Abstract
Due to the morphological variability, the identification of moss species can be difficult when the plant grows in submerged environments. The taxonomic status of an aquatic moss found in lakes of the Sôya Coast region, East Antarctica, had been controversial, and then, it was investigated by molecular phylogenetic and haplotype network analysis of two chloroplast regions (rps4 and trnL-F) and/or the nuclear ribosomal ITS region. Based on the results of the analyses, the moss was assigned to the genus Leptobryum and determined to be conspecific with Leptobryum wilsonii (Mitt.) Broth. described from South America. Almost no genetic variation was observed between all samples from Antarctic lakes and some samples of L. wilsonii from Chile. Molecular and geohistorical evidence suggests that immigration of L. wilsonii into Antarctic lakes took place during the Holocene via long-distance dispersal from South America. This study gives a clear example of the widespread assumption that most of the Antarctic moss species are post-glacial immigrants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
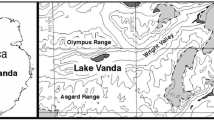
In various lakes of the Sôya Coast region, Dronning Maud Land, East Antarctica (Fig. 1), two aquatic moss species are currently recognized as components of the benthic vegetation (e.g., Imura et al. 2003). As reported by Kanda and Iwatsuki (1989), one is an aquatic form of Bryum pseudotriquetrum (Hedw.) P. Gaertn., B. Mey. & Scherb., a common species in Antarctic terrestrial environments. The identification of the other species is still controversial. The unidentified moss is sterile and is represented by non-characteristic filiform plants possessing oblong-lanceolate and unbordered leaves with short and weak costa and characteristic pale brown rhizoidal tubers (asexual propagules) (Fig. 2a–d). This moss is also the main component of “moss pillar,” a unique column or pillar-shaped vegetational structure consisting of two aquatic mosses, algae, and microorganisms (e.g., Nakai et al. 2012a, b), which has been found only at the bottom of some of the lakes in this region (Fig. 2e; Imura et al. 1999, 2003).
As summarized in Table 1, the moss has a long and complex taxonomic history. Nakanishi (1977) reported a moss with rhizoidal tubers from the bottom of several lakes in the Sôya Coast region and noted its external similarity to Bryum korotkevicziae Sav. & Smirn. and the variety hollerbachii Sav. & Smirn. that described from lakes in the Bunger Hills (66°18′ S, 100°45′ E), East Antarctica (Savich-Lyubitskaya and Smirnova 1959, 1960). Because the plants were sterile, Ochi (1979) treated the moss as a Bryum sp. and Imura and Kanda (1986) described its smooth rhizoids and spherical tubers but did likewise. Kanda and Iwatsuki (1989) considered it to be a Dicranella sp. The species was identified as Leptobryum pyriforme (Hedw.) Wilson by Imura et al. (1992) based on its rhizoidal tubers and synoicous inflorescences under culture conditions, but later Imura et al. (1999) treated it merely as a Leptobryum sp. In a comprehensive review of the genus Leptobryum, Arts (2001) recognized only two valid species, L. pyriforme and L. wilsonii. Using the illustration by Kanda and Iwatsuki (1989) as a reference, he considered the aquatic moss from Sôya Coast region to be L. wilsonii based on leaf characters. This species designation was questioned by Imura et al. (2003), however, because of the synoicous inflorescence—considered to be characteristic of L. pyriforme rather than L. wilsonii—that had been previously observed on cultured plants (Imura et al. 1992). The most recent taxonomic interpretation of this moss is that of Ochyra et al. (2008). These authors argued that sexuality (i.e., monoecy or dioecy) is not always a reliable diagnostic character for these species, especially under culture conditions, and recognized it as L. wilsonii. At the same time, based on type specimens of L. wilsonii from South America, they proposed the new combination Pohlia wilsonii (Mitt.) Ochyra, as they considered that there was a taxonomic affinity between L. wilsonii and Pohlia section Cacodon comprising the propaguliferous species in the genus (e.g., Shaw 1984). Morphological similarities between L. wilsonii and some species of Pohlia have also been noted by other authors (Shaw 1985 and Arts 1995). This species was actually even described previously as Pohlia integra (Cardot) A.J. Shaw (Shaw 1982). Recent molecular phylogenetic studies, however, suggest that Leptobryum and Pohlia are only distantly related (e.g., Cox et al. 2000; Goffinet et al. 2001; Guerra et al. 2011).
