Abstract
The feasibility and diagnostic value of real-time magnetic resonance imaging (RT-MRI) for the diagnosis of acute pulmonary embolism (PE) was evaluated by comparing RT-MRI and magnetic resonance angiography (MRA). In 39 consecutive patients with suspected PE real-time true fast imaging with steady-state precession (TrueFisp) was prospectively compared with contrast-enhanced MRA on a 1.5-T MR scanner. The TrueFisp sequence used allowed acquisition of T2-weighted images at 0.4 s per image so that the pulmonary vasculature could be visualized in three orientations in <3 min without the need for breath holding or contrast media application. Results of additional scintigraphic pulmonary perfusion examinations were available from 17 patients. All 39 primary RT examinations (100%) and 30 of 39 MRA examinations (77%) were of diagnostic quality. The reasons underlying failure to achieve diagnostic quality for MRA were breathing artifacts among dyspneic patients in all 9 cases. Compared with MRA, the sensitivities and specificities of RT sequences for PE were 93 and 100% (per examination), 96 and 100% (lobar artery PE), and 97 and 100% (segmental artery PE), respectively. Compared with scintigraphy, the sensitivity and specificity of RT-MRI were 83 and 100%, respectively. The MRA reached 100% sensitivity and specificity in this subgroup. The RT-MRI proved to be very robust and undisturbed by respiratory movements and patient cooperation. Its image quality assured fast diagnostic examinations, and its sensitivity and specificity, compared with MRA and scintigraphy, were sufficient to allow the diagnosis of acute central, lobar, and segmental PE; therefore, the emergency diagnosis of PE using RT-MRI is feasible and reliable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism (PE) is the third most common acute cardiovascular disease after cardiac ischemia and stroke [1]. As 1-year mortality rates from PE among treated patients is 2.5% [2], adequate, timely diagnosis is mandatory. Widely used perfusion-ventilation scintigraphy lacks specificity [3]. While pulmonary angiography was a long-standing gold standard, its restricted availability, the examination time required, the equipment and personnel demands, and a low percentage of major complications [4] all conspired to motivate a search for alternatives. Spiral CT, introduced for the diagnosis of PE by Remy-Jardin et al. in 1992 [5], has become the most widely used imaging modality [6]. Multi-slice CT allows imaging at the subsegmental level [7, 8] and visualizes simultaneous diseases such as atelectasis. This makes CT a very attractive tool for the referring physician since PE represents just one major differential diagnosis for thoracic pain or dyspnea. Due to its robustness, the accuracy of CT for diagnosing PE is regarded as being higher than that of magnetic resonance angiography (MRA) [9]. Inherent disadvantages of CT angiography (CTA) include exposure to ionizing radiation and the necessity to administer iodinated contrast media. For this reason, MRA has long been scrutinized as a procedure for diagnosing PE.
The MRA, although delivering a performance close to single-slice CT [10, 11], has played a limited role in evaluating PE up to now. Its lack of availability and limited patient access, as well as long examination time, have restricted its clinical use. Patients had to be able to sustain breath-holding periods of 15–27 s which limited indications to non-emergency diagnostics.
As real-time MR (RT-MRI), because of its design, is not susceptible to motion artifacts, this technique obviates the aforementioned disadvantages and appears promising for the evaluation of acute PE. True fast imaging with steady-state precession (TrueFISP, also called steady-state free precession) RT sequences produce predominantly T2-weighted contrasts which allow inherent discrimination of embolic material and patent pulmonary vessels/blood. We therefore evaluated for the first time the diagnostic value of RT TrueFISP MRI in patients with suspected acute PE and compared it with contrast-enhanced MRA and perfusion scintigraphy. The potential of RT-MRI for detecting non-embolic thoracic diseases was also evaluated.
Materials and methods
Patients
Thirty-nine consecutive in-house patients with suspected PE were prospectively examined using MR imaging (17 men and 22 women; mean age 57±13 years). They were referred to us by cardiologists. Suspicion arose from clinical symptoms, D-dimer tests, echocardiography, and duplex phlebosonography. Due to the focus of the radiology department on cardiac MRI, no CT scanner, and therefore no CTA, was available, and scintigraphy was available only during the daytime; thus, MRI was used as a primary diagnostic modality.
Informed consent was obtained, clinical condition permitting. The clinical condition of patients with suspected PE was graded according to the following scale: stage I, asymptomatic or mild dyspnea; stage II, severe dyspnea and agitation; and stage III, severe dyspnea and decreased blood pressure.
