Abstract
The aim of this study was to analyze the technical results, the extraosseous cement leakages, and the complications in our first 500 vertebroplasty procedures. Patients with osteoporotic vertebral compression fractures or osteolytic lesions caused by malignant tumors were treated with CT-guided vertebroplasty. The technical results were documented with CT, and the extraosseous cement leakages and periinterventional clinical complications were analyzed as well as secondary fractures during follow-up. Since 2002, 500 vertebroplasty procedures have been performed on 251 patients (82 male, 169 female, age 71.5 ± 9.8 years) suffering from osteoporotic compression fractures (n = 217) and/or malignant tumour infiltration (n = 34). The number of vertebrae treated per patient was 1.96 ± 1.29 (range 1–10); the numbers of interventions per patient and interventions per vertebra were 1.33 ± 0.75 (range 1–6) and 1.01 ± 0.10, respectively. The amount of PMMA cement was 4.5 ± 1.9 ml and decreased during the 5-year period of investigation. The procedure-related 30-day mortality was 0.4% (1 of 251 patients) due to pulmonary embolism in this case. The procedure-related morbidity was 2.8% (7/251), including one acute coronary syndrome beginning 12 h after the procedure and one missing patellar reflex in a patients with a cement leak near the neuroformen because of osteolytic destruction of the respective pedicle. Additionally, one patient developed a medullary conus syndrome after a fall during the night after vertebroplasty, two patients reached an inadequate depth of conscious sedation, and two cases had additional fractures (one pedicle fracture, one rib fracture). The overall CT-based cement leak rate was 55.4% and included leakages predominantly into intervertebral disc spaces (25.2%), epidural vein plexus (16.0%), through the posterior wall (2.6%), into the neuroforamen (1.6%), into paravertebral vessels (7.2%), and combinations of these and others. During follow-up (15.2 ± 13.4 months) the secondary fracture rate was 17.1%, including comparable numbers for vertebrae at adjacent and distant levels. The presence of intradiscal cement leaks was not associated with increased adjacent fracture rates. CT-guided vertebroplasty is safe and effective for treatment of vertebral compression fractures. CT-fluoroscopy provides an excellent control of the posterior vertebral wall. The number of cement leakages alone is not directly associated with clinical complications. However, even small volumes of pulmonary PMMA embolism might be responsible for the fatal outcome in cases with underlying cardiopulmonary insufficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vertebral compression fractures are frequent complications of osteoporosis and vertebral metastases, which might either cause minor complaints or result in substantial local pain, impaired physical function, reduced quality of life, and an increased mortality rate [1–4]. Conservative management of osteoporotic compression fractures includes analgesic drugs, surgical corsets, and physical therapy, which has been the standard treatment for decades. Percutaneous vertebroplasty has emerged as a widely accepted treatment modality for medically refractory osteoporotic compression fractures and is also used for metastatic vertebral fractures in order to stabilize the vertebral bodies parallel to radiotherapy or chemotherapy [5–10]. Numeous potential complications have been reported in the literature [6–12], including the hypothesis that the restored stiffness of the augmented vertebra itself might propagate secondary fractures in adjacent non-augmented vertebrae [13–15]. In our department, CT-guided percutaneous vertebroplasty was introduced in 2002 and has evolved as a valuable treatment option with significant benefit for the patients with respect to pain relief, reduced analgetics, and restoration of mobility. However, there have also been severe adverse events during this period. The purpose of this study is to describe a single-center experience with CT-guided vertebroplasty and to analyze frequency and causes of complications.
Methods
Indication for treatment included osteoporotic vertebral fractures with persistent medically refractory pain or osteolytic vertebral lesions due to primary or metastatic tumor disease. The study protocol was approved by the institutional ethical committee; all patients gave written informed consent. The diagnosis was confirmed by MRI using a 1.5-T system (Magnetom Vision, Siemens, Erlangen) or an open 0.2-T system (Magnetom Open, Siemens, Erlangen) using sagittal STIR and T1w spin echo sequences. Vertebral bodies proved positive for actual fractures if bone marrow edema was identified. Vertebral fractures with height loss but no bone marrow edema were judged as old and consolidated fractures and therefore not considered for treatment. In tumor cases, intra- and extravertebral tumor masses as well as infiltration of the spinal canal were also depicted or excluded with MRI and CT scans. In those cases, vertebroplasty was indicated in order to achieve primary stabilization and pain relief, which was followed by either radiotherapy and/or chemotherapy.
