Abstract
Antarctic climate changes influence environmental changes at both regional and local scales. Here we report Holocene paleolimnological changes in lake sediment core Sk4C-02 (length 378.0 cm) from Lake Skallen Oike in the Soya Kaigan region of East Antarctica inferred from analyses of sedimentary facies, a range of organic components, isotope ratios of organic carbon and nitrogen, and carbon-14 dating by Tandetron accelerator mass spectrometry. The sediment core was composed of clayish mud (378.0–152.5 cm) overlain by organic sediments (152.5 cm-surface). The age of the surface and the core bottom were 150 (AD1950-1640) and ca. 7,030 ± 73 calibrated years before present (cal BP), respectively, and the mean sedimentation rate was estimated to be 0.55 mm/year. Multi-proxy analyses revealed that the principal environmental change in the core is a transition from marine to lacustrine environments which occurred at a depth of 152.5 cm (ca. 3,590 cal BP). This was caused by relative sea level change brought about by ongoing retreat of glaciers during the mid-Holocene warming of Antarctica, and ongoing isostatic uplift which outpaced changes in global (eustatic) sea level. The mean isostatic uplift rate was calculated to be 2.8 mm/year. The coastal marine period (378.0–152.5 cm, ca. 7,030–3,590 cal BP) was characterized by low biological production with the predominance of diatoms. During the transition period from marine to freshwater conditions (152.5-approximately 135 cm, ca. 3,590–3,290 cal BP) the lake was stratified with marine water overlain by freshwater, with a chemocline and an anoxic (sulfidic) layer in the bottom of the photic zone. Green sulfur bacteria and Cryptophyta were the major photosynthetic organisms. The Cryptophyta appeared to be tolerant of the moderate salinity and stratified water conditions. The lacustrine period (approximately 135 cm-surface, ca. 3,290 cal BP-present) was characterized by high biological production by green algae (e.g. Comarium clepsydra and Oedegonium spp.) with some contributions from cyanobacteria and diatoms. Biological production during this period was 8.7 times higher than during the coastal marine period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies on paleoenvironmental changes are important to estimate the possible influence of future global warming induced by human activity. A lot of information on paleoenvironmntal changes on the Earth has been obtained by analyses of marine and lake sediment cores, and ice cores (Shackleton et al. 1990; Raymo and Ruddiman 1992; Kashiwaya et al. 2001; Matsumoto et al. 2003). High latitude areas are particularly sensitive to climatic changes (Short et al. 1991), and for periods beyond the instrumental record, these can be tracked using paleolimnological methods (Hodgson and Smol 2008). Since the Last Glacial Maximum (LGM), Antarctic climatic history, ice-sheet and relative sea-level changes have been studied in Maritime Antarctica, the Antarctic Peninsula and continental Antarctica. These have shown that there were two warm periods, one in the early Holocene, 11–9.5 cal ka BP, and one in the mid-Holocene called the mid-Holocene Hypsithermal, 4.5–2.8 cal ka BP (Bentley and Hodgson 2009). Lake sediment cores record global signals such as climatic change, regional signals such as the impact of changing temperature on biological production and relative sea level changes and local signals such as the advance and retreat of catchment glaciers and ice shelves, and changes in biological species composition (Roberts and McMinn 1998; Verleyen et al. 2003, 2004a, b; Hodgson et al. 2005, 2006; Smith et al. 2007).
A large number of lakes and ponds of varying salinity are distributed in ice-free areas of Antarctica including the Syowa Station area, East Antarctica (Murayama 1977; Murayama et al. 1988). Paleoenvironmental and paleolimnological changes in the postglacial period in the Soya Kaigan have been studied by Japanese Antarctic Research Expedition members (Yoshida and Moriwaki 1979; Miura et al. 1998a, b, c; Matsumoto et al. 2006; Ohzono et al. 2006). Matsumoto et al. (2006) studied organic components and sedimentation rates in sediment cores from Lakes Namazu Ike and Ô-ike, and suggested that aquatic moss showed a marked increase in relative abundance from 1,100 year BP (conventional age) to the core top in Lake Namazu Ike. Changes from marine to lacustrine environments are recorded in lower altitude lakes in the Soya Kaigan region due to the recession of glaciers and subsequent isostatic uplift during the mid Holocene (Seto et al. 2002).