Protocol
All patients with suspected PE were examined on a 1.5-T MR scanner (Magnetom Sonata, Siemens, Erlangen, Germany). The patients were placed within the MRI scanner, in the supine position, head first, with arms alongside the thorax. A quadrature phased-array coil was positioned on the patients’ chest. For contrast media application, a 20-G peripheral intravenous line was placed in an antecubital vein and was connected to a power injector (Spectris, Medrad, Pittsburgh, Pa.). Monitoring of blood oxygen saturation and ECG was applied if required by the clinical condition, but not routinely, since no cardiac-gated sequences were used. Mechanically ventilated patients could be examined if a respiration bag was used, since the strong external magnetic field of this MR scanner model interfered even with MR-compatible mechanical respirator models. Examination time was assessed retrospectively using DICOM tags for series, acquisition, and image time. Times required for the RT-MRI protocol and for the complete imaging protocol are given as mean±standard deviation (SD).
Real-time MRI
A non-cardiac-gated RT TrueFISP single-shot sequence similar to published techniques [12, 13] was used. Apart from the cardiac gating, basic image characteristics were similar to those of cardiac-gated TrueFISP sequences described previously [14, 15]. Parameters were adapted for minimal time of acquisition while maintaining a high spatial resolution (TR 3.1 ms, TE 1.5 ms, flip angle 59°). Bandwidth was increased to 1000 Hz for shorter acquisition times. In-folding artifacts in the coronal plane caused by a rectangular field of view (FOV) were permitted in order to achieve an acquisition time of below 0.5 s/image. Artifacts disturbed peripheral parts of the thorax, but not central, lobar, or segmental pulmonary artery (PA) branches. Since the acquisition of contiguous overlapping slices was not possible, two blocks of contiguous, non-overlapping slices were acquired interleaved, both blocks covering the same volume shifted by 50% slice thickness. The combination of both block’s slices resulted in the desired contiguous 50% overlapping slices. No extinction artifacts were detected in precursor studies or in the present study. Fat suppression was not applied so that a longer acquisition time could be avoided.
The RT TrueFISP sequence was applied according to the following protocol: (a) 100 contiguous slices in a coronal orientation, FOV 360 mm, 256×192 pixels, slice thickness 4 mm, 2-mm overlap, and acquisition time 0.52 s per slice; (b) 100 contiguous slices in a sagittal orientation, FOV 360 mm, 256×180 pixels, slice thickness 4 mm, 2-mm overlap, and acquisition time 0.45 s per slice; (c) 120 contiguous slices in a transverse orientation, FOV 340 mm, 256×156 pixels, slice thickness 3 mm, 1.5-mm overlap, and acquisition time 0.4 s per slice.
The slices covered the following regions to guarantee standardized comprehensive imaging of the pulmonary vasculature in three orthogonal projections: transverse orientation from the apex of the lung to the portal vein; coronal orientation from the manubrium sterni to the spinous process; and sagittal orientation from the right to the left medial clavicular line. Individual adaptation of slice geometry or position could be discarded since all three orthogonal stacks of slices covered both lungs entirely and standard head-first patient positioning placed the main PA in the center of the FOV and stack; thus, the sequences could be started immediately without adaptation or modification of any parameters. Images could be evaluated online after a delay of 1–2 s.
MRA
The RT images were used for proper planning of the consecutive MRA. Before starting contrast-enhanced (CE) MRA, a sagittal bolus timing sequence was performed after administration of 2-ml gadopentate dimeglumine (Gd-DTPA, Magnevist, Schering, Berlin, Germany) at 3 ml/s followed by a 30-ml saline flush at 3 ml/s. The delay was determined as the time from injection to peak pulmonary trunk enhancement minus 4 s.
The MRA was performed using a 3D fast low-angle shot (FLASH) sequence (TR 3.2 ms, TE 1.4 ms, flip angle 25°, fat saturation applied to avoid hampering infolding artifacts from the patients arms). A coronal FOV of 340 mm (512×384 pixels, 72 partitions, voxel size 0.7×1.2×1.5 mm) covered the pulmonary vasculature in 22 s. Twenty-millileters Gd-DTPA were injected at 3 ml/s after the previously determined delay followed by a 20-ml saline flush. Two sequential MRA breath-hold measurements were performed. A 10-s break allowed the patient to resume respiration. The second measurement served as backup if bolus timing, motion artifacts, or patient cooperation compromised the image quality of the first 3D data set.