Procedures were performed by one of two radiologists, who were trained in percutaneous vertebroplasty (MBP, SH). For the procedure, patients were carefully placed in prone position on the CT table by using pillows to create hyperextension. The blood pressure, heart rate, and oxygen saturation were continuously monitored. Conscious sedation with midazolam (2.5–5.0 mg i.v.) and piritramid (7.0–15 mg i.v.) was administered depending on the individual need. The intervention commenced with a standard CT acquisition using a four-row CT system (Somatom, Volume Zoom, Siemens, slice 2 mm, collimation 4 × 1 mm, 330 mAs, 120 kV) with multiplanar reformatted images of the spine. A mobile C-Arm fluoroscope (Siemens) was installed between the gantry and the base of the CT table. With this arrangement, cross-sectional imaging with CT-fluorospcopy and lateral fluoroscopy with C-arm could be alternated during the procedure. For CT-fluoroscopy, the voltage and the current of the CT tube was reduced to 80 kV and 20 mAs. After local anaesthesia, the 10-G cannula of the vertebroplasty set (Cemento set, OptiMed, Ettlingen) was advanced and steered by means of CT fluoroscopy using the transpedicular access as standard in lumbar vertebrae and an intercostotransverse access in thoracic vertebrae. Dorsolateral parapedicular access (direct puncture of the dorsolateral wall of the vertebral body omitting the pedicle) was only used when individual anatomy prohibited use of the standard access. The tip of the cannula was placed in the ventral third and in the midsagittal plane of the vertebral body. Three types of cements were used during the study period: (a) Osteopal 40, (b) Osteopal V and (c) Biomet Bone Cement-V (Biomet Merck GmbH, Berlin, Germany). The PMMA cement was applied under alternating CT-fluoroscopy and lateral fluoroscopy to maintain control of the cement distribution in both the in-plane cross-sectional and the lateral views, as has been previously described [7, 16]. If the cement distribution was unfavorable, for example, into the basal vertebral or epidural veins, the injection was interrupted for 30–60 s until the cement had polymerized at that point and was subsequently continued. Depending on the cement distribution, the bevel cut of the needle was directed towards the contralateral or the ipsilateral half of the vertebral body so that, ultimately, cement was deposited in the middle and to some extent both lateral thirds of the vertebral body. If the cement filled only the ipsilateral half of the vertebral body, an additional contralateral access was used to achieve a balanced deposit. The final result was documented by CT. All patients were monitored for 6 h after intervention, including testing for sensomotoric deficits and cardio-respiratory function. Patients were discharged after documentation of final clinical results. Tumor patients were discharged depending on their individual further treatments, e.g., radiation or chemotherapy.
All data were prospectively acquired in a comprehensive vertebroplasty data base. Pain relief was measured using a visual analogue score (VAS) with a range from 0 (no pain) to 10 (maximal pain). All peri-interventional clinical complications were registered with respect to their clinical and radiological preconditions and the causality with the interventional procedure. Extraosseous cement deposits were analyzed according to their source: (1) epidural venous plexus; (2) posterior vertebral wall; (3) neuroforamina; (4) anterior vertebral wall; (5) lateral vertebral wall; (6) upper endplate; (7) lower endplate; (8) both endplates; (9) paravertebral vessels; (10) pulmonary embolism; (11) access site. Every leak location was counted as a separate event. Follow-up consisted of clinical visits including radiographs or CT of the spine at 3, 6, and 12 months and yearly thereafter and MRI whenever the patient experienced newly discovered discomfort or pain. Symptomatic secondary vertebral fractures were treated with vertebroplasty and were included in the follow-up. Secondary fractures were classified as:
-
I.
adjacent fracture in the immediate vicinity of an upper or lower vertebral body that had been previously treated with vertebroplasty;
-
II.
secondary sandwich fracture, i.e., secondary fracture of a vertebral body located in-between two augmented vertebral bodies;
-
III.
secondary distant fracture, more than one segment distant from previously cemented vertebrae.
Statistical analysis was performed using SPSS for Windows, version 12. The quantitative descriptive data are given as mean and standard deviation.
Results
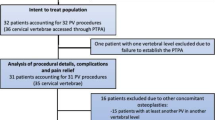
Between June 2002 and August 2007 a total of 500 vertebroplasty procedures were performed in 251 patients (82 male, 169 female, age 71.5 ± 9.8 years, range 37–95). Vertebral fractures were caused by malignant disease in 34 patients (62 vertebral levels) and osteoporotic in 217 patients (438 vertebral levels). This included nine patients with osteoporotic fractures for whom an additional surgical approach was scheduled. In these cases, the contiguous vertebrae were augmented (1) before operative corporectomy and posterior instrumentation (n = 6), (2) augmentation of adjacent non-fractured vertebrae before Kyphoplasty of severely compressed vertebrae in order to strengthen the adjacent segments for the ballooning during kyphoplasty (n = 2), or (3) before a decompression by laminectomy (n = 1) in order to achieve secure fixation of the pedicle screws. Patients entered the study with occurrence of primary treatment of 415 vertebral fractures. During follow-up (15.2 ± 13.4 months, range 0–48), an additional 85 secondary fractures occurred in 43 patients (Table 1). As results of the initial treatment, there remained 39 vertebrae in sandwich situations in 35 patients, meaning that there was one level untreated between two augmented levels. Eleven of such 39 sandwich vertebrae showed secondary failure during follow-up, 7 of which were treated with vertebroplasty. The number of secondary fractures adjacent to previously augmented levels was not increased compared to distant secondary fracture locations. 22 of 33 adjacent fractures occurred in the absence of any intradiscal cement leak from previous vertebroplasty. In secondary sandwich fractures, 7 of 11 fractures occurred with cement deposits in adjacent disc spaces. The number of vertebrae treated per patient was 1.96 ± 1.29 (range 1–10), one vertebra in 125 patients, two in 63, three in 32, four in 26, and six, eight, and ten vertebrae in 1 case, respectively. The number of interventions per patient and intervention per vertebra was 1.33 ± 0.75 (range 1–6) and 1.01 ± 0.10 (maximum 2 in 5 vertebrae), respectively (Table 1). The distribution of augmented vertebrae, the cement volumes per level, and the vertebral accesses are given in Table 2. There was a predominance at the thoracolumbar junction between Th11 and L3 covering about two thirds of the respective vertebral levels. The overall amount of PMMA cement was 4.5 ± 1.9 ml. It was slightly reduced with increasing experience since adequate treatment results could be achieved with smaller amounts of cement through the years (Table 3).