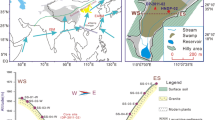
Lake Skallen Oike is located in the central part of Skallen in the southern part of the Soya Kaigan at an altitude of 10 m mean above sea level (asl, Figs. 1, 2). The length, width and area of the lake, and distance from the sea are 1,180 m, 275 m, 209,000 m2 and 135 m, respectively (Imura et al. 2003), and the maximum depth is 9.5 m (Seto et al. 2002). Presently the lake water is supplied from seasonal meltwater from snow. The lake surface is covered with thick ice except during the austral summer.
Locality of Lake Skallen Oike in the Soya Kaigan of East Antarctica (revised from Imura et al. 2003)
Organic components in lake sediments are supplied by living and dead organisms, and are the biomarkers of biological production, source organisms, and paleolimnological changes in the drainage basin (Matsumoto et al. 2003; Tani et al. 2009). Here we studied paleolimnological changes inferred from a multi-proxy data set of organic components (lipid biomarkers, photosynthetic pigments) and stable isotope ratios of organic carbon and nitrogen in a sediment core (Sk4C-02) from Lake Skallen Oike in the Soya Kaigan, along with sedimentary facies and carbon-14 dating by a Tandetron accelerator mass spectrometry (AMS). These are discussed in relation to Antarctic climatic and environmental changes.
Materials and methods
Sediment core
Sediment core (Sk4C-02, length 378.0 cm) was taken using a piston corer by KS on Dec. 26, 2004 in Lake Skallen Oike (69°40.300S, 39°24.647W) at a water depth of 9.42 m. The sediment core was analyzed at intervals from 365.7 cm to the surface, excluding the core catcher (378.0–365.7 cm).
Analytical methods
AMS dating and elemental analysis
AMS 14C dating of bulk organic carbon or algal debris was carried out by a Tandetron type-II instrument housed at Nagoya University (Watanabe et al. 2009). AMS radiocarbon data (14C/12C) were corrected to reflect the conventional age by simultaneous measurement of δ13C. Conventional ages were calibrated for lacustrine sediment using Intcal04 (Reimer et al. 2004) and for marine sediment using Marine04 (Hughen et al. 2004). A reservoir correction was applied to radiocarbon dates derived from marine samples by subtracting 1,300 year following recent conventions for the Southern Ocean (Berkman et al. 1998; Verleyen et al. 2005).
Total carbon (TC) and total sulfur (TS) contents were determined at 2.3 cm intervals by a Fisons NCS 2500 automatic elemental analyzer. Total organic carbon (TOC) and total nitrogen (TN) contents were determined by the same analyzer, after treatment with hydrochloric acid to remove carbonate carbon. Total inorganic carbon (TIC) content was calculated by subtracting the TOC content from the TC content.
Hydrocarbons, fatty acids and sterols
Hydrocarbons, fatty acids and sterols in sediment subsamples were analyzed at approximately 25 cm intervals by the methods of Matsumoto et al. (1979, 1982, 2003) and Matsumoto and Watanuki (1992). Briefly, organic components in the samples were extracted with ethyl acetate after saponification with 0.5 mol l−1 potassium hydroxide/methanol (80°C, 2 h). The ethyl acetate extracts were separated by chromatography on a silica gel column (160 mm × 6 mm i.d., 100 mesh, 5% water). Hydrocarbon and fatty acid-sterol fractions were obtained by elution with hexane and ethyl acetate, respectively. A half volume subsample of the fatty acid-sterol fraction was methylated with diazomethane. The other half volume of the fatty acid-sterol fraction was trimethylsilylated (TMS) with 25% N,O-bis(trimethylsilyl acetamide) acetonitrile solution, to obtain the sterol TMS derivatives. Hydrocarbons, fatty acid methyl esters and sterol-TMS derivatives were analyzed by a JEOL JMS K9 gas chromatograph–mass spectrometer (GC–MS) equipped with a fused silica capillary column (J&W DB5, 30 m × 0.25 mm i.d., film thickness 0.1 μm). Splitless mode was employed. Column oven temperature was programmed from 70 to 120°C at 30°C min−1, and from 120 to 320°C at 8°C min−1and kept at 320°C for 3.5 min. The flow rate of the helium carrier gas was 1.2 ml min−1. The temperatures of the injector, interface and ion source were maintained at 280, 300 and 250°C, respectively. Ionization energy, filament current and detector voltage were 70 eV, 200 μA and −1,000 V, respectively.