Apart from classification difficulties with respect to L. wilsonii versus Pohlia, much of the taxonomic confusion surrounding the aquatic moss from the Sôya Coast region is due to the sterile condition and phenotypic plasticity of the plant. It is well known that morphological characters of mosses often vary when the plants are submerged (e.g., Lodge 1959; Priddle 1979). In this study, we performed a molecular phylogenetic analysis to determine the taxonomic status of this puzzling moss. In addition, we conducted a haplotype network analysis to uncover detailed phylogenetic relationships and genetic variation within related taxa. Using the results of these analyses and geohistorical considerations, we also examined the origin of this species and its immigration history into Antarctic lakes.
Materials and methods
Plant materials
In the Sôya Coast region, an aquatic moss with rhizoidal tubers has been found in 26 lakes in three ice-free areas: Langhovde (one lake), Skallen (one lake), and Skarvsnes (24 lakes) (Imura et al. 2003 as Leptobryum sp.). In this study, 14 samples of this moss that collected from 11 lakes in the three ice-free areas were used for DNA sequencing as shown in Table 2. After collection, 12 of the samples were cultured under laboratory conditions to obtain high-quality DNA. The other two samples were kept frozen until DNA extraction.
Because the aquatic moss was thought to belong to Leptobryum (e.g., Arts 2001 as L. wilsonii; Imura et al. 1992 as L. pyriforme) or Pohlia (Ochyra et al. 2008 as P. wilsonii), we also included 10 samples of L. pyriforme from various regions of the world and four samples of L. wilsonii from South America (Table 2) to test their phylogenetic relationships to this species. The 10 samples of L. pyriforme were selected from a set of 49 samples used in a preliminary global phylogeographic study of L. pyriforme based on rps4, trnL-F, and ITS regions (Kato and Imura, unpublished data), and were chosen to represent the maximum intraspecific variation currently known for this species. Sequence data for Pohlia species were obtained from DNA databases (DDBJ/EMBL/GenBank) as shown in Table 3.
DNA extraction, PCR, and DNA sequencing
Total DNA was extracted using a modified version of the standard CTAB method (Murray and Thompson 1980). Nucleotide sequences of two chloroplast DNA (cpDNA) regions—the ribosomal protein S4 gene (rps4) and the trnL (UAA) 5′ exon–trnF (GAA) exon region (trnL-F)—and the internal transcribed spacer region of nuclear ribosomal DNA (ITS: ITS1-5.8S rDNA-ITS2) were amplified for each sample by polymerase chain reaction (PCR). PCR was performed using 0.5 units of Takara Ex Taq (Takara Bio, Shiga, Japan) or 0.4 units of Kod FX Neo (Toyobo, Osaka, Japan) in 20-μl reaction volumes according to each manufacturer’s instructions. Reaction conditions for PCRs using Takara Ex Taq consisted of 4 min of initial denaturation at 94 °C, followed by 30–35 cycles of denaturation (94 °C; 30 s), annealing (52 °C for cpDNA regions, 55 °C for ITS region; 30 s), and extension (72 °C; 60 s), ending with a final extension step (72 °C; 7 min). Samples for which PCR using Takara Ex Taq was unsuccessful were amplified using Kod FX Neo under the following reaction conditions: initial denaturation at 94 °C (2 min), followed by 30–35 cycles of denaturation (98 °C; 10 s), annealing (58 °C; 30 s), and extension (68 °C; 60 s). Primers used for amplification of the two cpDNA regions were rps5 (Nadot et al. 1995) and trnas (Souza-Chies et al. 1997) for rps4, and trnC and trnF (Taberlet et al. 1991) for trnL-F. From the cultured material, the entire ITS region was amplified using external primers ITS1 and ITS4 (White et al. 1990). From the uncultured material, the ITS region was amplified in two parts (i.e., ITS1 and ITS2 regions) using external primers ITSBF (5′-CATTAAACCTTATCATTTAGAGGAAGGAG-3′) or ITS1 for the forward primer, and ITS4 for the reverse primer, in combination with internal primers ITSC bryo and ITSD bryo (Sabovljevic et al. 2005) (We developed ITSBF to avoid fungal contamination problems.). Primers used for DNA sequencing were identical to those used for PCR amplification. Amplified fragments were purified with ExoSAP-IT (GE Healthcare, Waukesha, WI, USA), and DNA sequencing in both directions was accomplished using a BigDye Terminator v3.1 Cycle Sequencing Kit and an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The resulting sequences for each DNA region were assembled and edited using DNA Baser version 3 (Heracle Software, Lilienthal, Germany).
Detailed information of samples sequenced in this study, including voucher, sample ID, and GenBank accession number, is listed in Table 2. These samples are represented as their sample IDs throughout this paper (e.g., 14 samples of the aquatic moss from lakes of the Sôya Coast region are represented as AM1–AM14).
