Abstract
Computed tomography pulmonary angiography (CTA) has increasingly become accepted as a widely available, safe, cost-effective, and accurate method for a quick and comprehensive diagnosis of acute pulmonary embolism (PE). Pulmonary catheter angiography is still considered the gold standard and final imaging method in many diagnostic algorithms. However, spiral CTA has become established as the first imaging test in clinical routine due to its high negative predictive value for clinically relevant PE. Despite the direct visualization of clot material, depiction of cardiac and pulmonary function in combination with the quantification of pulmonary obstruction helps to grade the severity of PE for further risk stratification and to monitor the effect of thrombolytic therapy. Because PE and deep venous thrombosis are two different aspects of the same disease, additional indirect CT venography may be a valuable addition to the initial diagnostic algorithm—if this was positive for PE—and demonstration of the extent and localization of deep venous thrombosis has an impact on clinical management. Additional and alternate diagnoses add to the usefulness of this method. Using advanced multislice spiral CT technology, some practitioners have advocated CTA as the sole imaging tool for routine clinical assessment in suspected acute PE. This will simplify standards of practice in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its mostly unspecific clinical presentation, pulmonary embolism (PE) is often referred to as the great masquerader and remains a diagnostic challenge. PE is a common disorder occurring in some 600,000–630,000 patients per year in the United States [1–3]. It has been estimated that the diagnosis of PE will not be established in approximately 70% of patients who survive the initial thromboembolic event, accounting for 400,000 episodes per year. Of these patients, 120,000 will die from the disease (Fig. 1). The clinical scenario is crucial for assessing the likelihood of PE. Accordingly, distinct diagnostic algorithms are needed to assist clinical assessment and optimize the use of diagnostic tests, especially in an emergency department setting.
Incidence of pulmonary embolism in the United States (after [1]).
A chest radiograph is usually the first imaging study performed in patients with suspected PE [4]. However, conventional X-rays are of limited value in this respect [5]. For a long time, pulmonary catheter angiography has been advocated as the gold standard in the diagnostic work-up of suspected PE. Although the accuracy of classical pulmonary angiography is beyond dispute, only a small percentage of patients undergo study—even patients in whom diagnosis of PE was not adequately confirmed or excluded after ventilation/perfusion (V/Q) scintigraphy [6, 7]. Practically, this standard of reference has not been widely accepted [8].
Nuclear medicine V/Q scintigraphy has for long been the clinical mainstay for the evaluation of suspected PE. This traditional imaging test technique is noninvasive, inexpensive, and readily available in the acute care setting. It has a high sensitivity, which is why it remains a primary PE screening technique [9, 10]. However, the high sensitivity of perfusion scanning is associated with a relatively low specificity [11]. Furthermore, V/Q scintigraphy has a poor spatial resolution and provides only indirect evidence for PE [12]. Venous thromboembolism is not directly visualized but rather its effects on perfusion and ventilation [13]. These problems cause the need for probability criteria, categorized as high, intermediate, low, or very low probability and normal [14]. The main practical problem is evident in the large group of patients with intermediate or indeterminate probability when the classic PIOPED criteria are applied [15].
Single-slice CTA
Spiral CTA emerged in the 1990s as a new diagnostic technique. The basis of PE assessment on CTA is the direct visualization of clot material in the pulmonary arteries. The first CTA protocols with 5-mm-thick sections were suitable for visualization of the central (and segmental) pulmonary arteries. However, initial data suggested that this technique was not ideally suited as a first-line imaging tool: Drucker et al. [16] found an interobserver variation of 53–60% (sensitivity) and 81–97% (specificity) for diagnosing acute PE using pulmonary catheter angiography as the standard. Other CTA studies also had less than ideal sensitivity rates, especially on the subsegmental level [17, 18]. It is now known that 5-mm-thick sections are inaccurate for PE diagnostics [19, 20].
Despite a variety of scanning parameters, sophisticated protocols optimize the trade-off between effective slice thickness and the area covered. Thin collimation spiral CTA is mandatory for assessing peripheral PE [21]. However, only the central 10–15 cm of the pulmonary vasculature can be assessed within a single inspiration breath-hold acquisition.
Remy-Jardin et al. [22, 23] reported a higher sensitivity and specificity for thinner slices (3 vs 5 mm) using a high-pitch protocol (1.7). The main drawback of a 5-mm collimation is the partial volume effect, which may limit detection of subsegmental and segmental filling defects [24]. Therefore, the central portion of the thorax was examined within a 20-s inspiration breath-hold using 3-mm collimation protocols at 1-s tube rotation times (level of inferior pulmonary veins to aortic arch, z-coverage 10 cm). On the basis of a small prospective patient study with separate analysis of 20 different arterial territories, 3-mm collimated helical CTA provided good interobserver agreement for the main, lobar, and segmental level. Dedicated analysis of the subsegmental territories, however, had a high incidence of nonvaluable branches and poor interobserver agreement [25].