Pyrolysis methylation gas chromatography-mass spectrometry (PyMeGC–MS)
Water in the sediment samples (approximately 0.5 g) in glass vial (3.5 ml) was removed by aspirator (45°C). PyMeGC–MS was carried out by the method of Yamamoto et al. (2007). Dried sediment samples were homogenized in agate mortar, and wrapped in pyrofoil (curie point at 445°C), together with TMAH reagent (25% tetramethylammonium hydroxide methanol 10 μl) and nonadecanoic acid (10 ng) as internal standard. The solvent was removed by retained heat of the GC–MS column oven. PyMeGC–MS was carried out by a JAI-5 Curie point pyrolyzer at 445°C connected directly with a JMS K9 GC–MS. Oven and needle temperatures of the pyrolyzer were kept at 398 and 250°C, respectively. Column oven temperatures were programmed from 60 to 320°C at 8°C min−1 and kept at 320°C for 2.5 min. Other GC–MS conditions were same as for the lipid analysis stated above.
Sedimentary photosynthetic pigments
Sedimentary photosynthetic pigments were extracted using previously described methods (Tani et al. 2002, 2009; Nara et al. 2005; Soma et al. 2007). Photosynthetic pigments were ultrasonically extracted in 5.0 ml acetone at 0°C for 15 min three times. The combined extract was evaporated to dryness under N2, dissolved in 3 ml diethyl ether, and washed with 1 mol l−1 NaCl aqueous solution. After evaporating the ether phase to dryness under N2, the residue was dissolved into 200–500 μl acetone together with an internal standard, mesophorphyrin IX dimetyl ester (Sigma Chemical Co., USA, Soma et al. 1996, 2001), and analyzed by high-performance liquid chromatography (HPLC, LC-10A, Shimadzu, Japan) using photodiode array detection (SPD-M10AVP, Shimadzu, Japan).
A reversed-phase Navi C30-5 column (4.6 mm i.d. × 250 mm in length, Wako Pure Chemical Industries, Japan) was used to separate photosynthetic pigments as described previously (Tani et al. 2009). The mobile-phases were as follows: solvent A consisted of a mixture of acetonitrile and water (9:1 by volume) and solvent B was ethyl acetate. A linear gradient elution from 100% A to 100% B over 40 min was followed by an isocratic hold for 15 min at 100% B. The flow rate was 1.0 ml min−1. Absorption spectra (300–700 nm) were monitored with the photo-diode array detector. The assignment of pigments was based on their HPLC retention times and their absorption spectra compared with authentic compounds, or with literature data (Jeffrey et al. 1997; Britton et al. 2004; Porra 2006). Pheophytin a, pheophytin b, all-trans β,β-carotene from Wako Pure Chemical Co. (Japan), pyropheophytin a from Tama Biochemical Co. (Japan), and all-trans zeaxanthin, all-trans alloxanthin, all-trans diatoxanthin, and all-trans lutein from DHI Pigment Standards (Denmark) were used as standards.
Stable isotope analysis
To determine stable isotope ratios, sediment samples were combusted at 1,020°C in an elemental analyzer (Fisons Instruments EA1108), and the combustion products (CO2 and N2) were introduced with a helium carrier gas into an isotope-ratio mass spectrometer (Finnigan Delta Plus). For the carbon isotope analysis, sediment samples were combusted in a silver cup after being treated with drops of 1 mol l−1 hydrochloric acid to remove inorganic carbon. Ratios of 13C/12C and 15N/14N were expressed relative to the Vienna-PeeDee Belemnite (V-PDB) standard for carbon and N2 in air for nitrogen. Ratios of 13C/12C and 15N/14N were calculated as:
where R = 13C/12C or 15N/14N.
Instrument drift during analyses was checked with L-α-alanine (δ13C = −20.93‰, δ15N = 7.61‰) every three samples. The accuracy of the values was determined using inter laboratory-determined nitro arginine following the method of Minagawa et al. (1984) for δ13C (−22.27‰) and IAEA-N1 for δ15N (0.54‰).
Results
Lithology and geochronology
The Sk4C-02 sediment core was composed of clayish mud (378.0–152.5 cm) overlain by organic sediments (152.5 cm-surface, Fig. 3). The clayish mud was laminated and contained marine sediments, and glacio-marine sediments with echinoid spines. The organic freshwater sediments were stratified, with globular (pancake-like) and fragmental structures formed by microbial mats (Fig. 3). The radiocarbon (AMS 14C) chronology of Lake Skallen Oike is shown in Table 1. The age-depth relationship in the Sk4C-02 sediment core is shown in Fig. 4. The ages of surface (algal residue 0–2.3 cm), 141.5 cm (TOC), 141.5 cm (algal residue), 272.6 cm (TOC) and 364.6 cm depths (TOC) were 150 (AD1950-1640), 3,420 ± 32, 3,410 ± 40, 5,380 ± 57 and 6,820 ± 67 calibrated years before present (cal BP), respectively. The age of the core bottom (378.0 cm) was linearly extrapolated to 7,030 ± 59 cal BP.
