Abstract.
Our objective was to evaluate, in a routine clinical setting, the role of spiral CT as a second procedure in patients with clinically suspected pulmonary embolism (PE) and abnormal perfusion scan. We prospectively studied the role of spiral CT in 279 patients suspected of PE. All patients started their diagnostic algorithm with chest radiographs and perfusion scintigraphy. Depending on the results of perfusion scintigraphy, patients proceeded to subsequent levels in the algorithm: stop if perfusion scintigraphy was normal; CT and pulmonary angiography if subsegmental perfusion defects were seen; ventilation scintigraphy followed by CT when segmental perfusion defects were seen; and pulmonary angiography in this last group when results of ventilation/perfusion scintigraphy and CT were incongruent. Reference diagnosis was based on normal perfusion scintigraphy, high probability perfusion/ventilation scintigraphy in combination with abnormal CT, or pulmonary angiography. If PE was present, the largest involved branch was noted on pulmonary angiography, or on spiral CT scan in case of a high-probability ventilation/perfusion scan and a positive CT scan. A distinction was made between embolism in a segmental branch or larger, or subsegmental embolism. Two hundred seventy-nine patients had abnormal scintigraphy. In 27 patients spiral CT and/or pulmonary angiography were non-diagnostic and these were excluded for image analysis. Using spiral CT we correctly identified 117 of 135 patients with PE, and 106 of 117 patients without PE. Sensitivity and specificity was therefore 87 and 91%, respectively. Prevalence of PE was 53%. Positive and negative predictive values were, respectively, 91 and 86%. In the high-probability group, sensitivity and specificity increased to 97 and 100%, respectively, with a prevalence of 90%. In the non-high probability-group sensitivity and specificity decreased to 61 and 89%, respectively, with a prevalence of 25%. In a routine clinical setting single-detector spiral CT technology has limited value as a second diagnostic test because of low added value in patients with a high-probability lung scan and low sensitivity in patients with non-high-probability lung scan result.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various diagnostic algorithms in patients suspected of pulmonary embolism (PE) have recently been evaluated and advocated [1], but ventilation–perfusion (V/P) scintigraphy was for a long time the preferred initial test [2]. A recent survey performed in Austria revealed that spiral CT was the first-line imaging study for suspected PE in 56% of hospitals, followed by echocardiography and scintigraphy. The V/P scintigraphy served as second-line imaging test in 43% of hospitals [3]. Scintigraphy usually is an initial though not a final study since numerous reports, including the Prospective Investigators of Pulmonary Embolism Data (PIOPED), have shown that only a small number (33%) of patients will have an unequivocal diagnosis following V/P scintigraphy [4]. Additional tests are needed for the remaining majority (67%) with an inconclusive or non-high-probability scintigraphy. Spiral CT has been advocated as a primary test [3], or can be used as a second-line test [5].
Studies evaluating spiral CT have yielded contradictory results [6]. Van Erkel et al. have calculated that spiral CT is marginally cost-effective for any sensitivity [5]; however, when sensitivity dropped below 85%, conventional strategies without spiral CT had a lower mortality. Many groups have reported sensitivities for PE which were well above this threshold of 85% [7, 8, 9, 10, 11], whereas others have disputed this [12].
The purpose of this study was to determine, in a prospective multicenter study, the utility of spiral CT as a second procedure following V/P scintigraphy, in routine clinical practice.
Materials and methods
Patients
All 1162 patients from the six participating hospitals (four academic and two general), referred for tests to diagnose or exclude PE between the period May 1997 and March 1998, were eligible. The protocol was approved by the six local medical ethics committees and by the research committee of the National Health Insurance Council who subsidized the study. Inclusion criteria were clinical suspicion of PE as decided by the referring physician based on his or her judgment of signs and symptoms such as pleuritic or non-specific chest pain, and dyspnea. Exclusion criteria were anticoagulant treatment at the time of screening for more than 24 h, one or more diagnostic tests for pulmonary emboli already performed, younger than 18 years of age, and pregnancy as indicated by the patient. The initial tests had to be performed within 24 h after the initial suspicion of PE, and all diagnostic tests had to be completed within 48 h. The study was designed in such a way that patients with contraindications for spiral CT and/or conventional pulmonary angiography were not initially excluded from participation in the study. It was therefore possible to determine in how many patients a spiral CT scan could be performed.