Use of subsecond single-slice spiral CT scanners (tube rotation times 0.75 s) further reduced the partial volume effect [24]. Selecting a 2-mm collimation and a pitch of 2.0 significantly improved evaluation of subsegmental pulmonary arteries on CTA examination [23].
In addition, radiation exposure of helical CT was lower than that of classical pulmonary angiography [26]. Resten et al. [27] performed measurements of radiation dose using a predefined standardized CTA acquisition protocol and compared the results with a “standard” selective pulmonary arteriogram, using an anthropomorphic phantom and thermoluminescent dosimetry. Average dose was 4.375 times lower for CT (6.4±1.5 mGy vs 28±7.6 mGy).
It has been suggested [12, 28–30] that spiral CTA should replace ventilation–perfusion radionuclide scanning as the initial test for screening patients with suspected PE by using advanced single-slice CT examination protocols with 2- and 3-mm-thick sections. In 1999, Anderson and Wells [31] reported spiral CTA to be both a more sensitive and specific test (at least for central PE), although there are much more data on the use of diagnostic algorithms with lung scanning. A number of comparative studies led to a new definition on the impact of spiral CTA in this respect. Both techniques are rated equally highly in the literature [11, 32–34]. Due to limitations for accurate diagnosis of isolated peripheral PE with single-slice spiral CT, CTA has not yet become accepted as the reference standard [35]. Patients should therefore undergo either lung scanning or single-slice spiral CT imaging, depending on availability and expertise (Fig. 2). However, CTA as a second procedure after an abnormal V/Q scintigraphy had limited value in the clinical setting and vice versa [36, 37]. A survey by Schibany et al. [38] on equipment availability in Austria showed that CTA was the imaging tool of choice in 56% of hospitals, with V/Q scintigraphy in second place (43%). However, scintigraphy was not available in many hospitals (scintigraphy 19%, CTA 54%). Where available, it was used for a large number of patients each year.
Diagnostic algorithm including single-slice spiral CT (after [11]).
Multislice CTA
The introduction of multislice spiral CT was a milestone in CTA technology. Dual-section CT [39] and four-slice multi–detector row computed tomography (MDCT) [40–45] show promising results regarding PE diagnostics. Today, 16-slice MDCT is gaining in popularity because it allows a comprehensive evaluation in a short period of time [35]. Compared with single-slice CT, MDCT can more precisely delineate clots down to the subsegmental level: third subsegmental branches can be assessed with 4×2.5-mm collimation [40], and delineation of arteries down to the 5th and 6th order can be done with 4×1-mm collimation [42]. Using thin sections (approx. 1 mm) significantly decreases the number of arteries classified as indeterminate (approx. 70% less) [43] and improves interobserver agreement in detection of PE (1.25 vs 2.5-mm collimation) [44]. Combining such a dedicated examination protocol with additional 3D shaded-surface display reconstruction images allows precise anatomic analysis of peripheral pulmonary arteries [46].
Four-slice MDCT with 0.5-s rotation time and simultaneous acquisition of four slices already yields an up to eightfold increase in performance compared with 1.0-s single-slice spiral CT, which may be distributed over volume, time, and axial resolution [47]. Therefore, thin collimation CTA of the entire chest can now be done within a single breath-hold even in critically ill patients [45].
The overall image quality was rated as superior for four-slice MDCT compared with subsecond single-slice CT, allowing accurate interpretation down to the subsegmental arteries more often even in otherwise healthy patients (65% vs 10%) as well as in patients with underlying respiratory disease (47% vs 15.5%). Recently, using advanced tomographic imaging techniques, SPECT V/Q scintigraphy and four-slice MDCT both yielded an excellent and absolutely comparable diagnostic accuracy down to the subsegmental level [48]. CTA has been recommended as an initial imaging method, even for nonmassive PE. Technical parameters are becoming a more important issue in diagnostic algorithms (single-slice vs multislice) [49]. Effective dose equivalents according to ICRP 60 were calculated as 3.3 mSv (male patients) and 4.2 mSv (female) for CTA exams using four-slice MDCT technology (4×1-mm collimation, 120 kVp, 100 mAseff, z-coverage 293±26.8 mm for men, 282±27.8 for women) [50]. These dose equivalents are in accordance with another study performed by Kuiper et al. [51]. Using a 4×1-mm collimation protocol (120 kVp, 90–160 mAseff), effective dose equivalents were in the range of 2.2–6.0 mSv, based on the CTDI. In comparison to the dose–area product for pulmonary arteriograms, radiation dose was slightly lower for MDCT (average 4.2 vs 7.1 mSv, range 3.3–17.3 mSv).