Nuclear medicine
Perfusion lung scans were acquired additionally in 17 patients within 24 h of the respective MRI examination. Two-dimensional planar lung scans with eight standard projections using an Ecom 180° dual-head camera (Siemens, Erlangen, Germany) were performed in 11 patients. Six patients were examined using a single photon emission computed tomography (SPECT) technique. The data of a 3D SPECT perfusion lung scintigraphy were transformed into 64 transverse slices per examination. Tc-99m macroaggregated albumin with an activity of 148 MBq was used as the radiopharmaceutical.
Image interpretation
All examinations were evaluated by two radiologists (A.K., G.B.), and final diagnosis was made on the basis of consensus. Each pulmonary, lobar, and segmental artery of every patient was assessed separately with both sequence techniques. Every vessel was depicted on at least two RT images per plane, i.e., five to seven images per segmental vessel, and the evaluation referred to the plane, and the images showing the vascular wall and lumen without artifacts. Concordant results from two planes were required to confirm an RT-MRI finding.
For final evaluations, RT-MRI sequences and MRA examinations were evaluated separately to avoid any recall bias: firstly, all RT sequences from all patients were interpreted in random order before all the MRA data sets were evaluated. The MRA were assessed on non-reconstructed images as well as on multiplanar reconstructed (MPR) images and seldom on maximum intensity projection (MIP) images. The time required for RT image interpretation was assessed during this final evaluation and rounded off to the nearest completed 5-min period.
Signal-to-noise ratio [SNR; mean signal intensity (SI)/SD of SI], SI ratio (SI of surrounding blood/SI of embolic material), and contrast-to-noise ratio (CNR; SI of blood−SI of embolic material/noise) were calculated for all 5 central, all 18 left lobar, and all 22 segmental emboli of left and right S10. Values given are means±SD.
Subsegmental arteries, although usually depicted in RT-MRI and MRA, were not evaluated. The lingula stem of the left segmental arteries four and five, although anatomically part of the left upper lobe, was categorized as a lobar artery due to its size and origin. Segment seven (left medial basal or cardiac segment) of the left lung was not evaluated separately but regarded as part of the anteromedial segment, S8, of the left lung.
Both MRA and RT-MRI data sets were evaluated for coincidental mediastinal or pulmonary parenchymal findings not related to PE.
Diagnostic quality
Pulmonary artery imaging was classified on RT-MRI and MRA as being “non-diagnostic”, “diagnostic without thrombus,” and “diagnostic with thrombus.” Diagnostic quality was assumed if one of the following criteria were met: (a) discrimination of vessel wall and vessel lumen or thrombotic material was possible; or (b) the in-plane course of the vessel allowed simultaneous judgment of the relative blood flow (RT-MRI) or contrast enhancement (MRA) in different parts of the vessel.
Non-diagnostic quality of a single vessel was assumed if the vessel could not to be identified or exclusion of PE was not possible due to blurred vascular representation. Non-diagnostic quality of the examination was assumed if more than three lobar arteries or more than half the segmental arteries failed to achieve diagnostic quality.
Diagnostic criteria
Diagnosis of PE was established if (consistent with conventional pulmonary angiography and CTA criteria) either (a) thrombus could be directly visualized, or (b) cutoff of pulmonary vessel, or (c) abrupt changes in signal intensity during the course of PA were detected. These criteria applied for MRA and RT-MRI.
Statistical analysis
Vessels in an area with non-diagnostic image quality were excluded from analysis. A finding was classified as false negative if thrombus was clearly visible in MRA but not visible in RT-MRI. Although detectable, no discrimination was made between thrombotic material adherent to the vessel wall or floating freely in the vascular lumen, since determination of thrombus age was not an objective of this study. Evaluation was made at the segmental, lobar, and central PA level and per examination. Sensitivity and specificity, as well as positive and negative predictive values (PPV, NPV), were calculated using MRA as a reference standard. For this reason, examinations with non-diagnostic MRA sequences (9 of 39, 23%) had to be excluded from the evaluation. Due to the high percentage of non-diagnostic MRA examinations, a separate preliminary analysis used direct visualization of thrombotic material as “prima facie evidence” and, therefore, RT-MRI for reference purposes. The subgroup of patients with perfusion lung scans was additionally evaluated using scintigraphy as a reference standard.