The 30-day mortality was 0.4% (1 of 251 patients, 0.2% of all procedures, Table 4). One 78-year-old male with history of chemotherapy because of chronic lymphatic leukemia, pulmonary emphysema, and ischemic heart disease, suffered from steroid-induced osteoporosis with eight vertebral fractures, including fresh fractures at four thoracic and lumbar levels. During the second treatment session, there was some extraosseous cement displacement, and the injection was immediately stopped (3 ml type-c-cement) after detection with CT-fluoroscopy. Thirty minutes after the intervention, the patient presented with respiratory insufficiency with a O2-saturation of about 85–90% and bronchial spasm. At this stage, an overhang of midazolam was discussed. The patient improved immediately after medication with Beta-2-Mimetics, diuretics, and oxygen supply and could be transferred to the ward. During the next 48 h, the respiratory function deteriorated, and CT control demonstrated some small tubular cement emboli in the right pulmonary segmental arteries. However, CT also detected significant pulmonary congestion, pleural effusion, dystelectasis, and consolidation. The patient died because of cardiopulmonary insufficiency on the 7th post-interventional day (Fig. 1).
A 78-year-old male patient with pulmonary cement embolism (arrows) in segmental arteries of the upper und lower lobe during vertebroplasty. CT demonstrates some high attenuation solid emboli in the 3rd segmental artery of the right lung (arrow, Fig. 1 a-c) without evidence for segmental infarction or thrombus apposition. The basal segments demonstrate a more diffuse high attenuation area in segmental arteries with an accompanying infiltration and consolidation indicating subsegmental infarction (arrowhead, Fig. 1 c)
The overall procedure-related morbidity was 2.8% (7 of 251 patients, 1.4% of all vertebroplasties, Table 4). This included one patient with an associated but not procedure-related death. This 76-year-old male (hypopituitarism after extirpation of pituitary adenoma, secondary osteoporosis, recurrence of a carcinoma of the urinary bladder) presented with multiple fractures at nine vertebral levels. Four painful vertebral fractures with bone marrow edema in MRI were indicated for vertebroplasty. Treatment was performed in two sessions within 3 days, with two vertebral levels treated in each session (3 ml type-c-cement at both vertebral levels). Within 12 h following the second treatment session, he presented an acute coronary syndrome and was transferred to an intensive care unit. He subsequently developed a spontaneous retroperitoneal hematoma under low-weight heparin, pneumonia, and an adrenal crisis. The patient died at the 78th day after vertebroplasty, having been under long-term intensive care with ventilation, paralytic ileus with concomitant laparatomy because of perforation, renal insufficiency, and finally low-output heart failure.
One patient (85-year-old female) was scheduled for kyphoplasty because of severe compression fractures of the 2nd and 3rd lumbar vertebra with some displacement of the posterior vertebral wall. Prior to kyphoplasty, the adjacent non-fractured vertebrae L1 and L4 were augmented with vertebroplasty (1.5 ml, type-b-cement, level L1 and L4) in order to strengthen the adjacent segments for the ballooning during the scheduled kyphoplasty. However, the patient suffered from a fall during the night after intervention and subsequently developed a medullary conus syndrome. An emergency laminectomy was performed for decompression and was followed by kyphoplasty L2 and L3 (Fig. 2) (Table 4).
A 77-year-old woman with osteoporotic vertebral compression fracture of the lumbar levels 2 and 3. Vertebroplasty of level 1 and 4 was performed in order to stabilize these vertebrae before kyphoplasty of lumbar level 2 and 3. However, after a fall of the patient, a lateral X-ray and CT demonstrated a significant height loss of the 2nd lumbar vertebra with displacement of fragments and narrowing of the spinal canal (Fig. 2 a and b), resulting in an acute medullary conus syndrome. After laminectomy, the patient improved and was subsequently treated with kyphoplasty of lumbar vertebrae 2 and 3. Postoperative lateral X-ray demonstrates a restoration of the respective vertebral heights (Fig. 2 c)
One 65-year-old female patient displayed a missing patellar tendon reflex 1 day after vertebroplasty of metastatic vertebral fracture including the pedicle of L3. There was a leakage alongside with the puncture tract into the neuroforamen (cement c, 5 ml). Since the patient had no complaints during the treatment, the missing reflex was found during the routine post-interventional neurological examination. In retrospect, it could not be clarified whether or not this deficit was of an earlier nature because of the obvious infiltrating tumor mass at that site. However, because of the absence of complaints, no clinical conclusions could be drawn, and the deficit was rated as a procedure-related complication. Fortunately, she was clinically inconspicuous without sensomotoric deficits and without any need for surgical decompression (Fig. 3).