Sequence alignment and phylogenetic analysis
A molecular phylogenetic analysis was performed based on cpDNA (rps4 and trnL-F) regions. In addition to the 28 samples sequenced in this study, sequence data for 61 samples were obtained from DNA databases (DDBJ/EMBL/GenBank) and incorporated into the analysis. These additional samples included two accessions of L. wilsonii (Goffinet 5608 and MUB 28078), 10 species of Pohlia, and 49 samples representing species from 43 other genera (Table 3).
Sequences from each region were pre-aligned using the MUSCLE algorithm (Edgar 2004) as implemented in MEGA 5.05 (Tamura et al. 2011), followed by manual refinement. Incomplete data at the beginning and end of sequences as well as sites characterized by ambiguous alignment, the presence of insertion/deletions (indels), and mixed bases were excluded from further analysis. Samples with completely identical sequences in the final aligned matrix were treated as a single operational taxonomic unit (OTU).
Phylogenetic relationships were assessed using maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) methods as implemented in MEGA 5.05. Selection of nucleotide substitution models for ML was also performed using MEGA 5.05. Based on the lowest Akaike information criterion (AIC) value, GTR+G+I was selected as the best-fit model for constructing the ML tree. The ML tree was inferred from a close-neighbor-interchange heuristic search with an automatically generated initial tree. The MP tree was obtained using a close-neighbor-interchange algorithm set at search level 3, in which the initial trees were obtained using 10 random addition replicates. For the NJ tree, the maximum composite likelihood method was used to calculate evolutionary distances. In each method, branch support was assessed using bootstrap (1,000 replicates). Because all three methods (ML, MP, and NJ) produced largely congruent tree topologies, only the ML tree is presented. Bootstrap support values (BS) greater than 50 both on the MP and NJ trees were overlaid to assess the robustness of each branch of the ML phylogram.
Sequence alignment and haplotype network analysis
The results of sequence alignment and phylogenetic analysis suggested that the aquatic moss belongs to the genus Leptobryum and is conspecific with L. wilsonii (cf. Fig. 3). Some informative trnL-F sites were excluded during sequence alignment with phylogenetically distant species; however, phylogenetic resolution within Leptobryum was reduced. Consequently, only samples of Leptobryum species and the aquatic moss were used in subsequent analyses. Phylogenetic relationships and genetic variations among these samples were evaluated by constructing haplotype networks based on both cpDNA sequence data and a combined data set of sequences of cpDNA and the rapidly evolving nuclear ITS region. For the haplotype network based on cpDNA sequence data, we analyzed the 28 samples sequenced in this study (Table 2) and two samples of L. wilsonii from DNA databases (Goffinet 5608 and MUB 28078 in Table 3). For the haplotype network based on the cpDNA-ITS combined sequence data, only the 28 samples sequenced in this study were analyzed.
Maximum likelihood tree (ln-likelihood = −5198.97) based on cpDNA regions, with the phylogenetic position of the aquatic moss highlighted in gray. Number of samples and sample IDs are listed in parentheses. Numbers near branches are bootstrap support values above 50 for branches recovered using maximum likelihood, maximum parsimony, and neighbor-joining methods
Alignment of each genomic region was carried out as for the phylogenetic analysis, except that indels were included. Haplotype network analyses were performed using statistical parsimony as implemented in TCS (Clement et al. 2000), with the parsimony connection limit set to 95 % and indels were treated as fifth character states. Genetic variation among samples, represented as a percentage, was calculated based on the number of variable sites and alignment length. The calculation was performed using a pairwise sequence-identity matrix program implemented in BioEdit (Hall 1999), followed by manual confirmation.
Results
Sequence alignment and phylogenetic analysis
The final aligned cpDNA matrix for 86 samples contained 786 characters (518 rps4 and 268 trnL-F), of which 486 were constant, 143 were autapomorphic, and 157 were parsimony informative. Because two samples of L. wilsonii (CHL2 and CHL3) and 14 samples of the aquatic moss (AM1–AM14) had completely identical sequences, they were treated as one OTU. Of the remaining samples, four samples of L. wilsonii (BOL, CHL1, Goffinet 5608, and MUB 28078), nine samples of L. pyriforme, and two samples of Pohlia (P. annotina, and P. camptotrachela) were treated as each one OTU. Consequently, a total of 62 OTUs were used in the analysis. The ML tree (ln-likelihood = −5198.97) showing phylogenetic relationships among these samples is presented in Fig. 3.