In principle, retrospective ECG gating of the whole chest can now be done using MDCT during a single breath-hold. For PE assessment, reduction of motion artifacts might be advantageous, especially in the pericardiac arteries. However, no additional relevant diagnostic information was gained for four-slice MDCT. Longer scan times (35–42 s) and breath-holds make the procedure more difficult for the patient [52]. Even with eight-slice MDCT, ECG gating does not influence the diagnostic accuracy and interobserver agreement [53]. Furthermore, ECG-gated acquisitions require a considerably reduced (overlapping) pitch factor and usually higher tube–current time settings, which will result in a higher radiation dose to the patient. Therefore, ECG gating cannot currently be recommended for routine assessment of acute PE.
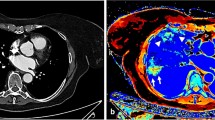
In daily clinical practice, standard MDCT with thin collimation—e.g., 4×1, 4×2.5, 8×1.25, 16×0.75 mm—has practically become the method of choice for imaging of the pulmonary circulation in patients suspected of having PE (Figs. 3 and 4) [35]. According to the updated guidelines of the British Thoracic Society, no further examination or treatment is needed for patients with a high-quality negative MDCT CTA [3, 49]. Moreover, the fast data acquisition and real-time image reconstruction allow for a distinct examination protocol. Because PE and deep venous thrombus are two different aspects of the same disease, a combined examination may be a valuable addition to the initial diagnostic algorithm [37]. Indirect CT phlebography (CTP) can therefore be an option for complete assessment of venous thromboembolism (Fig. 5). Several authors have stressed that the uncritical use of indirect CT venography may increase radiation exposure to the population [26, 50, 54]. CT venography should therefore only be performed in addition to CTA of the pulmonary arteries, if this was positive for PE and demonstration of the extent and localization of deep venous thrombosis has an impact on clinical management, as radiation exposure is quite high for MDCT (up to 9.3 mSv) [50, 54, 55].
Small subsegmental PE in the left anterior lobe artery. Small clot material can be delineated on CTA (white arrow), with corresponding areas of decreased aeration in the adjacent lung tissue (black arrows) (120 kVp, 100 mAseff; 16×0.75 mm; rotation time 0.5 s; reconstructed effective slice thickness 1 mm).
Accuracy
The clinical impact of isolated subsegmental PE has been a matter of controversy. On the one hand, small emboli may indicate a risk for recurrent, more significant emboli. Also, patients with underlying cardiorespiratory disease may be impaired. On the other hand, otherwise healthy patients with isolated subsegmental PE may not be at significantly increased risk for morbidity and mortality. The number of isolated subsegmental PEs falls in the range of 6–30% [56–58].
There is a recent trend to analyze the overall clinical accuracy of diagnostic tests. Although data on sensitivity and specificity will always be important, the clinical outcome of patients with negative diagnostics is even more important. A number of studies has shown that the risk of PE after an initial negative CTA is about 1%. Of 3,713 patients with a minimum clinical follow-up of 3 months, 44 recurrent cases of PE were documented. Interestingly, the examination technique itself did not have a significant impact in this regard. Studies were done with EBCT [59], single-slice CT [28, 60–70], and multislice CT [45, 71]. Secondary imaging rarely added clinically important findings, even for high-risk oncologic patients with initially negative CTA results [37].
Quantitative CT scores for PE are based on the overall amount of clot burden within the pulmonary vasculature and were adapted from angiographic indices. The degree of pulmonary artery obstruction may be relevant for patient treatment [72]. There is a good correlation with the clinical severity of PE [73–77]. The vascular obstruction index introduced by Qanadli et al. [74] was highly correlated with pulmonary artery obstruction on pulmonary angiography (r=0.867, p=0.0001; assessed by the Miller index [78]). It scores the level of obstruction (according to the anatomic subdivisions involved) and adds a weighting factor for the degree of vascular obstruction. This quantitative specific CT index showed an excellent concordance (CT: r=0.944, p<0.0001; pulmonary angiography: r=0.904, p<0.0001). Collomb et al. [77] suggested measuring four main criteria for assessment of the hemodynamic severity of PE: the vascular obstruction index, the diameter of the central pulmonary artery, the minimum diameter of the left ventricle, and the right ventricle to left ventricle ratio, respectively.
A pulmonary artery diameter of >30 mm indicates pulmonary artery pressure >20 mmHg [79], which is defined as acute pulmonary hypertension in this respect [80].
In spite of a direct demonstration of intravascular thrombi, secondary findings are common, such as wedge-shaped opacities within the adjacent lung parenchyma. CT can also identify characteristic heart changes, e.g., acute dilatation of the right heart as well as abnormal and characteristic interventricular septal shift, as the transseptal pressure gradient is reversed and the septum is bulged convex toward the left ventricle (Fig. 6) [81–84]. The consequence of severe PE on the heart can be accounted for in the following way: sudden obstruction of the pulmonary arteries results in increased vascular resistance, causing acute pulmonary hypertension and right ventricle afterloading [77, 85]. Collomb et al. [77] considered the right ventricle to left ventricle ratio as an easy assessable secondary sign for the severity of PE in everyday practice.
a Acute and subacute PE on the central, lobar, and segmental levels. b Distal to a, a pleural-based consolidation is seen (arrow). c Sixteen-slice MDCT also identified characteristic heart changes, with acute dilatation of the right heart, and abnormal and characteristic interventricular septal shift with bulging of the septum toward the left ventricle in this four-chamber view. d Additional indirect CT phlebography depicted bilateral femoral vein thrombosis (arrows).