A 65-year-old female patient with breast carcinoma and pathological fractures due to osteolytic tumor mass of the vertebra. CT demonstrates the osteolysis of the pedicle (arrow, Fig. 3 a). Fig. 3 b and c demonstrates the cement propagation from the pedicle into the neuroforamen with compression of the respective nerve root. Except for a missing patellar reflex, there were no other clinical symptoms
One 59-year-old female patient cancelled the second treatment session after one of two osteoporotic vertebral fractures had been augmented (2 ml type-b-cement) because of an inadequate effect of the peri-interventional analgetic drugs and conscious sedation. She refused a repeated treatment under general anesthesia and was therefore supplied with analgetic drugs for conservative treatment. Another 73-year-old male developed a transient hypoxemia with O2 saturation of 90% during conscious sedation before starting vertebroplasty. He was temporarily served with an oropharyngeal tube for 15 min, and the procedure was completed without further complications.
In another patient (71-year-old male, osteoporosis, actual history of a fall after climbing a ladder 2 months before), the pre-interventional MRI and CT demonstrated that the fracture line involved an pedicle of the 2nd lumbar vertebral body. After vertebroplasty, he suffered from persisting local pain although under analgetics for 2 months until consolidation of this pedicle (cement b, 4.5 ml). A 76-year-old male suffered from local pain of the ribs resulting from a rib fracture, most probably acquired during the prone position on the CT-table
The majority of patients had a major benefit at discharge from the hospital. Compared to the pre-interventional VAS score of 7.5 ± 1.5 (range 6–10), complete pain relief was achieved in 102 out of 251 patients (40.6%, VAS 1–3), and a significant improvement was stated in 144 out of 251 (57.4%, VAS 4–5), allowing for a reduction of analgesics. However, there were five patients with vertebral fractures and morphologically satisfying results, but without any pain relief. Two female patients suffered from some radiating pain that was initially attributed to the respective fracture, but was not resolved after augmentation. One male with osteoporotic vertebral fractures at level L2 to L5 needed infiltration therapy of Baastrup’s syndrome at level L4/L5 to achieve successful pain relief. In this case, Baastrup’s syndrome was induced by some height loss due to the vertebral compression. Another female patient with augmentation of a proven L2 vertebral fracture suffered from persistent back pain that finally had to be attributed to an additional spondylolisthesis at level L4/L5. In one male patient with vasculitis and multiple secondary steroid-induced osteoporotic vertebral fractures, vertebroplasty only resulted in short-term pain reduction with an overall disappointing treatment result with successive multiple fractures and death because of complications of the underlying vasculitis. In all these cases, however, symptomatic vertebral fractures had been proved by local pain on palpation, MRI with endplate impression and the respective bone marrow edema indicating the actual fracture. Despite adequate technical results, these procedures were not effective with repect to durable pain relief.
Cement leakages were detected by CT. There was an overall leakage rate of 55.4% (Table 5). Each small cement leakage detectable with CT was registered, single source leakages as well as complex combinations of leak sources. The cement volume applied per intervention decreased during the 5-year study period; however, leakage rates were not linked to the cement volumes used. Depending on the physicochemical conditions of the different types of PMMA cements, the overall leakage rate was 34.3% for cement a (usage from June 2002 to December 2003), 57.7% for cement b (August 2002 to January 2006), and 59.3% for cement c (December 2005 to August 2007), respectively. There were no differences between vertebrae with unilateral and with bilateral accesses. The leaks into the epidural space of the spinal canal included leakages into the epidural venous plexus (16%), cement propagation directly through the fractured posterior vertebral wall (2.6%), and cement propagation into the neuroforamina and the epidural space (1.6%). These numbers included five cases (1.1% of all vertebroplaties) with larger cement leaks into the spinal canal. Although there was no evidence for any neurological deficits, pain, or other clinical complications, these five deposits had reduced the sagittal spinal diameter and had therefore represented a potential cause of neurological complications (cement volume 3.6 ± 1.2). Three of these patients suffered from pathological vertebral fractures with metastatic posterior wall involvement. In one case with osteoporosis, the fracture directly involved the posterior vertebral wall and resulted in a direct cement dislocation into the spinal canal. The fifth case demonstrated a larger cement deposit in the epidural venous plexus with a narrowing of the spinal canal. However, fortunately none of these morphological findings were accompanied by any clinical symptoms (Fig. 4). The rate of intradiscal leaks through the upper, the lower, and both endplates was 16.2%, 6.6%, and 2.4%, respectively. There was no evidence that intradiscal cement leakage would promote the incidence of secondary adjacent fractures. Two thirds of adjacent fractures occurred in the absence of intradiscal cement leaks. Intravascular cement leakages into paravertebral vessels occurred in 7.2% of the cases. In these cases, the cement application was immediately interrupted and was continued after hardening of the cement in this location. However, in four cases (0.8%) a small volume cement embolism into the pulmonary artery was confirmed by CT. One of those was responsible for the fatal outcome described above.