Study design
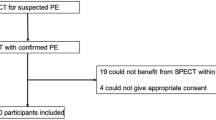
All patients started their diagnostic algorithm (Fig. 1) with perfusion scintigraphy in combination with chest radiography. A normal perfusion scintigram was considered to be enough evidence to exclude PE, and no further tests were performed. In patients with a segmental perfusion defect ventilation scintigraphy was obtained. Patients with subsegmental perfusion defects did not have ventilation scintigraphy, because there was no possibility of these scans becoming high probability. Subsequently. all patients with perfusion defects underwent spiral CT scanning and in case of a non-high-probability V/P scintigraphy result pulmonary angiography was added as reference. Pulmonary angiography was also added as reference when the CT scan was normal in case of a high-probability V/P scintigraphy. Patients with an inconclusive scintigraphic result, in whom CT findings were not verified by pulmonary angiography (protocol violators), were excluded. The final diagnosis in these patients were recorded for evaluation of possible bias; therefore, a normal perfusion scintigraphy, a pulmonary angiography, or a combination of a high-probability ventilation perfusion scan result and an abnormal CT scan result were taken as gold standard.
The responsible radiologist or nuclear physician in each participating center interpreted all test results. The diagnosis PE was used as reference diagnosis when pulmonary angiography was positive or when the combination of high-probability V/P scintigraphy with positive CT occurred. In the case of a negative pulmonary angiography or normal perfusion scintigraphy, PE was considered to be absent.
If PE was present, the largest involved branch was noted on the pulmonary angiography, or on the spiral CT scan in case of a high-probability V/P scan and a positive CT scan. A distinction was made between embolism in a segmental branch or larger, or subsegmental embolism.
Imaging studies
Chest radiographs were preferably made erect and in two directions (posterior–anterior and left lateral). If this was not possible, an anterior–posterior radiograph was made in bed.
Perfusion scintigraphy was performed after intravenous administration of 0.03 mCi/kg Tc-99m-labeled macro-aggregated serum albumin (Mallinckrodt, Petten, The Netherlands). Perfusion images were acquired with a minimum of 200,000 counts per view in at least four views: anterior; posterior; left anterior oblique; and right anterior oblique. Ventilation studies were performed using Krypton-81m (Mallinckrodt, Petten, The Netherlands) via Rb–Kr generator (Cygne-Amersham) in a single breath. A minimum of 200,000 counts was required for views in the same four directions as the perfusion scintigraphies. Gamma camera and collimator depended on local availability (Siemens Orbiter 3700 Digitrac, Siemens, Iselin, N.J.; ADAC Vertex and Argus, ADAC Laboratories, Maarsen, The Netherlands; Toshiba GCA501 and GCA9015a, Toshiba, Tokyo, Japan; Varicam, Elscint, Hackensack N.J.; collimators used MEAP, LEHR, MEHR, and LEGP)
Spiral CT scanners had to be capable of scanning at least 16 cm contiguously (Siemens Somatom Plus 4 and Somatom 6, Siemens, Erlangen, Germany; Elscint, Elscint, Haifa, Israel; Philips SR7000 and SR8000, Philips, Best, The Netherlands). Scanning was performed using a 5-mm table speed and 3-mm slice thickness. First the level of the aortic arch was determined on the scout view. Sixteen centimeters caudally the starting point was identified. The scanning process was then started from this position in a cranial direction, 20 s after intravenous injection of 900 mg/s of iodine for 40 s, either by injection of 100 ml of nonionic contrast agent with 35% iodine content (Iomeron 350, Bracco Byk Gulden, Konstanz, Germany) at an injection rate of 2.5 ml/s, or by injection of 120 ml of nonionic contrast agent 30% iodine content (Ultravist 300, Schering, Berlin, Germany) at an injection rate of 3.0 ml/s. The scan was performed during a single breath hold, although shallow breathing was allowed in very dyspneic patients. Images were reconstructed at 2-mm intervals. The radiologist responsible was allowed to make minor adjustments to the protocol such as adding a few seconds to the scanning delay time in cardiac-compromised patients and taking additional slices or small volumes in difficult areas.