Percentage of diagnostic quality at different clinical stages was analyzed using the chi-square test for linear trends.
Results
Clinical stage I of suspected PE was diagnosed in 23 of 39 patients, stage II in 11 examinations, and stage III in 5 patients.
Thirty-nine examinations were performed using RT-MRI and MRA. The examination time for RT-MRI was 209±25 s. The complete imaging protocol, including localization, bolus timing, preparation and MRA, required 11 min 10 s±1 min 57 s.
Diagnostic quality
Non-diagnostic PA depiction of RT sequences was caused by poor blood signal intensity in segmental arteries and flow artifacts in central and lobar PA. Evaluation of segmental vessels on RT images was simpler for vessels with a long in-plane course or for vessels depicted as being artifact free in a transverse section.
Direct thrombus visualization allowed lobar and central embolism to be easily detected on RT images by browsing the coronal stack of images, referring to transverse and sagittal orientation for confirmation. At the segmental level, images had to be evaluated in all three orientations several times in order to achieve diagnostic certainty. Diagnosis at the central and lobar level was therefore instantaneous, whereas evaluation of all segmental arteries took 5–20 min per patient.
All 9 cases of non-diagnostic MRA were caused by patient motion and breathing artifacts. Bolus timing was correct in all examinations since all MRA showed sufficient PA enhancement that was rarely associated with pulmonary vein enhancement. Evaluation of coronal raw images and postprocessing of MIP and MPR images took 5–10 min.
Table 1 shows the distribution of diagnostic quality with both imaging techniques for the regions evaluated. The MRA showed a percentage of diagnostic quality ranging from 77% (30 of 39) for all examinations to 70% at the segmental level, whereas the percentage of diagnostic quality in RT-MRI decreased slightly from 100% at the examination level to 96% at the segmental level. Patients with non-diagnostic MRA (n=9) were excluded from further evaluation.
The percentage of RT-MRI and MRA examinations with diagnostic quality at different clinical stages of suspected PE is shown in Table 2. All 39 RT-MRI examinations reached diagnostic quality including all 5 patients in stage III. Diagnostic quality of MRA, on the other hand, depended on patient co-operation: severe dyspnea appeared to impede diagnostic quality in MRA since 64.4% (7 of 11) of the MRA examinations in stage II and no MRA examination in stage III reached diagnostic quality. These differences were significant (p<0.05).
Pulmonary embolism
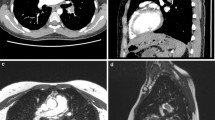
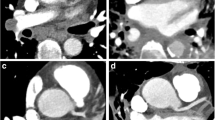
Figure 1 demonstrates PE as depicted by both imaging techniques in coronal views. Both sequences depicted all emboli. Figure 2 shows PE in a patient with severe dyspnea which led to respiratory artifacts and marked motion blurring in the MRA sequence, whereas image quality of real-time sequences was not affected by breathing artifacts. Figure 3 shows typical examples of PE presentation in RT sequences.
The MRA and RT-MRI were both capable of depicting embolic material adherent to the vessel wall as well as free floating thrombi. In 30 patients undergoing diagnostic examinations with both techniques, 60 main PA, 177 lobar, and 491 segmental arteries were evaluated; 1 patient had undergone a previous resection of the right lower lobe.
Frequency of PE varied with clinical stage. In MRA, 9 of 23 (39%) patients in stage I and 4 of 7 patients (57%) in stage II had PE. As MRA was not diagnostic in any stage-III examination and in 37% of stage-II examinations (Table 2), direct thrombus visualization in RT MRI was used as “prima facie evidence” in the following examinations: 4 of 5 patients (80%) at clinical stage III and 7 of 11 patients (64%) in stage II had visible thrombotic material and, therefore, PE. Of nine non-diagnostic MRA examinations, six had PE in RT-MRI. Two patients received systemic lysis using rTPA as a consequence of the MR examination.
Table 3 depicts the frequency of PE at different anatomical levels as well as sensitivity, specificity, and positive/negative predictive values for RT-MRI. Sensitivity per examination was 93 and 96% at the lobar level and 97% at the segmental level, and specificity was 100% for all three levels. In RT-MRI four false-negative segmental emboli and two false-negative lobar arteries in two examinations led to one false-negative examination. The MRA showed clear evidence of thrombotic material in two middle lobe (ML) arteries as well as in the arteries of segments 4 and 5, and RT sequences did not depict thrombotic material in either case. Final diagnosis changed in 1 patient with solitary embolism in the ML, whereas the other patient had massive PE with a large amount of thrombotic material in two lobar PAs.