In the ML tree, the OTU comprising two samples of L. wilsonii (CHL2 and CHL3) and 14 samples of the aquatic moss (AM1–AM14) is to be part of a well-supported clade (BS = 93 [ML]/95 [MP]/94 [NJ]) that also includes the remaining 14 Leptobryum samples (Fig. 3). This Leptobryum clade, which consists of two well-supported subclades corresponding to each species of the genus, L. pyriforme and L. wilsonii, is closely allied to samples of Meesiaceae and Splachnaceae, and together with them forms a well-supported larger clade (BS = 99/99/99) corresponding to Splachnales. On the other hand, 10 samples of Pohlia are included in a moderately supported clade (BS = 82/89/93) corresponding to the Mniaceae-Mielichhoferiaceae lineage reported by Guerra et al. (2011). Within this clade, samples of Pohlia are not monophyletic, although the three samples of Pohlia sect. Cacodon (P. andalusica, P. annotina, and P. camptotrachela) constitute a moderately supported clade (BS = 86/84/88).
Haplotype network analysis
The final matrix (including gaps) of cpDNA and ITS sequence data for the samples contained 1719 characters (570 rps4, 431 trnL-F, and 718 ITS). Statistical parsimony networks based on cpDNA and cpDNA-ITS combined sequence data are shown in Fig. 4. In the network based on cpDNA sequences (Fig. 4a), two samples of L. wilsonii (CHL2 and CHL3) and 14 samples of the aquatic moss (AM1–AM14) are assigned to one haplotype because they have completely identical sequences. Four samples of L. wilsonii (BOL, CHL1, Goffinet 5608, and MUB 28078) are assigned to two haplotypes, and 10 samples of L. pyriforme are to six haplotypes.
Statistical parsimony networks for studied samples of two Leptobryum species and the aquatic moss. (a) The network for 30 samples based on cpDNA sequences. Samples from DNA databases are underlined and abbreviated; G 5608 corresponds to Goffinet 5608 and M 28078 to MUB 28078. (b) The network for 28 samples based on cpDNA-ITS combined sequences. Circle size is proportional to the number of samples assigned to an identical haplotype. Haplotypes with gray backgrounds consist of samples of L. wilsonii and the aquatic moss. Haplotypes with white backgrounds consist of samples of L. pyriforme. A filled circle represents a hypothetical haplotype. The straight and wavy line connecting each haplotype represents a single base substitution and a single indel between them. The colors of straight and wavy lines indicate whether the variations occurred in cpDNA regions (black) or the ITS region (white). Sample IDs of the aquatic moss are indicated in bold type. Bidirectional arrows with comments show the genetic variation between designated haplotypes
In the network based on cpDNA-ITS combined data (Fig. 4b), four unconnected subnetworks were obtained under the 95 % connection limit (= 18). Subnetworks-1 and subnetworks-2 contain the five haplotypes associated with samples of L. wilsonii and the aquatic moss; subnetworks-3 and subnetworks-4 comprise eight haplotypes representing L. pyriforme. Within subnetwork-1 (Fig. 4b), the three haplotypes are closely related, being separated from one another only by variation in the ITS region. Thirteen samples of the aquatic moss (AM2–AM14) are assigned to one of the haplotypes, which is separated from another haplotype, consisting of a single sample of the aquatic moss (AM1), by one indel. The other haplotype, consisting of two samples of L. wilsonii (CHL2 and CHL3), is separated from the two aquatic moss haplotypes (AM1 and AM2–AM14) by one base substitution and three or four indels. Subnetwork-2 contains two haplotypes, each corresponding to a single sample of L. wilsonii (BOL and CHL1), which are separated by five base substitutions and 12 indels. The most distant haplotypes of L. wilsonii and the aquatic moss, CHL2-CHL3 and BOL, vary by seven cpDNA sites (base substitutions) and 62 ITS sites (21 base substitutions and 41 indels), which correspond to L. wilsonii intraspecific variation of 4.1 % (69 variable sites out of 1681 total).Within L. pyriforme, haplotypes, consisting of one sample each, AK3 and CHN or USA, are the most distantly separated. They are separated by variations occurring in three cpDNA sites (base substitutions) and 56 ITS sites (19 base substitutions and 37 indels), corresponding to L. pyriforme intraspecific variation of 3.6 % (59 variable sites out of 1630 total). The closest haplotypes between L. wilsonii (including the aquatic moss) and L. pyriforme, AM1 and BEL-ZAF, or MEX-WANT are separated by changes in 13 cpDNA sites (12 base substitutions and one indel) and 119 ITS sites (29 base substitutions and 90 indels). This is equivalent to 7.8 % interspecific variation (132 variable sites out of 1688 total).