Pulmonary angiography was performed by introducing a 7.1-F Grollmann catheter (Cook, Eindhoven, The Netherlands) in a femoral vein under local anesthesia. The left and right main pulmonary arteries were selectively catheterized in turn, and angiographic images were digitally acquired in antero-posterior and left anterior oblique views while injecting contrast material. Depending on the patient and the anticipated cardiac output, a protocol of 15 ml/s (total 30 ml) or 20 ml/s (40 ml total) was used. Occasionally, additional images were obtained in areas of special interest matching the perfusion images. Interpretation was done on a viewing station.
Image analysis
Ventilation–perfusion scintigraphies were interpreted in conjunction with the chest radiographs using the revised PIOPED criteria [13] and were classified as normal, inconclusive (non-high), or high probability.
The CT scans were interpreted by the lead radiologist on duty in each participating center. The CT and V/P scintigraphy were not independent tests. The observers were blinded to the detailed results of V/P scintigrams but were aware of the presence of abnormal scintigraphic test results. The radiologist was allowed to make occasional multiplanar reconstructions in problematic areas.
A workstation allowing cine-mode viewing with various window and level settings was always used. Standard settings were window width 350 HU, window level 50 HU for mediastinal structures and pulmonary vasculature, and window width 1500 HU and window level –500 HU for comparison of opacified lung vasculature and anatomical relation to bronchi and lung parenchyma. Of all CT scans with the final diagnosis of PE, the largest involved branch of the pulmonary vasculature was noted and categorized into two categories: segmental or larger artery involved; or subsegmental artery PE.
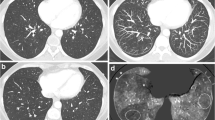
For the detection of PE on CT, the previously described criteria [9] were used. Pulmonary embolism was considered to be present if in the case of a well-opacified scan there was an intraluminal filling defect on more than one slice. A filling defect could be seen as a complete occlusion of the vessel, an eccentric partial-filling defect, or a partial central filling defect surrounded by contrast agent. A CT scan was considered negative if in the case of a good-quality scan no filling defects could be seen. A scan was considered to be equivocal when insufficient opacification of vessels or imaging artifacts were observed.
The interpretation of the angiograms was also done on a viewing station. For the detection of PE the diagnostic criteria described by Sagel and Greenspan [14] were used. If present, the largest branch involving PE was noted and as with CT-scan analysis categorized in two categories.
Statistical analysis
Statistical analyses were performed using statistical software (SPSS, Cary, N.C.). Data management and these analyses were performed by a specially assigned group of biostatisticians and epidemiologists. Sensitivity and specificity (including their 95% confidence intervals) for CT were calculated. In addition, the sensitivity for CT was calculated separately for both the subsegmental and segmental or larger PEs.
Results
Of 1162 patients suspected of having PE, 179 did not meet our criteria. Sixteen patients were below the age of 18 years, 11 were pregnant, in 6 there was an indication to treat immediately with thrombolytic therapy at the time of screening (1 of these 6 patients was also below the age of 18 years), and 43 patients had diagnostic tests performed prior to start of the study protocol. One hundred four patients could not be included in the study because the diagnostic work-up could not be started within 24 h after screening due to logistical problems during weekends and holidays. Informed consent was obtained from 627 patients of the remaining 983 eligible patients (64%).
In 101 patients the study protocol was not completed because not all imaging studies were performed within 48 h due to the following reasons: logistical problems or hardware down time (17 patients); withdrawal of informed consent (12 patients); withdrawal by the treating physician (21 patients); medical reasons (28 patients) such as contrast allergy; deterioration; or withdrawal by the treating physician because of alternative diagnosis (18 patients). In 5 patients the reason for not completing the protocol was unknown (Table 1 ).
Ultimately 526 patients with a mean age of 51.2 years were included. Male-to-female ratio was 0.66. Only 18% were inpatients, and 82% were outpatients. Eighty-three patients (16%) had had a venous thrombo-embolic event in the past. Median duration of symptoms was 3 days. The characteristics of a sample of 141 consecutive patients who were excluded during 2 months of the study were evaluated for comparison with all 526 study patients. The group of patients included had no different male-to-female ratio, frequency of comorbidity, or risk factors for PE such as immobilization, previous trauma or surgery, use of oral contraceptives, familial risks or inherited blood disorders, as compared with the excluded group.