Table 4 shows the occurrence of PE and the distribution of thrombotic material per examination, and at the central pulmonary, lobar, and segmental artery level. Since most patients with PE had more than one affected pulmonary segment, with one segment sufficing for the diagnosis of PE, the frequency of PE increased from 26% of segmental PA and 27% of lobar PA to 47% of all examinations. Quantitative measurements (SNR, SI ratio, and CNR) of representative embolic material are given as means±SD. The SNR of blood was 18.1±6.7. For central pulmonary arteries SNR of embolic material (n=5) was 5.7±2.1, the SI ratio blood/embolic material amounted to 4.3±1.7, and the CNR was 11.9±4.1. For lobar emboli (n=18) the values were as follows: SNR 8.7±4.7; SI ratio 3.2±0.6; and CNR 10.2±3.2. The respective values for segmental emboli (n=22) were as follows: SNR 5.2±2.3; SI ratio 6.6±3.2; and CNR 13.5±3.4.
Nuclear medicine
Seventeen perfusion scintigraphies were available in patients with clinical stage-I PE. All MRA and RT-MRI examinations reached diagnostic quality in this subgroup. Scintigraphy was positive for acute PE in 6 of 17 patients (35%), and RT-MRI was positive in 5 of these 6 patients (5 of 17, 29%). Sensitivity, specificity, PPV, and NPV for RT-MRI, therefore, were 83% (5 of 6), 100% (11 of 11), 100% (5 of 5), and 92% (11 of 12), respectively. The MRA depicted all 6 patients with PE correctly; thus, sensitivity, specificity, PPV, and NPV was 100% for MRA in this subgroup.
Coincidental findings
Coincidental findings included three pleural bleedings, three gastric herniations, segmental and lobar atelectasis in 5 patients, vertebral hemangioma in 1 patient, and rupture of an ascending aortic graft in 1 patient. All these findings were detected by RT-MRI alone, and no further sequences (e.g., T1-weighted spin-echo sequences) were required to establish the diagnosis. Apart from the aortic graft rupture, when MRA was performed to present findings in a way clinicians are more familiar with, all other coincidental findings would have been overlooked by MRA.
Discussion
The RT-MRI with TrueFISP sequences was used for diagnosis of PE for the first time. Diagnosis of central and lobar PE was immediate and diagnosis of segmental PE was reliable. Patients in this study presented more severe stages of PE, which led to a high rate of inconclusive MRA examinations. Among these severely ill patients in particular, reliable performance of RT-MRI ideally complemented MRA and allowed emergency diagnosis and fast therapeutic decisions.
Presently, however, CTA is the most commonly used imaging modality for diagnosing PE [6]. Sensitivity of CTA reaches up to 94% for segmental PE [16] but depends on vessel size [17]. Combined assessment of PE and peripheral thrombosis is feasible [18]; however, radiation exposure and the necessity to administer iodinated contrast media are disadvantages of CTA. The MRA does not share these disadvantages and has been repeatedly evaluated for PE diagnosis. Sensitivity is comparable to single-slice CTA [10, 11, 19] and was even slightly superior in autoptic controlled porcine studies [20, 21], ranging from 60 [22] and 85% [19] to 100% [10]. This evolving role of MRA justified its use as a reference method in the present study; however, long periods of apnea necessary for CE-MRA limit its indication to non-emergency examinations.
Unlike MRA, RT imaging is not, by definition, susceptible to motion artifacts. Due to the non-segmented acquisition of the image, motion artifacts occur only during the acquisition of each individual image (0.4–0.5 s for the TrueFISP sequence applied); thus, TrueFISP sequences are suitable for fast whole-body [23] and emergency imaging [15]. In addition to this robustness, True-FISP’s inherent T2 contrast led to a CNR (embolus/blood) sufficient to depict PE without use of contrast agent, thereby reducing preparation time and error sources in urgent diagnostics.
