabu H, Takenaka D, Adachi S, Kono M, Sugimura K (2002) Solitary pulmonary nodules: potential role of dynamic MR imaging in management initial experience. Radiology 224:503–511
Swensen SJ, Viggiano RW, Midthun DE, Müller NL, Sherrick A, Yamashita K, Naidich DP, Patz EF, Hartman TE, Muhm JR, Weaver AL (2000) Lung nodule enhancement at CT: multicenter study. Radiology 214:73–80
Marom EM, Sarvis S, Herndon JE 2nd, Patz EF Jr (2002) T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose PET. Radiology 223:453–459
Kim SK, Allen-Auerbach M, Goldin J, Fueger BJ, Dahlbom M, Brown M, Czernin J, Schiepers C (2007) Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 48:214–220
Jeong YJ, Lee KS, Jeong SY, Chung MJ, Shim SS, Kim H, Kwon OJ, Kim S (2005) Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology 237:675–683
Kono R, Fujimoto K, Terasaki H, Müller NL, Kato S, Sadohara J, Hayabuchi N, Takamori S (2007) Dynamic MRI of solitary pulmonary nodules: comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol 188:26–36
Hashimoto Y, Tsujikawa T, Kondo C, Maki M, Momose M, Nagai A, Ohnuki T, Nishikawa T, Kusakabe K (2006) Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5. J Nucl Med 47:426–431
Vogt FM, Herborn CU, Hunold P, Lauenstein TC, Schröder T, Debatin JF, Barkhausen J (2004) HASTE MRI versus chest radiography in the detection of pulmonary nodules: comparison with MDCT. AJR Am J Roentgenol 183:71–78
Schroeder T, Ruehm SG, Debatin JF, Ladd ME, Barkhausen J, Goehde SC (2005) Detection of pulmonary nodules using a 2D HASTE MR sequence: comparison with MDCT. AJR Am J Roentgenol 185:979–984
Ohno Y, Sugimura K, Hatabu H (2002) MR imaging of lung cancer. Eur J Radiol 44:172–181
Both M, Schultze J, Reuter M, Bewig B, Hubner R, Bobis I, Noth R, Heller M, Biederer J (2005) Fast T1- and T2-weighted pulmonary MR-imaging in patients with bronchial carcinoma. Eur J Radiol 53:478–488
Kersjes W, Mayer E, Buchenroth M, Schunk K, Fouda N, Cagil H (1997) Diagnosis of pulmonary metastases with turbo-SE MR imaging. Eur Radiol 7:1190–1194
Ohno Y, Hatabu H, Takenaka D, Higashino T, Watanabe H, Ohbayashi C, Yoshimura M, Satouchi M, Nishimura Y, Sugimura K (2004) Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative and qualitative assessment with STIR turbo spin-echo MR imaging. Radiology 231:872–879
Walker R, Kessar P, Blanchard R, Dimasi M, Harper K, DeCarvalho V, Yucel EK, Patriquin L, Eustace S (2000) Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging 11:343–350
Yi CA, Jeon TY, Lee KS, Lee JH, Seo JB, Kim YK, Chung MJ (2007) 3-T MRI: usefulness for evaluating primary lung cancer and small nodules in lobes not containing primary tumors. AJR Am J Roentgenol 18:386–392
Bruegel M, Gaa J, Woertler K, Ganter C, Waldt S, Hillerer C, Rummeny EJ (2007) MRI of the lung: value of different turbo spin-echo, single-shot turbo spin-echo, and 3D gradient-echo pulse sequences for the detection of pulmonary metastases. J Magn Reson Imaging 25:73–81
Romano PS, Mark DH (1992) Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest 101:1332–1337
Ginsberg RJ, Hill LD, Eagan RT, Thomas P, Mountain CF, Deslauriers J, Fry WA, Butz RO, Goldberg M, Waters PF et al (1983) Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 86:654–658
Nagasaki F, Flehinger BJ, Martini N (1982) Complications of surgery in the treatment of carcinoma of the lung. Chest 82:25–29
Wiener JI, Chako AC, Merten CW, Gross S, Coffey EL, Stein HL (1986) Breast and axillary tissue MR imaging: correlation of signal intensities and relaxation times with pathologic findings. Radiology 160:299–305
Fossel ET, Brodsky G, deLayre JL, Wilson RE (1983) Nuclear magnetic resonance for the differentiation of benign and malignant breast tissues and axillary lymph nodes. Ann Surg 198:541–545
Bottomley PA, Hardy CJ, Argersinger RE, Allen-Moore G (1987) A review of 1H nuclear magnetic resonance relaxation in pathology: are T1 and T2 diagnostic? Med Phys 14:1–37
Shuman WP, Baron RL, Peters MJ, Tazioli PK (1989) Comparison of STIR and spin-echo MR imaging at 1.5 T in 90 lesions of the chest, liver, and pelvis. AJR Am J Roentgenol 152(4):853–859
Fink C, Puderbach M, Biederer J, Fabel M, Dietrich O, Kauczor HU, Reiser MF, Schönberg SO (2007) Lung MRI at 1.5 and 3 Tesla: observer preference study and lesion contrast using five different pulse sequences. Invest Radiol 42(6):377–383
Obuchowski NA, Graham RJ, Baker ME, Powell KA (2001) Ten criteria for effective screening: their application to multislice CT screening for pulmonary and colorectal cancers. AJR Am J Roentgenol 176:1357–1362
Black WC, Welch HG (1993) Advances in diagnostic imaging and overestimation of disease prevalence and the benefits of therapy. N Engl J Med 328:1237–1243
Black WC, Welch HG (1997) Screening for disease. AJR Am J Roentgenol 168:3–11
Acknowledgement
The authors thanks Munenobu Nogami, M.D., Ph.D. (Division of PET, Institute of Biomedical Research and Innovation), Yoshikazu Kotani, M.D. (Division of Cardiovascular and Respiratory Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine), Masahiro Yoshimura, M.D. (Division of Pulmonary Surgery, Hyogo Cancer Center), Takanori Higashino, M.D., Ph.D. (Department of Radiology, Tenri Hospital), Hideaki Kawamitsu, B.S., and Masahiko Fujii, M.D. (Division of Radiology, Kobe University Hospital) for their contribution to this work. This work was supported by Philips Medical Systems.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Philips Medical Systems and the Knowledge Cluster Initiative of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Rights and permissions
About this article
Cite this article
Koyama, H., Ohno, Y., Kono, A. et al. Quantitative and qualitative assessment of non-contrast-enhanced pulmonary MR imaging for management of pulmonary nodules in 161 subjects. Eur Radiol 18, 2120–2131 (2008). https://doi.org/10.1007/s00330-008-1001-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1001-2