Discussion
In this study, we found that two samples of L. wilsonii from Chile (CHL2 and CHL3) and 14 samples of the aquatic moss from Antarctic lakes (AM1–AM14) had completely identical cpDNA sequences (Fig. 4a) and almost identical ITS sequences (Fig. 4b; subnetwork-1). The phylogenetic analysis accordingly placed the aquatic moss in the L. wilsonii subclade of the genus Leptobryum (Fig. 3). The aquatic moss is clearly conspecific with L. wilsonii as previously suggested by morphological examination (e.g., Arts 2001). In contrast, the new combination P. wilsonii proposed by Ochyra et al. (2008) is unacceptable because the molecular phylogenetic evidence indicates that Pohlia is distantly separated from Leptobryum at the order level (Fig. 3).
Members of the genus Leptobryum are defined by their shiny pear-shaped inflated capsules, long-pointed perichaetial leaves, and abundant characteristic rhizoidal tubers (e.g. Arts 2001; Brotherus 1924). In the taxonomic revision of Arts (2001), L. pottiaceum Dusén, L. escomelii Thér., L. stellatum (Herzog) Broth., and P. integra (Cardot) A.J. Shaw were reduced into synonymy under L. wilsonii; as a consequence, only two species—L. pyriforme and L. wilsonii—were recognized in the genus. Arts (2001) also recognized no intraspecific taxa in any of the two Leptobryum species. In the haplotype network analysis in our study, samples of L. wilsonii (including the aquatic moss) were separated into two subnetworks based on cpDNA-ITS combined sequences (Fig. 4b). These results suggest the possibility that each subnetwork may correspond to an independent taxon in the genus. We therefore attempted to evaluate the intraspecific variation in L. wilsonii by comparing it with intraspecific variation in L. pyriforme and interspecific variation between L. pyriforme and L. wilsonii. The L. wilsonii intraspecific variation, calculated as 4.1 %, was slightly higher than that observed within L. pyriforme (3.6 %) but lower than between the two species (7.8 %). It is thus inconclusive to recognize the two subnetworks of L. wilsonii as independent infrageneric taxa based on the current molecular data; however, the monospecificity of L. pyriforme also seems to be uncertain, as shown by the existence of unconnected networks in the species. To achieve a more precise taxonomic circumscription of L. wilsonii and also L. pyriforme, further molecular and morphological examinations with numerous samples, including type specimens of described taxa, may be needed.
Leptobryum pyriforme is widely known as a cosmopolitan weedy species and a pioneer of bare or disturbed land (e.g., Bradbury 2006). This species is even reported from maritime Antarctica: from Deception Island (63°00′ S, 60°40′ W) (specimen WANT in this study) (Lewis Smith 1984) and from Galindez Island (65°14′ S, 64°14′ W) (Ochyra and Tyshchenko 2006). In contrast, the distribution of L. wilsonii is more restricted. Outside Antarctica, occurrences of this species are concentrated in several South American countries (i.e., Argentina, Bolivia, Chile, Ecuador, Peru, and Uruguay), with only one known collection from Lesotho, Southern Africa (Arts 1995 as Pohlia integra), and Mexico, North America (Shaw 1982 as P. integra). In these regions, most specimens of L. wilsonii have been collected from moist and wet habitats as river-bank, ditch-side, seepage slope or wet rock in high elevation areas above 1,300 m up to 4,600 m (Arts 1995; Churchill et al. 2000; Arts 2001). Since this species seems to prefer wet condition but has never been collected from totally submerged environments, it is typically regarded as terrestrial moss species. In Antarctica, however, L. wilsonii has never been reported from terrestrial environments, having been found only in lakes of the Sôya Coast region and probably of the Schirmacher Oasis (70°45′ S, 11°38′ E). In the latter region, located over 1,000 km west of the Sôya Coast region, a scant collection of Leptobryum has been reported once from Lake Zub (Tewari and Pant 1996). Their voucher specimen was not available during our study, but judging from their short description and illustrations of its leaves and tubers, this moss appears to be conspecific with the aquatic moss from the Sôya Coast region, namely L. wilsonii. Populations of L. wilsonii in these Antarctic lakes are geographically separated, and their habitat type also varies from non-Antarctic populations.