All 39 RT-MRI examinations reached diagnostic quality regardless of clinical stage or respiratory motion even in patients with massive PE. Preceding volunteer examinations showed robust RT image quality for breath rates up to 30 breaths/min, and artifacts increased markedly at >40 breaths/min. The amount of embolic material, however, influenced the diagnostic certainty: central and lobar PE was diagnosed immediately, whereas isolated segmental PE required meticulous analysis. Systolic blood flow caused in-flow artifacts in the main PA on several images, but usually this area was artifact free on directly preceding or following images, and diagnostic confidence was not affected. Due to 31 non-diagnostic (mainly middle lobe) segmental arteries in RT-MRI, two segmental PE were missed and 1 patient in clinical stage I was therefore classified as a false negative. As patients with massive dyspnea had advanced stages of PE, the diagnostic performance (sensitivity 93%) was sufficient for immediate diagnosis in an emergency setting. After a learning curve in image interpretation, the number of 320 RT images assuring the depiction of every segmental PA in three planes on several images could be markedly reduced to achieve an acquisition time of 1 min.
The MRA showed the following complementary characteristics: the diagnostic performance of MRA did not depend on the location, but on the amount of embolic material, i.e., the clinical condition of patients. The MRA provided reliable results, particularly in (mostly non-dyspneic) patients with a smaller embolic burden, where diagnostic confidence was better than it was with RT-MRI.
The PE prevalence of 47% in our study was markedly higher than previously reported (35% diagnosed by pulmonary angiography [4]: 19.6–44% found by CTA [5, 24, 25] and 26.6–36% for MRA [10, 11, 19], while the distribution of emboli (predominantly right and multiple; Table 4) paralleled autoptic findings [26]. Part of the high prevalence is credited to the clinical judgment of the referring cardiologists (complemented by ECG, D-dimer, echocardiography and duplex sonography). More importantly, the previously very limited availability of MR as the sole imaging modality (no CT, daytime availability of scintigraphy), and the initiation of RT-MRI as an emergency approach, resulted in initial self-restriction. Patients were probably referred after a more specific clinical selection, augmenting the pretest probability.
This higher proportion of advanced clinical stages of PE and of dyspneic patients explains the considerably higher number of non-diagnostic MRA examinations (23%). The clinical condition of patients, however, is seldom stated in published studies, but has to be deduced from the rate of non-diagnostic examinations or positive findings. Non-diagnostic quality is reported for MRA in 4–15% [10, 11, 27], for pulmonary angiography in 3% [4, 28], and for CTA in 2.7–10.4%, depending on technique [24, 29]. Shallow breathing degrades the quality of single-slice CTA only slightly [5, 30] but hinders subsegmental PA evaluation more substantially [24]. Considering the prevalence of PE among dyspneic patients, the robustness of our MRA may be within the reported range. Artificial ventilation did not hamper RT-MRI, but this applies to breath-hold CE-MRA or CTA examinations also, as image acquisition can be acquired in breath-hold position in these patients.
Several approaches to overcome long breath-holding periods for CE-MRA have been proposed: high-power gradients allow the acquisition time to be limited to 4 s [31]; however, for ultrafast acquisition, temporal resolution has to be traded for spatial resolution. In our experience with this approach dyspnea is unproblematic, but lower spatial resolution makes unambiguous evaluation of all segmental PA difficult. Parenchymal perfusion may be accessible this way [32] since the limited spatial resolution averages otherwise undetectable (sub)subsegmental vasculature. Navigator-echo sequences allowed free breathing, but an acquisition time of up to 16 min required non-approved intravascular contrast agents [33]. We nevertheless chose a 512-matrix-size MRA for better diagnostic confidence for segmental PE, a factor which outweighed the slightly longer acquisition time: motion blurring increased only a little from a matrix size of 256×256 (17 s) to 512×512 (22 s).
As the mentioned coincidental findings indicate, pleural and pericardial fluid collection and aortic dissection or acute rupture could be easily diagnosed by RT-MRI, which is consistent with a study on aortic aneurysm and dissection [13]. Afflictions of the pulmonary parenchyma (bleeding, aspiration, infiltration, and atelectasis) could be localized and quantified accurately. This advantage over pulmonary angiography, scintigraphy, and CE-MRA matches the ability of CTA to detect side diagnoses.
A possible algorithm using MRI as a first-line test for PE (wherever symptoms, d-dimer, and/or ECG give rise to suspicion) should start with RT-MRI. The examination can be terminated immediately after embolic material or a significant side diagnosis is detected (in dyspneic patients usually after 20–40 s). Patients with negative results after the first RT slab are typically not dyspneic and tolerate the protocol’s continuation to allow increases in sensitivity (two more RT-MRI orientations and MRA). Segmental or larger PE is excluded if all RT-MRI and MRA images reveal no PE.