Large cement deposits in the spinal canal. Fig. 4 a and b: 73-year-old man with history of bronchial carcinoma and metastatic vertebral fracture. Parasagittal cement deposit (arrows) through the destroyed posterior vertebral wall into the spinal canal with reduction of the diameters. Fig. 4c and d: 60-year-old male with COPD, osteoporosis, and vertebral fracture. CT after vertebroplasty demonstrates a significant cement leak (arrows) through the posterior vertebral wall, covered by the longitudinal ligament, but with reduction of the spinal diameters. Both patients were asymptomatic
Discussion
Over the past 5 years, vertebroplasty has become a standard clinical procedure in our hospital using combined CT-guidance and lateral fluoroscopy [7, 16]. This set-up provides a straightforward and safe positioning of the cannula and gives excellent online control of the posterior vertebral wall during cement application. CT allows for an early detection of cement spread into the basivertebral and epidural veins or direct cement leakage through fracture lines. The alternating lateral conventional fluoroscopy gives additional intermittent control over the cranio-caudal cement spread and helps to realize the distribution pattern of the cement within the vertebral body in all directions (x, y, and z-axis). In our study, the majority of patients undergoing vertebroplasty suffered from compression fractures caused by severe osteoporosis, and two thirds of our patients were female. Despite the obviously beneficial clinical results of these treatments, the clinical complications prompted a critical review of our cases.
The overall complication rate in our series was acceptable low. However, there were two severe fatal adverse events, one of which was directly related to the procedure. In this case, a small venous cement leak developed, which prompted an immediate interruption of the injection. However, although the displaced cement volume was very small, in the context with underlying severe COPD and coronary heart disease, it could have contributed to the deleterious outcome. From the technical aspect, there was no increased pressure at cement injection, and the injection was immediately interrupted. The volume of cement was 3 ml, which is in the lower range of the volumes we normally used, and at that time, there was a sufficiently large experience with this type of cement (437th procedure overall, 78th procedure with the particular cement). Moreover, during the treatment, there was no evidence for pulmonary deterioration detectable by either clinical symptoms, change of oxygen saturation, or altered heart rate. Therefore, the procedure was continued and completed as a routine. The clinical symptoms started with delay and may not be exclusively related to the small volume of cement. Beside the mechanical occlusion of few subsegmental pulmonary arteries, the cement might have induced additional toxic effects to the bronchi and the vascular system, resulting in bronchospasm and pulmonary congestion. The vascular occlusion might have induced apposition thrombi in the tributary smaller vessels and/or in the immediate adjacent vessel segments; however, the larger diameter pulmonary vessels were patent at CT scans (Fig. 1). Beside PMMA-cement embolism, a concomitant fatty bone marrow embolism might be discussed as a potential cofactor, since the insertion of cement volume inevitably causes some expulsion of bone marrow [17]. Cement propagation via paravertebral veins into the inferior vena cava and pulmonary embolism has been described in several case reports as possible cause for hypotension, arrhythmia, and hypocapnia [17–21]. In our particular case, the beginning of the clinical symptoms was 30 min after completion of the procedure. Therefore, a combination of the mechanical vascular embolic obstruction, toxic reactions, and the underlying pulmonary diseases might have contributed to the fatal outcome. In retrospective analysis, pulmonary cement embolism has been described in 4.6% to 8.1% of the cases [10, 22], with one of eight patients being symptomatic [10]. Experimental data have demonstrated that high-viscosity cements could probably reduce the leakage rate to avoid those complications completely in future. However, this would be associated with increased injection forces, which is not yet technically solved [23]. The second severe adverse event coincided with the vertebroplasty procedure, but was not procedure-related. This patient suffered from acute coronary syndrome within 12 after the procedure and was transferred to the intensive care unit. He died 78 days later because of various associated complications.
The overall clinical morbidity was 2.1%. The one case with a missing patellar tendon reflex occurred in a patient with a metastatic destruction of a vertebral pedicle. In addition to direct cement propagation through defective bone, the area of the intervertebral foramina may also be reduced by retrograde filling of intraforaminal radicular veins and thereby induce radicular complaints [10, 24]. In our study, neuroforaminal cement deposits occurred in 1.6%, which had a potential risk for radiculopathy, but there was no evidence for respective complaints in these cases. Two more procedure-related complications were related to conscious sedation, one hypoxia already prior to the start of the intervention and one ineffective peri-interventional analgesia. Although procedure-related, these were not linked to the interventional technique itself. Carrying out the whole procedure under local anesthesia has considerable advantages compared to general anesthesia and is therefore preferred in our department. Besides avoiding the intrinsic risks of cardiovascular and respiratory complications of general anesthesia in older patients, local anesthesia allows for a continuous communication with the patient in order to early detect radicular pain, neurological deficits, and other complications during the procedure. Moreover, this proceeding is cost effective and easy to perform without a need for expensive intermediate care or even intensive care in most cases. The medullary conus syndrome was induced by post-interventional collapse of the patient and was therefore associated, but not procedure-related, as the missing pain relief in a case with a persistent pedicle fracture was not. However, in this particular case we could have performed a pediculoplasty in the same procedure in order to stabilize this fracture. Concomitant fractures of ribs, transverse process fractures, and pedicle fractures have been described in the literature [10, 17]. Pedicle fractures may be a primary finding of the vertebral compression or might be induced by the passage of the cannula during the procedure. New osteoporotic rib fractures are supposed to occur when the patient is placed in prone position on the table for and during the procedure. However, they might significantly bias the clinical outcome regarding pain relief and should be treated with analgetic drugs for an appropriate period.