In Antarctica, as well as L. wilsonii, occurrences of mosses in submerged environments have been well known. At present, totally 12 species, which correspond to ca. 11 % of Antarctic moss flora, are listed as aquatic mosses in Antarctica, although they also exist as terrestrial mosses elsewhere in the world (reviewed in Li et al. 2009). Nine of them have been observed both in Antarctic lakes and on their surrounding lands, and therefore, it seems to be reasonable to presume that these aquatic mosses have been derived from terrestrial populations of same species as suggested by Priddle (1979). The other three species [Drepanocladus longifolius (Mitt.) Paris, Plagiothecium orthocarpum Mitt. and L. wilsonii] have been known to be exclusively submerged in Antarctica. In comparison with the latter two species, populations of D. longifolius which have been found in some lakes in the northern Antarctic Peninsula region are geographically close to non-Antarctic (e.g., subantarctic and southern South American) populations (Ochyra et al. 2008; Li et al. 2009). In contrast, a population of P. orthocarpum, which has been collected once from Lake Glubokoye in the Schirmacher Oasis (Savich-Lyubitskaya and Smirnova 1964 as P. simonovii Sav. & Smirn.), is geographically distant to non-Antarctic (e.g., subantarctic South Georgia and Kerguelen islands) populations (Ochyra et al. 2008), as well as L. wilsonii. Thus, these two species could be regarded as highly isolated in Antarctic lakes, and therefore, the immigration processes of them are the topic of high interest.
Almost no genetic variation was observed between samples of L. wilsonii from Chile (CHL2 and CHL3) and those from Antarctic lakes (AM1–AM14) (Fig. 4b; subnetwork-1). In bryophyte taxa, such high sequence similarity among disjunct populations has been explained by either relictualism combined with slow evolutionary rates (e.g., stenoevolution sensu Frey et al. 1999, 2010) or recent long-distance dispersal (e.g., Shaw et al. 2003; Vanderpoorten et al. 2008). In the case of this study, the latter theory is more applicable to the distribution of L. wilsonii in Antarctic lakes, as the former theory (i.e., the persistence of this species in Antarctica before the last glacial period) must be rejected based on several lines of evidence. First, extant lakes of the Sôya Coast region are believed to have been formed during the Holocene (Iwasa et al. 2000; Seto et al. 2002; Matsumoto et al. 2006, 2010). For example, the oldest record of lake sediment cores so far reported in this region is 7,030 ± 59 years before present (BP) in Lake Skallen-Oike, which was inferred by radiocarbon dating (Matsumoto et al. 2010). The aquatic moss communities in this region are also thought to have been established relatively recently. The radiocarbon age of the oldest layer containing moss debris has been confirmed by Seto et al. (2002) as 3,200 years BP in Lake Akebi-ike and by Matsumoto et al. (2006) as 1,110 years BP in Lake Namazu-ike. Second, L. wilsonii has never been reported from terrestrial habitats in Antarctica, only from lakes. If L. wilsonii had survived in some Antarctic refugia during the last glacial period prior to lake formation in the Sôya Coast region, it might be expected to persist in present terrestrial habitats rather than lakes. These considerations, taken together, suggest that the immigration of L. wilsonii into Antarctic lakes took place during the Holocene by means of long-distance dispersal from other continents, presumably South America where this species has its current maximum occurrence. Based on their very low rates of endemism, most of Antarctic moss species have typically been assumed to be post-glacial immigrants from other continents (e.g., Peat et al. 2007; Convey et al. 2008; Ochyra et al. 2008). The immigration process of L. wilsonii into Antarctic lakes suggested by the molecular and geohistorical evidence in this study is to be an example supporting this widespread assumption.
A remaining important subject is the process of range expansion, which shapes the present distribution of L. wilsonii in Antarctic lakes. This process, which presumably corresponds to the frequency of dispersal events into Antarctic lakes from other continents and dispersal events among Antarctic lakes, may be detectable by analysis of genetic diversity and population genetic structure of this species. In this study, samples from Antarctic lakes had almost identical sequences. This suggests that Antarctic populations of this species are genetically nearly homogeneous, but this is not conclusive because of the small sample size in this study (14 samples) and the presumed slow evolutionary rate of the Antarctic plants due to their asexual reproduction. Population genetic analysis based on hypervariable DNA markers (e.g., microsatellites) along with exhaustive sampling of Antarctic and additional non-Antarctic samples of L. wilsonii would be needed to clarify this question.