Further studies need to address the sensitivity of RT-MRI compared with established CTA since the evolving, but still controversial, role of MRA as a reference method, represents the major limitation of the present study. Only a part of our collective could be examined by perfusion scintigraphy. In our study the accuracy of RT-MRI was comparable to that of established techniques, but because of a selection bias, the results are only indicative (lower prevalence of PE in the scintigraphy subgroup and daytime availability of scintigraphy).
Conclusion
Our results demonstrated the feasibility of MRI in a study population unfavorable for CE-MRA, as well as the strength of RT-MRI, which proved to be very robust and reliable for diagnosing PE regardless of the patient’s clinical condition. The RT-MRI required neither venous access nor ECG, and predefined protocols with oversized examination volumes obviated proper positioning or error-prone parameter adaptation; hence, emergency diagnostics are feasible within 1–3 min. A combination of RT-MRI for acute central or lobar PE and differential diagnosis, and MRA—more vulnerable to motion artifacts—for reliable diagnosis at the segmental level covers the whole range of clinical presentations of acute PE including artificially ventilated patients. The RT-MRI is, therefore, included in our work-up for PE. Future indications for MRI-based diagnosis of PE may no longer be restricted to niche indications (such as contraindication against iodinated contrast media), but may also include emergency diagnostics.
References
Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Forcier A, Patwardhan NA (1991) A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worchester DVT study. Arch Intern Med 151:933–938
Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE, Spera MA, Abass Alavi RN, Terrin ML (1992) The clinical course of pulmonary embolism. N Engl J Med 326:1240
The PIOPED investigators (1990) Value of ventilation/perfusion scan in acute pulmonary embolism: results of prospective investigation of pulmonary embolism diagnosis (PIOPED). J Am Med Assoc 263:2753–2759
Stein PD, Athanasoulis C, Alavi A (1992) Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 85:462–468
Remy-Jardin M, Remy J, Wattinne L, Giraud F (1992) Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique: comparison with pulmonary angiography. Radiology 185:381–387
Schibany N, Fleischmann D, Thallinger C, Schibany A, Hahne J, Ba-Ssalamah A, Herold CJ (2001) Equipment availability and diagnostic strategies for suspected pulmonary embolism in Austria. Eur Radiol 11:2287–2294
Remy-Jardin M, Remy J, Artaud D, Deschildre F, Duhamel A (1997) Peripheral pulmonary arteries: optimization of the spiral CT acquisition protocol. Radiology 204:154
Schoepf UJ, Kessler MA, Rieger CT, Herzog P, Klotz E, Wiesgigl S, Becker CR, Exarhos DN, Reiser MF (2001) Multislice-CT imaging of pulmonary embolism. Eur Radiol 11:2278
Ghaye B, Remy J, Remy-Jardin M (2002) Non-traumatic thoracic emergencies: CT diagnosis of acute pulmonary embolism—the first 10 years. Eur Radiol 12:1886–1905
Meaney JFM, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR (1997) Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 336:1422–1427
Oudkerk M, van Beek EJR, Wielopolski P, van Ooijen PMA, Brouwers-Kuyper EMJ, Bongaerts AHH, Berghout A (2002) Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet 359:1643–1647
Duerk JL, Lewin JS, Wendt M, Petersilge C (1998) Remember true FISP? A high SNR, near 1-second imaging method for T2-like contrast in interventional MRI at 2 T. J Magn Reson Imaging 8:203–208
Nitz WR (2002) Fast and ultrafast non-echo-planar MR imaging techniques. Eur Radiol 12:2866–2882
Miller S, Simonetti OP, Carr J, Kramer U, Finn JP (2002) MR imaging of the heart with cine true fast imaging with steady-state precession: influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology 223:263–269