In our study, the 55.4% extraosseous leak rate was CT-based and comprises very small leakage volumes in the majority of cases. However, it is well known that the rate of extraosseous leakage obviously depends on the sensitivity of the imaging modality used. CT-based analysis results in much higher leak rates compared to conventional X-ray analysis [9, 12]. Reported leakage rates of studies should therefore not be compared directly without mentioning the method of leak detection. Layton et al. reported an overall leak rate of 25% based on fluoroscopically visualized cement deposits [10]. The authors stated that their leak rate would have been higher if CT scans had been used. Our results and those of other groups demonstrate that morphological findings alone should not be rated as complications since the numbers and the amount of an extraosseous cement deposit alone do not correlate with clinical complications. Therefore, the amount of leakage was not calculated in our study, but was very small in the vast majority of cases. Large paravertebral leakages may be completely asymptomatic, whereas small volume intraspinal leaks increase the risk for catastrophic results. CT-fluoroscopy does allow for an early detection of unintended cement distribution through the posterior wall and might thereby contribute to safety. In the present study, leakages within the spinal canal included cement spreading into the epidural veins and direct leaks through fracture lines of the posterior vertebral walls. During the liquid phase of the bone cement distribution, some leakage into the epidural veins does not necessarily cause clinical complications since cement in epidural veins fills pre-existing anatomical structures and does not reduce or compress the dural sac [9]. However, cement polymerization is accompanied with heat effects that might possibly affect the dural structures. Using CT, the epidural venous leak rate was 16% in our study. The number of larger intraspinal cement deposits as shown in Fig. 4 could be restricted to the five reported cases that could have had a potential risk for neurological complications, but in fact did not have any clinical complaints. In concordance with literature, those clinical complications are fortunately rare, and emergency surgery has been reported to be below 1% [11, 25]. Control of the posterior vertebral wall is, however, crucial for the outcome of the procedure. With respect to anatomy, the boundary of the concave posterior wall surface is hardly detectable on lateral fluoroscopy alone. CT-fluoroscopy permits an online cross-sectional imaging of the posterior vertebral wall. The lateral conventional fluoroscopy in front of the CT gantry provides a monitoring of cranio-caudal cement spread. The combination of both with alternating cross-sectional or lateral online imaging proved to be highly effective to control posterior vertebral wall.
In order to avoid cement extravasation, osseous phlebography has been proposed [26–28]. Since the viscosity of contrast medium differs significantly from the cement, we feel that a pre-treatment contrast injection might only give the impression of putative additional safety. We assume that online imaging with combined cross-sectional and lateral fluoroscopy may facilitate the steerability of the procedure. Large cement volumes and lower viscosity cements have been demonstrated to be associated with an increased incidence of epidural leakages [23, 29]. In a recent paper, Kaufmann et al. reported that there was no association between the cement volume and the clinical results with respect to pain relief and medication use [30]. This is consistent with our experience. The mean cement volume in our patient population has therefore been consequently decreased over the years and is actually about 3.5 ± 1.0 ml. Besides venous leakages, cement leakages have also been reported for paravertebral lumbar arteries [31, 32]. This is also consistent with our results (Table 5), but has so far remained without clinical complications. However, there is a potential for associated arterial embolism into peripheral arteries and might even affect vessels supplying the spinal cord [17]. Because of the small sizes, those arterial cement deposits may be difficult to detect on fluoroscopy. Moreover, there have been reports on medial or lateral deviation of the needle path, which lead to thecal injury, epidural bleeding or intrathecal cement leakage, or paravertebral cement deposits [17]. Those complications have not been found in our patient group and might be avoided by using the CT fluoroscopy.
With our patient population there has been no evidence for peri-procedural infections. However, there have been reports on infected vertebral bodies or epidural abscesses after vertebroplasty, which might be life threatening. Needle biopsy for culture and administration of specific antibiotics has been proposed, and surgical decompression was necessary in particular cases [33–35]. Vats et al. described a fluid-filled cavity within the vertebral bodies surrounding the polymethylmethacrylate as one sign of potential infection. In the light of negative cultures, these authors discussed the importance of polymerase chain reaction (PCR), which finally confirmed the definite diagnosis of bacterial infection [33].