Depiction of cardiac and pulmonary function in combination with the quantification of pulmonary obstruction helps to grade the severity of PE for further risk stratification and to monitor the effect of thrombolytic therapy.
Demonstration of ancillary findings [86] and suggestion of the true alternate diagnoses are clinically extremely beneficial (Fig. 7) [54, 87–90]. Garg et al. [61] reported that CT provided either additional findings or alternate diagnoses in more than half of the examinations interpreted as negative for PE.
Recently, more generally applicable protocols have been introduced. CTA has been evaluated more comprehensively toward a one-stop shopping strategy, including assessment of deep venous thrombosis using indirect CT phlebography [40, 50, 54, 91–93], right-heart geometrical assessment [84], and interactive analysis of nearly isotropic data sets using advanced postprocessing methods [94–96].
Lung scintigraphy remains the first imaging modality where CTA is not available and for patients with contraindications for iodinated contrast media (anaphylaxis, renal failure), as well as in pregnant patients [3]. Real-time and contrast-enhanced magnetic resonance angiography [97–99] may represent a valuable alternative in this respect.
CTA is an appropriate initial test in patients with intermediate and high clinical suspicion of PE under emergency conditions [100]. In patients with a low clinical probability of PE, the most cost-saving strategy involves plasma D-dimer assessment, a degradation product of cross-linked fibrin [101, 102]. Due to its high sensitivity, D-dimer ELISA has been advocated as the first-line test for ruling out PE in outpatients, provided the assay has been validated in an outcome study [33]. D-dimer is very specific for fibrin. However, the specificity of fibrin for venous thromboembolism is poor. Fibrin is produced in a wide variety of conditions, such as cancer, inflammation, infection, and necrosis. Additionally, it is not useful in elderly patients or inpatients, as it is normal in less than 10% of patients [33].
From a radiological point of view, CTA has effectively become the de facto first imaging test in clinical routine, as patients with a high-quality negative CTA do not require further examination or treatment for suspected PE (Fig. 8). The 32-, 40-, and 64-slice MDCT, which are already available, will further improve the overall image quality, especially in the pericardial region (Figs. 9 and 10). We are likely to see further technical developments in CT technology in the near future.
Maximum intensity projection (MIP) from a standard 64-slice MDCT data set without ECG pulsing: Note the sharp and distinct anatomic detail of the pericardial region, without cardiac motion artifacts (120 kVp, 100 mAseff; 64 (2×32)×0.6 mm; resolution 0.4 mm, tube rotation time 0.5 s; total time for data acquisition 7 s for 350 mm).
Nevertheless, our clinical partners who refer patients for diagnostic testing must still be convinced. Hopefully, not only the British Thoracic Society [49], but many others will create new standards of practice and release updates of existing guidelines.
References
Dalen JE, Alpert JS (1975) Natural history of pulmonary embolism. Prog Cardiovasc Dis 17:259–270
Lilienfeld DE, Chan E, Ehland J, Godbold JH, Landrigan PJ, Marsh G (1990) Mortality from pulmonary embolism in the United States. Chest 98:1067–1072
Goldhaber SZ, Elliott CG (2003) Acute pulmonary embolism, Part 1: epidemiology, pathophysiology, and diagnosis. Circulation 108:2726–2729
Coche E, Verschuren F, Hainaut P, Goncette L (2004) Pulmonary embolism findings on chest radiography and multislice spiral CT. Eur Radiol 14:1241–1248
Greenspan RH, Ravin CE, Polansky SM, McLoud TC (1982) Accuracy of chest radiograph in diagnosis of pulmonary embolism. Invest Radiol 17:539–543
Fishman EK, Horton KM (2000) CT of suspected pulmonary embolism: study design optimization. Am J Roentgenol 175:1002–1003
Sostman HD, Ravin CE, Sullivan DC (1982) Use of pulmonary angiography for suspected pulmonary embolism: influence of scintigraphic diagnosis. Am J Roentgenol 139:673–677
Harvey RT, Gefter WB, Hrung JM, Langlotz CP (2000) Accuracy of CT angiography versus pulmonary angiography in the diagnosis of acute pulmonary embolism: evaluation of the literature with summary ROC curve analysis. Acad Radiol 7:786–797
Banks BF (1998) Diagnosis and treatment of acute pulmonary thromboembolic disease. Appl Radiol 27: S27–S33