Of the 526 patients available for complete analysis, 247 (47%) had a normal perfusion scintigraphy. The findings in the entire group with abnormal scintigraphies and the findings in two subgroups (high probability V/P scintigraphy vs non-high-probability V/P scintigraphy) are given below and in Table 2 .
Entire population
Two hundred seventy-nine patients had an abnormal perfusion scintigram. Results could not be analyzed in 27 patients. In 18 of these patients CT was equivocal (pulmonary angiography positive for PE in 3), in 7 patients pulmonary angiography was non-conclusive (CT positive for PE in 2), and in 2 patients both CT and angiography were non-conclusive. Eighteen patients were considered to have no PE, 9 patients were treated for PE on the results of CT, pulmonary angiography, or a high-probability V/Q scan result.
When compared with either a concordant high-probability VP scintigraphy or abnormal pulmonary angiography, spiral CT correctly identified 117 of 135 patients with PE, and 106 of 117 patients without PE. Calculated sensitivity and specificity was therefore 87 and 91%, respectively (Table 2 ).
Prevalence of PE in the group of patients that had a CT scan was 53%. Positive and negative predictive values were 91 and 86%, respectively (Table 2 ).
High-probability group
Perfusion–ventilation scintigraphy resulted in high probability in 113 patients. The CT was non-diagnostic in 2 patients and angiography was non-diagnostic in 3 patients. In 94 of 108 patients spiral CT confirmed the presence of pulmonary emboli. The CT was negative (discordant) in the remaining 14 patients. Angiography was negative in 11 patients and positive for PE in 3 patients. There were thus 11 true- and 3 false-negative CT diagnoses (Table 2 ).
Non-high-probability group
Ventilation–perfusion scintigraphy was inconclusive in 166 patients (32%) because of the presence of segmental perfusion defects matched by ventilation defects (76 patients), or because of subsegmental perfusion defects only (90 patients). In 4 patients pulmonary angiography was inconclusive and in 17 patients the CT scan result was inconclusive. Pulmonary angiography was positive for PE in 2 and negative in 15 of these 17 patients with non-conclusive CT scan results. In 1 patient both pulmonary angiography and CT scan were of insufficient non-conclusive quality; thus, in 144 patients CT was compared with angiography (Table 2 ). There were 22 true-positive, 96 true-negative, 12 false-positive, and 14 false-negative CT results.
Segmental analysis of CT-scan results
Pulmonary embolism was found in 135 patients of 252 patients with conclusive CT scans. A segmental analysis was available in all of these 135 scans. The results are summarized in Table 3 . In 111 patients PE was found in segmental or larger vessels: in 104 patients CT confirmed the presence of PE, and in 7 patients no PE was considered to be present. In 24 patients we found evidence of PE in subsegmental vessels. In 13 patients the CT scan was considered positive for PE, and the other 11 again were considered normal.
We also compared the results of the 18 false-negative CT scans (7 segmental and 11 subsegmental) with the initial result of V/P scintigraphy. Fifteen CT scans were from patients with non-high V/P scintigraphy results. Only 3 were from patients with an initial high-probability result.
Discussion
The role of spiral CT in the diagnostic algorithm for PE is still controversial. It is likely that various strategies, depending on population characteristics, local expertise, and availability of diagnostic tests, are appropriate. Our detailed description of the population and registration of patients who were excluded allows comparison with various other studies and indicates that our population is representative of a general unselected population. The prevalence of PE in this study group (26%) was similar to that found in the PIOPED group (28%) and other studies [6] using conventional angiography as gold standard. The ratio of out- vs inpatients was somewhat higher than reported before [1, 5]. Also, we did not find any differences with regard to comorbidity, risk factors for PE, and demographic characteristics between patients who participated in the study and a random sample of consecutive patients that were excluded.