References
Arts T (1995) Pohlia integra (Card.) Shaw a neglected species, recorded from South Africa and South America. J Bryol 18:791–796
Arts T (2001) The moss genus Leptobryum and the identity of Pohlia integra. J Bryol 23:325–330
Bradbury SM (2006) Response of the post-fire bryophyte community to salvage logging in boreal mixedwood forests of northeastern Alberta, Canada. For Ecol Manag 234:313–322
Brotherus VF (1924) Musci (Laubmoose). In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 10, 2nd edn. pp 143–478
Churchill SP, Griffin D III, Munõz J (2000) A checklist of the mosses of the tropical Andean countries. Ruizia 17:1–203
Clement M, Posada D, Crandall K (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
Convey P, Gibson JAE, Hillenbrand CD, Hodgson DA, Pugh PJA, Smellie JL, Stevens MI (2008) Antarctic terrestrial life—challenging the history of the frozen continent? Biol Rev 83:103–117
Cox CJ, Goffinet B, Newton AE, Shaw AJ, Hedderson TAJ (2000) Phylogenetic relationships among the diplolepideous-alternate mosses (Bryidae) inferred from nuclear and chloroplast DNA sequences. Bryologist 103:224–241
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Frey W, Stech M, Meißner K (1999) Chloroplast DNA-relationship in palaeoaustral Lopidium concinnum (Hypopterygiaceae, Musci). An example of stenoevolution in mosses. Studies in austral temperate rain forest bryophytes 2. Plant Syst Evol 218:67–75
Frey W, Pfeiffer T, Stech M (2010) Geomolecular divergence patterns of Gondwanan and Palaeoaustral bryophytes—an overview. Studies in austral temperate rain forest bryophytes 34. Nova Hedwigia 91:317–348
Goffinet B, Cox CJ, Shaw AJ, Hedderson TAJ (2001) The bryophyta (mosses): systematic and evolutionary inferences from an rps4 gene (cpDNA) phylogeny. Ann Bot 87:191–208
Guerra J, Jiménez-Martínez JF, Cano MJ, Jiménez-Fernández JA (2011) A contribution to the phylogenetic study of Mielichhoferiaceae-Mniaceae (Bryophyta) based on molecular sequence data. Nova Hedwigia 93:47–56
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Imura S, Kanda H (1986) The gemmae of the mosses collected from the Syowa Station area, Antarctica. Mem Natl Inst Polar Res Spec Issue 44:241–246
Imura S, Higuchi M, Kanda H, Iwatsuki Z (1992) Culture of rhizoidal tubers on an aquatic moss in the lakes near the Syowa station area, Antarctica. Polar Biosci 5:177–179
Imura S, Bando T, Saito S, Seto K, Kanda H (1999) Benthic moss pillars in Antarctic lakes. Polar Biol 22:137–140
Imura S, Bando T, Seto K, Ohtani S, Kudoh S, Kanda H (2003) Distribution of aquatic mosses in the Sôya Coast region, East Antarctica. Polar Biosci 16:1–10
Iwasa T, Bando T, Nakamura T, Imura S (2000) The environmental changes presumed by AMS 14C ages of algal sediments in Antarctic lakes, near the Syowa Station. Sum Res Using AMS Nagoya Univ 11:74–80 (in Japanese with English Abstract)
Kanda H, Iwatsuki Z (1989) Two aquatic mosses in the lakes near Syowa Station, Continental Antarctica. Hikobia 10:293–297
Lewis Smith RI (1984) Colonization and recovery by cryptogams following recent volcanic activity on Deception Island, South Shetland Islands. Br Antarct Surv Bull 62:25–51
Li SP, Ochyra R, Wu PC, Seppelt RD, Cai MH, Wang HY, Li CS (2009) Drepanocladus longifolius (Amblystegiaceae), an addition to the moss flora of King George Island, South Shetland Islands, with a review of Antarctic benthic mosses. Polar Biol 32:1415–1425
Lodge E (1959) Effects of certain cultivation treatments on the morphology of some British species of Drepanocladus. J Linn Soc Lond Bot 56:218–224
Matsumoto GI, Komori K, Enomoto A, Imura S, Takemura T, Ohyama Y, Kanda H (2006) Environmental changes in Syowa Station area of Antarctica during the last 2300 years inferred from organic components in lake sediment cores. Polar Biosci 19:51–62
Matsumoto GI, Tani Y, Seto K, Tazawa T, Yamamuro M, Watanabe T, Nakamura T, Takemura T, Imura S, Kanda H (2010) Holocene paleolimnological changes in Lake Skallen Oike in the Syowa Station area of Antarctica inferred from organic components in a sediment core (Sk4C-02). J Paleolimnol 44:677–693