Paleolimnological sedimentary changes found in the Sk4C-02 sediment core from Lake Skallen Oike. The transition from marine to saline lake sediments, and from saline lake to freshwater lake sediments occurred at depths of 152.5 cm (ca. 3,590 cal BP) and approximately 135 cm (ca. 3,290 cal BP), respectively
Depth-age relationship in the Sk4C-02 sediment core from Lake Skallen Oike. Conventional ages were calibrated for terrestrial sediment (Reimer et al. 2004) and for marine sediment (Hughen et al. 2004). A reservoir correction was done for marine samples by subtracting 1,300 years (Berkman et al. 1998). The ages of surface (algal residue 0–2.3 cm), 141.5 cm (TOC), 141.5 cm (algal residue), 272.6 cm (TOC) and 364.6 cm depths (TOC) were 150 (AD1950-1640), 3,420 ± 32, 3,410 ± 40, 5,380 ± 57 and 6,820 ± 67 calibrated year before present (cal BP), respectively. The age of the core bottom (378.0 cm) was linearly extrapolated to be 7,030 ± 59 cal BP
Elemental data (TC, TOC, TN, TS and TIC)
TC, TOC, TN and TIC contents near the bottom (363.4 cm) of the sediment core were approximately 0.6, 0.4, 0.08 and 0.2%, respectively, increased gradually to a depth of 153.0 cm, and increased dramatically from this depth to the surface, attaining values of approximately 24, 20, 2 and 4%, respectively (Fig. 5). TS contents between 364.6 and 153.0 cm depth were approximately 1%, but those between 150.7 cm and the surface were relatively high (1–3%). TOC/TS weight ratios near the bottom of the core (363.4 cm) were approximately 0.5, gradually increased to a depth of 153.0 cm, and abruptly increased from this depth to the surface, reaching approximately 10 (Fig. 5).
Total carbon (TC), total organic carbon (TOC), total nitrogen (TN), total inorganic carbon (TIC) and total sulfur (TS) contents, TOC/TC, TOC/TN and TS/TOC weight ratios in the Sk4C-02 sediment core from Lake Skallen Oike. Depths between surface-135 cm (light green), 135–152.5 cm (light brown) and 152.5–365.7 cm (light blue) are freshwater lake, saline lake and marine sediments, respectively
Hydrocarbons, fatty acids and sterols
A suite of n-alkanes ranging in carbon chain length from n-C15 to n-C35 were found in the Sk4C-02 sediment core with bimodal distributions maximizing at n-C17 and n-C23, together with isoprenoid-alkanes [i-C18, i-C19, i-C20 and i-C30 (squalane)]. Major hydrocarbons in the sediment core were n-C17, n-C21, n-C23, and/or n-C25 alkanes. A series of n-alkanoic acids ranging in carbon chain length from n-C12 to n-C32 maximizing at n-C16 were detected in the sediment core, along with n-alkenoic and branched (iso and anteiso) acids, as in the case of sediment core from Lake Ô-ike in the Syowa Station area (Matsumoto et al. 2006). The major fatty acids were n-C16:0 (carbon chain length: number of unsaturation), n-C16:1, and/or n-C18:1n9c acids. Branched acids were found in all the samples, but changes in their percentages in the sediment core were small. Percentages of long-chain (n-C20–n-C32) n-alkanes and n-alkanoic acids between depths of near the bottom and approximately 150 cm were 60–70 and 5–10%, respectively, were much lower than those of depths between approximately 150 cm and the surface (70–80 and 20–40%, respectively, Fig. 6).