We evaluated the performance of spiral CT in a routine clinical situation, following an abnormal V/P scan result. Patients were, without selection, recruited in two academic and four general hospitals. The initial test was perfusion scintigraphy. Perfusion scintigraphy is a readily available robust uncontroversial technique with a high negative predictive value (87%) [13] that can be used to safely reduce considerably the volume of patients needing additional tests. In our population 47% of patients had a normal perfusion scintigram and did not require further testing for PE. The patients, who had perfusion abnormalities on the scintigram, were analyzed according to our predefined protocol using standard equipment and local expertise available at the time of analysis. The radiologists knew that scintigrams were not normal but had no information regarding the category of scintigraphic findings. In 27 patients the interpreting radiologists were uncertain about the results of the CT scan, the pulmonary angiogram, or both. A final clinical consensus diagnosis made in these patients based on all available information combined showed that two-thirds of these patients did not have pulmonary emboli. It is unlikely that exclusion of these incomplete data sets introduces significant bias because these data were incomplete, not only because of non-conclusive test results, but also because of inconclusive results of the gold standard. In a clinical situation equivocal test results also do not have a major impact because they are identified as equivocal. They are therefore no basis for clinical management and other information, or additional tests will be used.
The sensitivity (87%) and specificity (91%) of spiral CT in our population relative to our gold standard was lower than that of reports from specialized centers [8, 9]. The sensitivity and specificity were, on the other hand, higher than some of the more skeptical reports [11, 12]. Our sensitivity was similar to the threshold of a 85% sensitivity required to avoid higher mortality than conventional strategies not using CT. We think that the performance of spiral CT in our routine clinical setting is realistic. We consider our data as baseline, in view of the current technical multi-detector ring CT revolution that is generally expected to result in better performance [16, 17], although more prospective studies are clearly needed. Increased technical quality will probably even decrease the problem of not detected subsegmental emboli, which is the main cause of false-negative CT results. In 24 of our 135 patients with pulmonary emboli, a subsegmental artery was the largest artery involved. Eleven of these 24 patients (46%) had a false-negative CT, whereas only 7 of 104 patients (7%) with involvement of at least a segmental artery had a false-negative CT. When considering only the results of segmental PE our sensitivity increased to 94%. The clinical relevance of isolated subsegmental embolism, however, remains unclear. Some authors have mentioned that the detection of subsegmental PE in otherwise healthy patients might be less important [18].
The accuracy of our algorithm can also be improved by implementing stricter quality-control methods (CT scanning technique, educating radiologists) [19, 20] and by adding additional tests; however, the design of a new algorithm is beyond the scope of our current study.
In view of our routine clinical setting we think that the performance of our algorithm with CT as a second-line test is acceptable if we look at the entire population. We were, however, disappointed with the added value of CT relative to the prevalence in the two subpopulations. The prevalence of pulmonary emboli in the group with high probability scintigrams was 90%. The CT test results are accurate, but the gain in terms of sensitivity (97%), specificity (100%), and positive predictive value (100%) can only be small. The negative predictive value of 78%, on the other hand, is low. With CT we were able to identify 11 (10%) patients who did not have PE at the cost of 3 (3%) patients that had a true-positive V/P scintigraphy but a false-negative CT. The CT test results in this subpopulation were accurate with the exception of a relative high fraction of false-negative results. We could not determine the potential impact of a known high-probability scintigraphic test result on the accuracy of CT [7, 10]
The yield of CT in patients with a non-high-probability scintigrams is more substantial in view of the prevalence of 25%, but test results of CT are poor in this group. Only the relatively high fraction of true negatives result in somewhat better specificity (89%) and negative predictive value (87%). Many false negatives result unfortunately in unacceptable low sensitivity (61%) and positive predictive value (65%).
Apart from studies determining sensitivities and specificities of spiral CT [9, 10], several groups have reported on the performance of CT in various specific clinical settings [21, 22]. Ferretti et al. reported a false-negative rate for CT of 5.4% in a population of 164 with intermediate V/P scintigraphy in combination with normal duplex ultrasound of the legs [7]. Our 7.1% rate of false negatives for the entire population and 9.7% for the non-high-probability scintigraphy group fit within their 95% confidence interval (1.3–9.7%). There are obviously various differences, such as the use of ultrasound, and the use of clinical follow-up as gold standard, that prohibit comparison of results in detail.