Goldhaber SZ (1998) Pulmonary embolism. N Engl J Med 339:93–104
Kauczor HU, Heussel CP, Thelen M (1999) Update in diagnostic strategies of pulmonary embolism. Eur Radiol 9:262–275
Garg K, Welsh CH, Feyerabend AJ, Subber SW, Russ PD, Johnston RJ, Durham JD, Lynch DA (1998) Pulmonary embolism: diagnosis with spiral CT and ventilation–perfusion scanning—correlation with pulmonary angiographic results or clinical outcome. Radiology 208:201–208
Smith TP (2000) Pulmonary embolism: what’s wrong with this diagnosis? Am J Roentgenol 174:1489–1497
Parker J, Coleman R, Siegel B, Sostman H, McKusick K, Royal H (1996) Procedure guideline for lung scintigraphy: 1.0 Society of Nuclear Medicine. J Nucl Med 37:1906–1910
PIOPED Investigators (1990) Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis. J Am Med Assoc 263:2753–2759
Drucker EA, Rivitz SM, Shepard JAO, Boiselle PM, Trotman-Dickenson B, Welch TJ, Maus TP, Miller SW, Kaufman JA, Waltman AC, McLoud TC, Athanasoulis CA (1998) Acute pulmonary embolism: assessment of helical CT for diagnosis. Radiology 209:235–241
Goodman LR, Curtin JJ, Mewissen MW, Foley WD, Lipchik RJ, Crain MR, Sagar KB, Collier BD (1995) Detection of pulmonary embolism in patients with unresolved clinical and scintigraphic diagnosis: helical CT versus angiography. Am J Roentgenol 164:1369–1374
VanRossum AB, Pattynama PMT, Ton ER, Treurniet FE, Arndt JW, VanEck B, Kieft GJ (1996) Pulmonary embolism: validation of spiral CT angiography in 149 patients. Radiology 201:467–470
Rathbun SW, Raskob GE, Whitsett TL (2000) Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review. Ann Intern Med 132:227–232
VanBeek EJ, Brouwers EM, Song B, Bongaerts AH, Oudkerk M (2001) Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta-analysis. Clin Appl Thromb Hemost 7:87–92
Remy-Jardin M, Baghaie F, Bonnel F, Masson P, Duhamel A, Remy J (2000) Thoracic helical CT: influence of subsecond scan time and thin collimation on evaluation of peripheral pulmonary arteries. Eur Radiol 10:1297–1303
Remy-Jardin M, Remy J, Deschildre F, Artaud D, Beregi JP, Hossein-Foucher C, Marchandise X, Duhamel A (1996) Diagnosis of acute pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 200:699–706
Remy-Jardin M, Remy J, Artaud D, Deschildre F, Duhamel A (1997) Peripheral pulmonary arteries: optimization of the spiral CT acquisition protocol. Radiology 204:157–163
Remy-Jardin M, Remy J, Artaud D, Fribourg M, Beregi JP (1998) Spiral CT of pulmonary embolism: diagnostic approach, interpretive pitfalls and current indications. Eur Radiol 8:1376–1390
Ruiz Y, Caballero P, Caniego JL, Friera A, Olivera MJ, Tagarro D, Alvarez-Sala R (2003) Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis—interobserver agreement. Eur Radiol 13:823–829
Diederich S (2003) Radiation dose in helical CT for detection of pulmonary embolism. Eur Radiol 13:1491–1493
Resten A, Mausoleo F, Valero M, Musset D (2003) Comparison of doses for pulmonary embolism detection with helical CT and pulmonary angiography. Eur Radiol 13:1515–1521
Blachere H, Latrabe V, Montaudon M, Valli N, Couffinhal T, Raherisson C, Leccia F, Laurent F (2000) Pulmonary embolism revealed on helical CT angiography: comparison with ventilation–perfusion radionuclide scanning. Am J Roentgenol 174:1041–1047
Cross JJL, Kemp PM, Walsh CG, Flower CDR, Dixon AK (1998) A randomized trial of spiral CT and ventilation perfusion scintigraphy for the diagnosis of pulmonary embolism. Clin Radiol 53:177–182
Goodman LR (1998) Helical CT for initial imaging of pulmonary embolus. Am J Roentgenol 171:1153–1154
Anderson DR, Wells PS (1999) Improvements in the diagnostic approach for patients with suspected deep vein thrombosis or pulmonary embolism. Thromb Haemost 82:878–886
Tapson VF, Carroll BA, Davidson BL, Elliott CG, Fedullo PF, Hales CA, Hull RD, Hyers TM, Leeper KV Jr, Morris TA, Moser KM, Raskob GE, Shure D, Sostman HD, Taylor Thompson B (1999) The diagnostic approach to acute venous thromboembolism: clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 160:1043–1066
Task Force on Pulmonary Embolism, European Society of Cardiology (2000) Task force report: guidelines on diagnosis and management of acute pulmonary embolism. Eur Heart J 21:1301–1336
Fedullo PF, Tapson VF (2003) The evaluation of suspected pulmonary embolism. N Engl J Med 349:1247–1256
Schoepf UJ, Goldhaber SZ, Costello P (2004) Spiral computed tomography for acute pulmonary embolism. Circulation 109:2160–2167