Clinical and experimental data suggest that the cement rigidity reduces the local spinal flexibility, increases the intradiscal pressure, and propagates secondary adjacent fractures [13, 36]. Our data demonstrated secondary fractures in 17.1% of patients. Since the actual analysis included all procedures up to now, it might be anticipated that the fracture rate will somewhat increase during follow-up. However, the numbers of secondary fractures at adjacent locations was comparable to those at distant locations, meaning that there was no increased risk for secondary fractures in the immediate vicinity of an already augmented vertebra. This is consistent with Trout et al., who reported on 186 secondary vertebral fractures in 86 (19.9%) of 423 patients. These authors demonstrated a 41.4% rate of adjacent fractures with a shorter time to fracture compared to non-adjacent fractures [37]. Syed et al. reported on 41 adjacent fractures among all 78 secondary fractures. Only 13 of 41 adjacent fractures occurred in the presence of pre-existing intradiscal cement leaks [38]. Our data confirm these findings and showed no increased rate of secondary adjacent fractures as well as no increased secondary fracture rate in cases with intradiscal cement deposits. There has been no separate calculation of the intradiscal leak volumes in those cases. However, since the majority of secondary adjacent fractures occurred in the absence of those leakages, the knowledge of the leak volumes would not have a relevant impact on the secondary adjacent fracture rate.
Lindsay et al. have analyzed the risk of new vertebral fractures in the year following the first osteoporotic fracture [1]. The authors demonstrated that the intrinsic risk for secondary osteoporotic vertebral fractures increased with the number of fractures at baseline. In cases with ≥2 fractures at baseline, the secondary fracture rate was 24% during the 1st year [1]. We, therefore, suggest that the secondary fracture rate in our patient population was caused by the underlying osteoporosis rather than by the previously performed vertebral augmentation. Particularly in the specific situation of sandwich vertebrae, the secondary fracture rate was about one third without evidence for secondary fractures in the other two thirds, meaning that even this particular anatomical situation is not necessarily the only precondition for secondary fractures.
Conclusion
CT-guided vertebroplasty is safe and effective for treatment of vertebral compression fractures. CT-fluoroscopy provides an excellent control of the posterior vertebral wall and thereby contributes to the safety of the procedure. The number of extraosseous cement leakages is not directly associated with clinical complications and depends on the imaging modality used. Pulmonary embolism of PMMA cement, however, may be associated with a fatal outcome in cases with underlying cardiopulmonary insufficiency, even with small volumes of displaced cement.
References
Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E (2001) Risk of new vertebral fracture in the year following a vertebral fracture. JAMA 285:320–323
Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR (1999) Vertebral fractures and mortality in older women. Arch Intern Med 159:1215–1220
Silverman SL (1992) The clinical consequences of vertebral compression fracture. Bone 13(suppl2):S27–S31
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population based study of survival after osteoporotic fractures. Am J Epidemiol 137:1001–1005
Debussche-Depriester C, Deramond H, Fardellone P (1991) Percutaneous vertebroplasty with acrylic cement in the treatment of osteoporotic vertebral crush fracture syndrome. Neuroradiology 33:149–152
Deramond H, Depriester C, Galibert P, Le Gars D (1998) Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications, and results. Radiol Clin North Am 36:533–546
Gangi A, Dietmann JL, Guth S, Steib JP, Roy C (1999) Computed tomography (CT) and fluoroscopy-guided vertebroplasty: Results and complications in 187 patients. Sem Intervent Radiol 16:137–142
Alvarez L, Alcaraz M, Perez-Higueras A, Granzio JJ, deMiguel I, Rossi RE, Quinones D (2006) Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine 31:1113–1118
Mousavi P, Roth S, Finkelstein J, Cheung G, Whyne C (2003) Volumetric quantification of cement leakage following percutaneous vertebroplasty in metastatic and osteoporotic vertebrae. J Neurosurg 99:56–59
Layton KF, Thielen KR, Koch CA, Luetmer PH, Lane JI, Wald JT, Kallmes DF (2007) Vertebroplasty, first 1000 levels of a single center: evaluation of the outcomes and complications. Am J Neuroradiol 28:683–689
Guglielmi G, Andreula C, Muto M, Gilula MA (2005) Percutaneous vertebroplasty: indications, contraindications, technique, and complications. Acta Radiol 46:256–268
Schmidt R, Cakir B, Mattes T, Wegener M, Puhl W, Richter M (2005) Cement leakage during vertebroplasty: an underestimated problem? Eur Spine 14:466–473