Our study design was similar to that used in the single-center prospective study performed by van Rossum et al. [10]. We believe our lower sensitivity and specificity relative to those reported by them (sensitivity 95%, specificity 97%) show that the reported high results cannot be maintained in a routine clinical environment.
Various gold standards have been used by previous researchers. We believe that our gold standard is acceptable in view of the power of concordance of two positive tests (spiral CT and high-probability V/P scintigraphy), both with high positive predictive values [9, 10], as well as the high predictive values of pulmonary angiography. Disadvantages of our definition of gold standard include the interobserver variability of pulmonary angiography (reaching 34%) [23], and the dependence of tests and gold standards upon each other. All current imaging techniques directly or indirectly visualize the effects of occluded or non-occluded vessels. The quality of our gold standard, however, is similar to that used by others and, because of practical and ethical reasons, difficult to improve. The potential impact of angiographic interobserver variability on gold standard and subsequently on study results can be demonstrated by eliminating subsegmental emboli. When considering only the results of segmental PE our sensitivity increased to 94%.
This study showed that spiral CT in patients without a normal perfusion scintigram is an acceptable test. The sensitivity of 87% was within the previously calculated range that does not increase mortality [5]. There remain, however, problems with too many false negatives. When compared with V/P scintigraphy and pulmonary angiography, spiral CT does have an important advantage: Because of the ability of transverse imaging, spiral CT is also able to give information on the other structures of the thorax and therefore a possible alternative explanation of the complaints in the absence of PE. In patients with uncertain clinical signs in whom the differential diagnosis includes other chest abnormalities, CT may be used as a first-line test.
We therefore conclude that in a routine clinical setting current CT technology has limited value as a second diagnostic test because of low added value in patients with a high-probability lung scan and low sensitivity in patients with non-high-probability lung scan result.
References
Kauczor HU, Heussel CP, Thelen M (1999) Update on diagnostic strategies of pulmonary embolism. Eur Radiol 9:262–275
Hull RD, Hirsh J, Carter CJ, Raskob GE et al. (1985) Diagnostic value of ventilation–perfusion lung scanning in patients with suspected pulmonary embolism. Chest 88:819–828
Schibany N, Fleischman D, Thallinger C, Schibany A, Hane J, Ba-Ssalamah A, Herold CJ (2001) Equipment availability and diagnostic strategies for suspected pulmonary embolism in Austria. Eur Radiol 11:2287–2294
The PIOPED investigators (1990) Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). J Am Med Assoc 263:2753–2759
Van Erkel AR, van Rossum AB, Bloem JL, Kievit J, Pattynama PMT (1996) Spiral CT angiography for suspected pulmonary embolism: a cost-effectiveness analysis. Radiology 201:29–36
Rathbun SW, Raskob GE, Whitsett TL (2000) Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review. Ann Intern Med 132:227–232
Ferretti GR, Bosson PD, Buffaz PD et al. (1997) Acute pulmonary embolism: role of helical CT in 164 patients with intermediate probability at ventilation–perfusion scintigraphy and normal results at duplex US of the legs. Radiology 205:453–458
Mayo JR, Remy-Jardin M, Müller NL et al. (1997) Pulmonary embolism: prospective comparison of spiral-CT with ventilation–perfusion scintigraphy. Radiology 205:447–452
Remy-Jardin M, Remy J, Deschildre F et al. (1996) Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 200:699–706
Van Rossum AB, Treurniet FE, Kieft GJ, Smith SJ, Schepers-Bok R (1996) Role of spiral volumetric computed tomographic scanning in the assessment of patients with clinical suspicion of pulmonary embolism and an abnormal ventilation/perfusion lung scan. Thorax 51:23–28