Van Strijen MJL, De Monyé W, Kieft GJ, Pattynama PMT, Huisman MV, Smith SJ, Bloem JL (2003) Diagnosis of pulmonary embolism with spiral CT as a second procedure following scintigraphy. Eur Radiol 13:1501–1507
Ginsberg MS, Oh J, Welber A, Panicek DM (2002) Clinical usefulness of imaging performed after CT angiography that was negative for pulmonary embolus in a high-risk oncologic population. Am J Roentgenol 179:1205–1208
Schibany N, Fleischmann D, Thallinger C, Schibany A, Hahne J, Ba-Ssalamah A, Herold CJ (2001) Equipment availability and diagnostic strategies for suspected pulmonary embolism in Austria. Eur Radiol 11:2287–2294
Qanadli SD, El Hajjam M, Mesurolle B, Barré O, Bruckert F, Joseph T, Mignon F, Vieillard-Baron A, Dubourg O, Lacombe P (2000) Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 217:447–455
Müller C, Kopka L, Funke M, Funke C, Grabbe E (2001) Diagnosis of lung embolism and underlying venous thrombosis in multi-slice spiral CT. Fortschr Röntgenstr 173:528–535
Raptopoulos V, Boiselle PM (2001) Multi-detector row spiral CT pulmonary angiography: comparison with single-detector row spiral CT. Radiology 221:606–613
Ghaye B, Szapiro D, Mastora I, Delannoy V, Duhamel A, Remy J, Remy-Jardin M (2001) Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology 219:629–636
Schoepf UJ, Holzknecht N, Helmberger TK, Crispin A, Hong C, Becker CR, Reiser MF (2002) Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology 222:483–490
Patel S, Kazerooni EA, Cascade PN (2003) Pulmonary embolism: optimization of small pulmonary arteries visualization at multidetector row CT. Radiology 227:455–460
Remy-Jardin M, Tillie-Leblond I, Szapiro D, Ghaye B, Cotte L, Mastora I, Delannoy V, Remy J (2002) CT angiography of pulmonary embolism in patients with underlying respiratory disease: impact of multislice CT on image quality and negative predictive value. Eur Radiol 12:1971–1978
Coche E, Pawlak S, Dechambre S, Maldague B (2003) Peripheral pulmonary arteries: identification at multi-slice spiral CT with 3D reconstruction. Eur Radiol 13:815–822
Klingenbeck-Regn K, Schaller S, Flohr T, Ohnesorge B, Kopp AF, Baum U (1999) Subsecond multi-slice computed tomography: basics and applications. Eur J Radiol 31:110–124
Reinartz P, Wildberger JE, Schaefer W, Nowak B, Mahnken AH, Buell U (2004) Tomographic imaging in the diagnosis of pulmonary embolism: comparison between V/Q lung scintigraphy in SPECT technique and multi-slice spiral CT. J Nucl Med 45:1501–1508
British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group (2003) British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 58:470–483
Wildberger JE, Mahnken AH, Sinha AM, Stargardt A, Haage P, Schaller S, Günther RW (2002) A differentiated approach to the diagnosis of pulmonary embolism and deep venous thrombosis using multi-slice CT. Fortschr Röntgenstr 174:301–307
Kuiper JW, Geleijns J, Matheijssen NAA, Teeuwisse W, Pattynama PMT (2003) Radiation exposure of multi-row spiral computed tomography of the pulmonary arteries: comparison with digital subtraction pulmonary angiography. Eur Radiol 13:1496–1500
Schuster A, Frank R, Pallwein L, Gotwald T, Daniaux M, ZurNedden D (2001) Retrospectively ECG-gated multislice CT angiography for the detection of pulmonary embolism: comparison to non-ECG-gated spiral CT imaging. Radiology 221:P256
Marten K, Engelke C, Funke M, Obenauer S, Baum F, Grabbe E (2003) ECG-gated multislice spiral CT for diagnosis of acute pulmonary embolism. Clin Radiol 58:862–868
Ghaye B, Dondelinger RF (2002) Non-traumatic thoracic emergencies: CT venography in an integrated diagnostic strategy of acute pulmonary embolism and venous thrombosis. Eur Radiol 12:1906–1921
Begemann PG, Bonacker M, Kemper J, Guthoff AE, Hahn KE, Steiner P, Adam G (2003) Evaluation of the deep venous system in patients with suspected pulmonary embolism with multi-detector CT: a prospective study in comparison to Doppler sonography. J Comput Assist Tomogr 27:399–409
Stein PD, Henry JW (1997) Prevalence of acute pulmonary embolism in central and subsegmental pulmonary arteries and relation to probability interpretation of ventilation/perfusion lung scans. Chest 111:1246–1248
DeMonyé W, VanStrijen MJL, Huisman MV, Kieft GJ, Pattynama PMT, Advances in New Technologies Evaluating the Localisation of Pulmonary Embolism (ANTELOPE) Group (2000) Suspected pulmonary embolism: prevalence and anatomic distribution in 487 consecutive patients. Radiology 215:184–188