Murray MG, Thompson WF (1980) Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Nadot S, Bittar G, Carter L, Lacroix R, Lejeune B (1995) A phylogenetic analysis of monocotyledons based on the chloroplast gene rps4, using parsimony and a new numerical phenetics method. Mol Phylogenet Evol 4:257–282
Nakai R, Abe T, Baba T, Imura S, Kagoshima H, Kanda H, Kanekiyo A, Kohara Y, Koi A, Nakamura K, Narita T, Niki H, Yanagihara K, Naganuma T (2012a) Microflorae of aquatic moss pillars in a freshwater lake, East Antarctica, based on fatty acid and 16S rRNA gene analyses. Polar Biol 35:425–433
Nakai R, Abe T, Baba T, Imura S, Kagoshima H, Kanda H, Kohara Y, Koi A, Niki H, Yanagihara K, Naganuma T (2012b) Eukaryotic phylotypes in aquatic moss pillars inhabiting a freshwater lake in East Antarctica, based on 18S rRNA gene analysis. Polar Biol 35:1495–1504
Nakanishi S (1977) Ecological studies of the moss and lichen communities in the ice-free areas near Syowa Station, Antarctica. Antarct Rec 59:68–96
Ochi H (1979) A taxonomic review of the genus Bryum, Musci in Antarctica. Mem Natl Inst Polar Res Spec Issue 11:70–80
Ochyra R, Tyshchenko O (2006) Leptobryum pyriforme (Hedw.) Wilson. In: Blockeel T (ed) New national and regional bryophyte records, 13. J Bryol 28:151–152
Ochyra R, Lewis Smith RI, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Peat HJ, Clarke A, Convey P (2007) Diversity and biogeography of the Antarctic flora. J Biogeogr 34:132–146
Priddle J (1979) Morphology and adaptation of aquatic mosses in an Antarctic lake. J Bryol 10:517–529
Sabovljevic M, Frahm JP, Herbiniaux U (2005) Taxonomic value, systematic position and the origin of German populations of Isothecium holtii Kindb, based on molecular data. Lindbergia 30:107–112
Savicz-Lyubitskaya LI, Smirnova ZN (1959) New species of Bryum Hedw. from the Bunger Hills. Inf Byull Sov Antarkt Eksped 7:34–39 (in Russian)
Savicz-Lyubitskaya LI, Smirnova ZN (1960) New variety of Bryum korotkevicziae Sav.-Ljub. et Z. Smim. Inf Byull Sov Antarkt Eksped 17:25–27 (in Russian)
Savicz-Lyubitskaya LI, Smirnova ZN (1964) A deep-water member of the genus Plagiothecium Br. et Sch. in Antarctica. Inf Byull Sov Antarkt Eksped 49:33–39 (in Russian)
Seto K, Imura S, Bando T, Kanda H (2002) Paleoenvironment of Holocene recorded in Antarctic lakes. Gekkan Chikyu 24:31–36 (in Japanese)
Shaw AJ (1982) Pohlia Hedw. (Musci) in North and Central America and the West Indies. Contr Univ Mich Herb 15:219–295
Shaw AJ (1984) Character analysis, phylogeny, and classification of the moss genus Pohlia. Can J Bot 62:219–229
Shaw AJ (1985) The correlation between taxonomy and peristome structure in the Bryaceae. J Hattori Bot Lab 59:79–100
Shaw AJ, Werner O, Ros RM (2003) Intercontinental Mediterranean disjunct mosses: morphological and molecular patterns. Am J Bot 90:540–550
Souza-Chies TT, Bittar G, Nadot S, Carter L, Besin E, Lejeune B (1997) Phylogenetic analysis of Iridaceae with parsimony and distance methods using the plastid gene rps4. Plant Syst Evol 204:109–123
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tewari SD, Pant G (1996) Some moss collections from Dakshin Gangotri, Antarctica. Bryol Times 91:7
Vanderpoorten A, Devos N, Goffinet B, Hardy OJ, Shaw AJ (2008) The barriers to oceanic island radiation in bryophytes: insights from the phylogeography of the moss Grimmia montana. J Biogeogr 35:654–663
White T, Burns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols. A guide to methods and applications. Academic Press, Inc, San Diego, pp 315–322
Acknowledgments
We gratefully acknowledge Dr. Jonathan Shaw and Molly McMullen (DUKE), Dr. Tomio Yamaguchi (HIRO), and Dr. Helen Peat (AAS) for loans of herbarium specimens and Dr. Satoshi Kobayashi for providing one of the Antarctic samples. We thank Kenichi Watanabe (NIPR) for his technical assistance. This research was supported by a Grant-in-Aid for Scientific Research (No. 23247012) from JSPS. We also thank two anonymous reviewers for their constructive remarks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kato, K., Arikawa, T., Imura, S. et al. Molecular identification and phylogeny of an aquatic moss species in Antarctic lakes. Polar Biol 36, 1557–1568 (2013). https://doi.org/10.1007/s00300-013-1373-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1373-x