Goodman LR, Curtin JJ, Mewissen MW et al. (1995) Detection of pulmonary embolism in patients with unresolved clinical and scintigraphic diagnosis: helical CT versus angiography. Am J Roentgenol 164:1369–1374
Drucker EA, Rivitz SM, Shepard JA et al. (1998) Acute pulmonary embolism: assessment of helical CT for diagnosis. Radiology 209:235–241
Worsley DF, Alavi A (1995) Comprehensive analysis of the results of the PIOPED study. J.Nucl Med 36:2380–2387
Sagel SS, Greenspan RH (1970) Nonuniform pulmonary arterial perfusion: pulmonary embolism. Radiology 99:541–548
Blum AG, Delfau F, Grignon B et al. (1994) Spiral-computed tomography versus pulmonary angiography in the diagnosis of acute massive pulmonary embolism. Am J Cardiol 74:96–98
Qanadli SD, El Hajjam M, Mesurolle B et al. (2000) Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 217:447–455
Schoepf UJ, Kessler MA, Rieger CT, Herzog P, Klotz E, Weisgigl S, Becker CR, Exarhos DN, Reiser MF (2001) Multislice CT imaging of pulmonary embolism. Eur Radiol 11:2278–2286
Stein PD, Goldhaber SZ, Gottschalk A et al. (1998) Opinions/hypotheses: opinions regarding the diagnosis and management of venous thromboembolic disease. Chest 113:499–504
Remy-Jardin M, Remy J, Artaud D, Deschildre F, Duhamel A (1997) Peripheral pulmonary arteries: optimization of the spiral CT acquisition protocol. Radiology 204:157–1563
Beigelman C, Chartrand-Lefebvre C, Howarth N, Grenier P (1998) Pitfalls in diagnosis of pulmonary embolism with helical CT angiography. Am J Roentgenol 171:579–585
Miniati M, Pistolesi M, Marini C, Ricco G di, Formichi B, Prediletto R et al. (1996) Value of perfusion lung scan in the diagnosis of pulmonary embolism: results of the Prospective Investigative Study of Acute Pulmonary Embolism diagnosis (PISA-PED). Am J Respir Crit Care Med 154:1387–1393
Garg K, Welsh CH, Feyerabend AJ et al. (1998) Pulmonary embolism: diagnosis with spiral CT and ventilation–perfusion scintigraphy: correlation with pulmonary angiographic results or clinical outcome. Radiology 208:201–208
Van Beek EJ, Bakker AJ, Reekers JA (1996) Pulmonary embolism: interobserver agreement in the interpretation of conventional angiographic and DSA images in patients with nondiagnostic lung scan results. Radiology 198:721–724
Acknowledgements.
Financial support for this study was provided by the Dutch National Health Insurance Council (Ziekenfondsraad), grant nr. D94–090. The results of this study are part of the results of the ANTELOPE study group (Advances of New Technologies Evaluating Localisation Of Pulmonary Embolism), a Dutch prospective multicenter trial on pulmonary embolism. Participants: Academic Medical Center, Amsterdam, Dept. of Vascular Medicine, Dept. of Nuclear Medicine, Dept. of Radiology: B.J. Sanson, H.R. Büller, H.J. Baarslag, and J.A. Reekers. Leiden University Medical Center, Leiden. Dept. of Radiology, Dept. of General Internal Medicine, Dept. of Nuclear Medicine: W. de Monyé, P.M.T. Pattynama, M.V. Huisman, and A.E. Meinders. Leyenburg Hospital, The Hague: Dept. of Radiology, Dept. of General Internal Medicine: M.J.L. van Strijen, G.J. Kieft, F.E.E. Treurniet, and S.J. Smith. Slotervaart Hospital, Amsterdam. Dept. of Internal Medicine: M.R. MacGillavry, D.P.M. Brandjes, and F. Turkstra. University Hospital VU, Amsterdam: Dept. of Pulmonary Medicine, Dept. of Nuclear Medicine, Dept. of Radiology: P.J. Hagen, P.E. Postmus, F.G. van den Berg, R.P. Golding, and R.A. Manoliu. University Hospital Utrecht, Utrecht: Dept. of Radiology, Dept. of General Internal Medicine, Dept. of Pulmonary Medicine: I.J.C. Hartmann, J.D. Banga, T.H. Lo, and P.F.G.M. van Waes. The authors thank A. van de Berg for her help with statistical analysis of the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Strijen, M.J.L., de Monyé, W., Kieft, G.J. et al. Diagnosis of pulmonary embolism with spiral CT as a second procedure following scintigraphy. Eur Radiol 13, 1501–1507 (2003). https://doi.org/10.1007/s00330-002-1709-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-002-1709-3