Oser RF, Zuckerman DA, Gutierrez FR, Brink JA (1996) Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology 199:31–35
Swensen SJ, Sheedy PF 2nd, Ryu JH, Pickett DD, Schleck CD, Ilstrup DM, Heit JA (2002) Outcomes after withholding anticoagulation from patients with suspected acute pulmonary embolism and negative computed tomographic findings: a cohort study. Mayo Clin Proc 77:130–138
Lomis NN, Yoon HC, Moran AG, Miller FJ (1999) Clinical outcomes of patients after a negative spiral CT pulmonary angiogram in the evaluation of pulmonary embolism. J Vasc Interv Radiol 10:707–712
Garg K, Sieler H, Welsh CH, Johnston RJ, Russ PD (1999) Clinical validity of helical CT being interpreted as negative for pulmonary embolism: implications for patient treatment. Am J Roentgenol 172:1627–1631
Goodman LR, Lipchik RJ, Kuzo RS, Liu Y, McAuliffe TL, O’Brien DJ (2000) Subsequent pulmonary embolism: risk after a negative helical CT pulmonary angiogram—prospective comparison with scintigraphy. Radiology 215:535–542
Gottsäter A, Berg A, Centergard J, Frennby B, Nirhov N, Nyman U (2001) Clinically suspected pulmonary embolism: is it safe to withhold anticoagulation after a negative spiral CT? Eur Radiol 11:65–72
Ost D, Rozenshtein A, Saffran L, Snider A (2001) The negative predictive value of spiral computed tomography for the diagnosis of pulmonary embolism in patients with nondiagnostic ventilation–perfusion scans. Am J Med 110:16–21
Tillie-Leblond I, Mastora I, Radenne F, Paillard S, Tonnel AB, Remy J, Remy-Jardin M (2002) Risk of pulmonary embolism after a negative spiral CT angiogram in patients with pulmonary disease: 1-year clinical follow-up study. Radiology 223:461–467
Nilsson T, Olausson A, Johnsson H, Nyman U, Aspelin P (2002) Negative spiral CT in acute pulmonary embolism. Acta Radiol 43:486–491
Musset D, Parent F, Meyer G, Maitre S, Girard P, Leroyer C, Revel MP, Carette MF, Laurent M, Charbonnier B, Laurent F, Mal H, Nonent M, Lancar R, Grenier P, Simonneau G, Evaluation du Scanner Spirale dans l’Embolie Pulmonaire study group (2002) Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet 360:1914–1920
VanStrijen MJ, DeMonye W, Schiereck J, Kieft GJ, Prins MH, Huisman MV, Pattynama PM, Advances in New Technologies Evaluating the Localisation of Pulmonary Embolism Study Group (2003) Single-detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study in 510 patients. Ann Intern Med 138:307–314
Donato AA, Scheirer JJ, Atwell MS, Gramp J, Duszak R Jr (2003) Clinical outcomes in patients with suspected acute pulmonary embolism and negative helical computed tomographic results in whom anticoagulation is withheld. Arch Intern Med 163:2033–2038
Friera A, Olivera MJ, Suarez C, Gimenez N, Caballero P, Thromboembolic Study Group of La Princessa Hospital (2004) Clinical validity of negative helical computed tomography for clinical suspicion of pulmonary embolism. Respiration 71:30–36
Kavanagh EC, O’Hare A, Hargaden G, Murray JG (2004) Risk of pulmonary embolism after negative MDCT pulmonary angiography findings. Am J Roentgenol 182:499–504
Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE et al (1992) The clinical course of pulmonary embolism. N Engl J Med 326:1240–1245
Bankier AA, Janata K, Fleischmann D, Kreuzer S, Mallek R, Frossard M, Domanovits H, Herold CJ (1997) Severity assessment of acute pulmonary embolism with spiral CT: evaluation of two modified angiographic scores and comparison with clinical data. J Thorac Imaging 12:150–158
Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, Barre O, Bruckert F, Dubourg O, Lacombe P (2001) New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. Am J Roentgenol 176:1415–1420
Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, Remy J (2003) Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 13:29–35
Wu AS, Pezzulo JA, Cronan JJ, Hou DD, Mayo-Smith WW (2004) CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome—initial experience. Radiology 230:831–835
Collomb D, Paramelle PJ, Calaque O, Bosson JL, Vanzetto G, Barnoud D, Pison C, Coulomb M, Ferretti G (2003) Severity assessment of acute pulmonary embolism: evaluation using helical CT. Eur Radiol 13:1508–1514
Miller GA, Sutton GC, Kerr IH, Gibson RV, Honey M (1971) Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br Med J 19:681–684
Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH (1984) CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 19:16–22