Pereles FS, McCarthy RM, Carr JC, Kapoor V, Krupinski EA, Finn JP (2002) Thoracic aortic dissection and aneurysm: evaluation with non-enhanced TrueFISP MR angiography in less than 4 minutes. Radiology 223:270–274
van Rossum AB, Pattynama PM, Ton ER, Treurniet FE, Arndt JW, van Eck B, Kieft GJ (1996) Pulmonary embolism: validation of spiral CT angiography in 149 patients. Radiology 201:467–470
Ruiz Y, Caballero P, Caniego JL, Friera A, Olivera MJ, Tagarro D, Alvarez-Sala R (2003) Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement. Eur Radiol 13:823–829
Ciccotosto C, Goodman LR, Washington L, Quiroz FA (2002) Indirect CT venography following CT pulmonary angiography: spectrum of CT findings. J Thorac Imaging 17:18–27
Gupta A, Frazer CK, Ferguson JM, Kumar AB, Davis SJ, Fallon MJ, Morris IT, Drury PJ, Cala LA (1999) Acute pulmonary embolism: diagnosis with MR angiography. Radiology 210:353–359
Reittner P, Coxson HO, Nakano Y, Heyneman L, Ward S, King GG, Baile EM, Mayo JR (2001) Pulmonary embolism: comparison of gadolinium-enhanced MR angiography with contrast-enhanced spiral CT in a porcine model. Acad Radiol 8:343–350
Seo JB, Im JG, Goo JM, Chung MJ, Moon WK, Lee KH, Kim IO (2003) Comparison of contrast-enhanced CT angiography and gadolinium-enhanced MR angiography in the detection of subsegmental-sized pulmonary embolism. Acta Radiol 44:403–410
Bergin CJ, Sirlin CB, Hauschildt JP, Huynh TV, Auger WR, Fedullo PF, Kapelanski DP (1997) Chronic thromboembolism: diagnosis with helical CT and MR imaging with angiographic and surgical correlation. Radiology 204:695–702
Barkhausen J, Quick HH, Lauenstein T, Goyen M, Ruehm SG, Laub G, Debatin JF, Ladd ME (2001) Whole-body MR imaging in 30 seconds with real-time TrueFISP and a continuously rolling table platform: fesasibility study. Radiology 220:252–256
Remy-Jardin M, Tillie-Leblond I, Szapiro D, Ghaye B, Cotte L, Mastora I, Delannoy V, Remy J (2002) CT angiography of pulmonary embolism in patients with underlying respiratory disease: impact of multislice CT on image quality and negative predictive value. Eur Radiol 12:1971–1978
Teigen CL, Maus TP, Sheedy PF, Stanson AW, Johnson CM, Breen JF, McKusick MA (1995). Pulmonary embolism: diagnosis with contrast-enhanced electron-beam CT and comparison with pulmonary angiography. Radiology 194:313–319
Morpurgo M, Schmid C (1995) The spectrum of pulmonary embolism: clinicopathologic correlations. Chest 107:18S–20S
Ghaye B, Szapiro D, Mastora I, Delannoy V, Duhamel A, Remy J, Remy-Jardin M (2001) Peripheral pulmonary arteries: How far in the lung does multi-detector row spiral CT allow analysis? Radiology 219:629–636
Van Beek EJR, Reekers JA (1999) Pulmonary angiography in the differential diagnosis of pulmonary embolism. Eur Radiol 9:1310–1316
Remy-Jardin M, Baghaie F, Bonnel F, Masson P, Duhamel A, Remy J (2000) Thoracic helical CT: influence of sub-second scan time and thin collimation on evaluation of peripheral pulmonary arteries. Eur Radiol 10:1297
Gotway MB, Patel RA, Webb WR (2000) CT for the evaluation of suspected acute pulmonary embolism: diagnostic pitfalls. J Comput Assist Tomogr 24:267–273
Goyen M, Laub G, Ladd ME, Debatin JF, Barkhausen J, Truemmler KH, Bosk S, Ruehm SG (2001) Dynamic 3D MR angiography of the pulmonary arteries in under four seconds. J Magn Reson Imaging 13:372–377
Matsuoka S, Uchiyama K, Shima H, Terakoshi H, Nojiri Y, Oishi S, Ogata H (2001). Detectability of pulmonary perfusion defect and influence of breath holding on contrast-enhanced thick-slice 2D and 3D MR pulmonary perfusion images. J Magn Reson Imaging 14:580–585
Abolmaali N, Hietschold V, Appold S, Ebert W, Vogl TJ (2002) Gadomer-17-enhanced 3D navigator-echo MR angiography of the pulmonary arteries in pigs. Eur Radiol 12:692–697
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kluge, A., Müller, C., Hansel, J. et al. Real-time MR with TrueFISP for the detection of acute pulmonary embolism: initial clinical experience. Eur Radiol 14, 709–718 (2004). https://doi.org/10.1007/s00330-003-2164-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2164-5