Komemushi A, Tanigawa N, Kariya S, Kojima H, Shomura Y, Komemushi S, Sawada S (2006) Percutaneous vertebroplasty for osteoporotic compression fracture: multivariate study of predictors of new vertebral body fracture. Cardiovasc Intervent Radiol 29:580–585
Tanigawa N, Komemushi A, Kariya S, Kojima H, Shomura Y, Sawada S (2006) Radiological follow-up of new compression fractures following percutaneous vertebroplasty. Cardiovasc Intervent Radiol 29:92–96
Berlemann U, Ferguson SJ, Nolte L-P, Heini PF (2002) Adjacent vertebral failure after vertebroplasty. J Bone Joint Surg (Br) 84-B:748–752
Pitton MB, Herber S, Bletz C, Drees P, Morgen N, Koch U, Böhm B, Eckardt A, Düber C (2008) CT-guided vertebroplasty in osteoprotic vertebral fracturesIncidence of secondary fractures and impact of intradiscal cement leakages during follow-up. European Radiology 18:43–50
Baumann C, Fuchs H, Kiwit J, Westphalen K, Hierholzer J (2007) Complications in percutaneous vertebroplasty associated with puncture or cement leakage. Cardiovasc Intervent Radiol 30:161–168
Baumann A, Tauss J, Baumann G, Tomka M, Heussinger M, Tiesenhausen K (2006) Cement embolization into the vena cava and pulmonal arteries after vertebroplasty: interdiciplinary management. Eur J Vasc Endovasc Surg 31:558–561
Lim KJ, Yoon SZ, Jeon YS, Bahk JH, Kim CS, Lee JH, Ha JW (2007) An intraatrial thrombus and pulmonary thromboembolism as a late complication of percutaneous vertebroplasty. Anesth Analg 104:924–926
Padovani B, Kasriel O, Brunner P, Peretti-Viton P (1999) Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. Am J Neuroradiol. 20:375–377
Freitag M, Gottschalk A, Schuster M, Wenk W, Wiesner L, Standl TG (2006) Pulmonary embolism caused by polymethylmetacrylate during percutaneous vertebroplasty in orthopedic surgery. Acta Anaesthesiol Scand 50:248–251
Choe DH, Marom EM Ahrar K (2004) Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. Am J Roentgenol 183:1097–1102
Baroud G, Crookshank M, Bohner M (2006) High-viscosity cement significantly enhances uniformity of cement filling in vertebroplasty: an experimental model and study on cement leakage. Spine 31:2562–2568
Chen JK, Lee HM, Shih JT, Hung ST (2007) Combined extraforminal and intradiscal cement leakage following percutaneous vertebroplasty. Spine 32:E358–E362
Hochmuth K, Proschek D, Schwarz W, Mack M, Kurth AA, Vogl TJ (2006) Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol 16(5):998–1004
Vasconcelos C, Gailloud P, Beauchamp NJ, Heck DV, Murphy KJ (2002) Is percutaneous vertebroplasty without pretreatment venography safe? Evaluation of 205 consecutive procedures. Am J Neuroradiol 23:913–917
Mc Graw JK, Heatwole EV, Strnad BT, Silber JS, Patzilk SB, Boorstein JM (2002) Predictive value of intraosseous venography before percutaneous vertebroplasty. J Vasc Interv Radiol 13:149–153
Gaughen JR Jr, Jensen ME, Schweickert PA, Kaufmann TJ, Marx WF, Kallmes DF (2002) Relevance of antecedent venography in percutaneous vertebroplasty fort he treatment of osteoporotic compression fractures. Am J Neuroradiol 23:594–600
Ryu KS, Park CK, Kim MC, Kang JK (2002) Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg 96(1 Suppl):56–61
Kaufmann TJ, Trout AT, Kallmes DF (2006) The effects of cement volume on clinical outcome of percutaneous vertebroplasty. Am J Neuroradiol 27:1933–1937
Amoretti N, Hovorka I, Marcy PY, Grimaud A, Brunner P, Bruneton JN (2007) Aortic embolism of cement: a rare complication of lumbar percutaneous vertebroplasty. Skeletal Radiol 36:685–687
Hodler J, Peck D, Gilula LA (2003) Midterm outcome after vertebroplasty: predictive value of technical and patient-related factors. Radiology 227:662–668
Vats HS, McKiernan FE (2006) Infected vertebroplasty: case report and review of the literature. Spine 31:E859–E862
Söyüncü Y, Ozdemir H, Söyüncü S, Bigat Z, Gür S (2006) Posterior spinal epidural abscess: an unusual complication of vertebroplasty. Joint Bone Spine 73:753–755
Olmos AM, González SA, Clemente DJ, Tomé VC (2006) Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure: report of a case and a review of the literature. Spine 31:E770–E773
Baroud G, Bohner M (2006) Biomechanical impact of vertebroplastyPostoperative beiomechanics of vertebroplasty. Joint Bone Spine 73:144–150
Trout AT, Kallmes DF, Kaufmann TJ (2006) New fractures after vertebroplasty: adjacent fractures occur significantly sooner. Am J Neuroradiol 27:217–223
Syed MI, Patel NA, Jan S, Harron MS, Morar K, Skaikh A (2005) Intradiscal extravasation with low-volume cement filling in percutaneous vertebroplasty. Am J Neuroradiol 26:2397–2401
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pitton, M.B., Herber, S., Koch, U. et al. CT-guided vertebroplasty: analysis of technical results, extraosseous cement leakages, and complications in 500 procedures. Eur Radiol 18, 2568–2578 (2008). https://doi.org/10.1007/s00330-008-1020-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1020-z