Come PC (1992) Echocardiographic evaluation of pulmonary embolism and its response to therapeutic interventions. Chest 101:S151–S162
Reid JH, Murchison JT (1998) Acute right ventricular dilatation: a new helical CT sign of massive pulmonary embolism. Clin Radiol 53:694–698
Oliver TB, Reid JH, Murchison JT (1998) Interventricular septal shift due to massive pulmonary embolism shown by CT pulmonary angiography: an old sign revisited. Thorax 53:1092–1094
Wintersperger BJ, Stäbler A, Seemann M, Holzknecht N, Helmberger T, Fink U, Reiser MF (1999) Evaluation of right ventricular performance in patients with acute pulmonary embolism by helical CT. Fortschr Röntgenstr 170:542–549
Quiroz R, Kucher N, Schoepf UJ, Kipfmueller F, Solomon SD, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: prognostic role in acute pulmonary embolism. Circulation 109:2401–2404
Elliott CG (1992) Pulmonary physiology during pulmonary hypertension. Chest 101:S163–S171
Ghaye B, Remy J, Remy-Jardin M (2002) Non-traumatic thoracic emergencies: CT diagnosis of acute pulmonary embolism: the first 10 years. Eur Radiol 12:1886–1905
Kim KI, Müller NL, Mayo JR (1999) Clinically suspected pulmonary embolism: utility of spiral CT. Radiology 210:693–697
Remy-Jardin M, Remy J (1999) Spiral angiography of the pulmonary circulation. Radiology 212:615–636
Garg K, Macey L (2003) Helical CT scanning in the diagnosis of pulmonary embolism. Respiration 70:231–237
MacDonald SL, Mayo JR (2003) Computed tomography of acute pulmonary embolism. Semin Ultrasound CT MR 24:217–231
Cham MD, Yankelevitz DF, Shaham D, Shah AA, Sherman L, Lewis A, Rademaker J, Pearson G, Choi J, Wolff W, Prabhu PM, Galanski M, Clark RA, Sostman HD, Henschke CI, Pulmonary CT Angiography–Indirect CT Venography Cooperative Group (2000) Deep venous thrombosis: detection by using indirect CT venography. Radiology 2000:216:744–751
Loud PA, Katz DS, Bruce DA, Klippenstein DL, Grossman ZD (2001) Deep venous thrombosis with suspected pulmonary embolism: detection with combined CT venography and pulmonary angiography. Radiology 219:498–502
Garg K, Kemp JL, Russ PD, Barón AE (2001) Thromboembolic disease: variability of interobserver agreement in the interpretation of CT venography with CT pulmonary angiography. Am J Roentgenol 176:1043–1047
Simon M, Boiselle PM, Choi JR, Rosen MP, Reynolds K, Raptopoulos V (2001) Paddle-wheel CT display of pulmonary arteries and other lung structures: a new imaging approach—technical innovation. Am J Roentgenol 177:195–198
Wildberger JE, Niethammer MU, Klotz E, Schaller S, Wein BB, Günther RW (2001) Multi-slice CT for visualization of pulmonary embolism using perfusion weighted color maps. Fortschr Röntgenstr 173:289–294
Jeong YJ, Lee KS, Yoon YC, Kim TS, Chung MJ, Kim S (2004) Evaluation of small pulmonary arteries by 16-slice multidetector computed tomography: optimum slab thickness in condensing transaxial images converted into maximum intensity projection images. J Comput Assist Tomogr 28:195–203
Oudkerk M, VanBeek EJ, Wielopolski P, VanOoijen PM, Brouwers-Kuyper EM, Bongaerts AH, Berghout A (2002) Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography in the diagnosis of pulmonary embolism: a prospective study. Lancet 359:1643–1647
Kluge A, Müller C, Hansel J, Gerriets T, Bachmann G (2004) Real-time MR with TrueFISP for the detection of acute pulmonary embolism: initial clinical experience. Eur Radiol 14:709–718
Ohno Y, Higashino T, Takenaka D, Sugimoto K, Yoshikawa T, Kawai H, Fujii M, Hatabu H, Sugimura K (2004) MR angiography with sensitivity encoding (SENSE) for suspected pulmonary embolism: comparison with MDCT and ventilation–perfusion scintigraphy. Am J Roentgenol 183:91–98
Ryu JH, Swensen SJ, Olson EJ, Pellikka PA (2001) Diagnosis of pulmonary embolism with use of computed tomographic angiography. Mayo Clin Proc 76:59–65
Perrier A, Nendaz MR, Sarasin FP, Howarth N, Bounameaux H (2003) Cost-effectiveness analysis of diagnostic strategies for suspected pulmonary embolism including helical computed tomography. Am J Respir Crit Care 167:39–44
Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J, Hayoz D, Bounameaux H (2004) Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med 116:291–299
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wildberger, J.E., Mahnken, A.H., Das, M. et al. CT imaging in acute pulmonary embolism: diagnostic strategies. Eur Radiol 15, 919–929 (2005). https://doi.org/10.1007/s00330-005-2643-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2643-y