Depth profiles of selected molecular markers in the Sk4C-02 sediment core from Lake Skallen Oike. Relative abundance of selected n-alkanes, n-alkanoic acids and sterols reveals percentages in total alkanes, alkanoic acids and sterols, respectively. Depths between surface-135 cm (light green), 135–152.5 cm (light brown) and 152.5–365.7 cm (light blue) are freshwater lake, saline lake and marine sediments, respectively
Stenols (cholest-5-en-3β-ol, 24-methylcholetsa-5,22-dien-3β-ol, 24-methylcholest-5-en-3β-ol, 24-ethylcholeta-5,22-dien-3β-ol and 24-ethylcholest-5-en-3β-ol) and stanols (5α-cholestan-3β-ol, 24-methyl-5α-choletan-3β-ol and 24-ethyl-5α-cholestan-3β-ol) were found in the Sk4C-02 sediment core. The major sterols were 24-methylcholest-5-en-3β-ol, 24-ethylcholeta-5,22-dien-3β-ol and/or 24-ethylcholest-5-en-3β-ol. Percentages of 24-methylcholest-5-en-3β-ol in total sterols from the bottom of the core to approximately 150 cm (20–80%) were much higher than those from 150 cm to the surface (0–20%, Fig. 6).
Pyrolysis products
PyMeGC–MS of sediment core samples yielded a series of n-alkanoic acids (n-C10–n-C32) with the predominance of even-carbon numbers and branched (iso and anteiso) acids (Fig. 6). Long-chain n-alkanoic acids in the pyrolysis products in depths of 364.6–153.0 cm were low, but those in depths of 150.7 cm-surface were abundant, as in the case of extracted fatty acids described above (Fig. 6).
Photosynthetic pigments
Intact chlorophyll a, pheophytin a and pyropheophytin a were detected as major chlorophyll a derivatives (Fig. 7). All trans-lutein, all-trans zeaxanthin, and all-trans β,β-carotene were detected as the major intact carotenoids. Besides these intact (all-trans type) carotenoids, cis-isomers of several carotenoids such as 9-cis alloxanthin and 9-cis diatoxanthin, were detected (Fig. 7) as described earlier (Tani et al., 2009). These carotenoids were identified by comparing their retention times, UV–Vis spectra and mass spectra with those of authentic standards. Bacteriochlorophyll d identification was based on the observed adsorption spectrum with Soret and Qy (red) maxima at 426 and 652 nm, respectively, and their ratio of 1.20 (Porra 2006). A HPLC peak with its absorption maximum at 438 (as a shoulder), 463, and 494 nm was tentatively assigned as chlorobactene (Britton et al. 2004). These pigments are specific to phototrophic green sulfur bacteria, Chlorobiaceae (Pfennig 1967; Borrego and Garcia-Gil. 1994; Spuier et al. 2002), and were simultaneously detected between 199.0 and 139.2 cm depth with a maximum at 139.2 cm (Fig. 7).
Depth profiles of photosynthetic pigments in the Sk4C-02 sediment core from Lake Skallen Oike. Chlorophyll a, pheophythin a and pyrophyophytin a: ubiquitous (Verleyen et al. 2004b; Tani et al. 2009). Fucoxanthin and cis-diatoxanthin: Diatoms, Dinophyta, Chrysophyta, brown algae and Heptophycae (Chihara 1997; Verleyen et al. 2004b). Zeaxanthin: Cyanobacteria, Chlorophyta and mosses (Verleyen et al. 2004b; Hodgson et al. 2006). Lutein: Chlorophyta, red algae, Charophyceae and vascular plants (Chihara 1997; Verlayen et al. 2004b; Tani et al. 2009). Pheophobide b, pyropheophorbide b: Similar to lutein. cis- Alloxanthin: Cryptophyta (Jeffrey et al. 1997; Leavitt and Hodgson 2001). Bacteriochlorophyll a, chlorobactene: Green sulfur bacteria (Pfennig 1967; Borrego and Garcia-Gil. 1994; Spuier et al. 2002). Depths between surface-135 cm (light green), 135–152.5 cm (light brown) and 152.5–365.7 cm (light blue) are freshwater lake, saline lake and marine sediments, respectively
Stable isotope ratios
δ13C and δ15N values in the sediment core showed marked shifts at 150.7 cm depth (Fig. 8). Variations in δ13C values were relatively small ranging from −21.8 to −17.1‰ with a mean of −20.2 ± 1.2‰ (standard deviation) for depths between 350.8 cm and 139.2 cm. The δ13C value at 120.8 cm depth was −15.6‰, increased steadily toward the surface and attained −12.0‰ at the near surface (3.5 cm depth). Like δ13C, variations in δ15N values were relatively small ranging from 4.1 to 6.3‰ with a mean of 4.98 ± 0.60‰ between 350.8 cm and 139.2 cm depth. The δ15N value of 5.5‰ at 150.7 cm depth suddenly decreased to 1.3‰ by 120.8 cm depth, and stayed at lower values (0.4–1.7‰) between 120.8 cm and the near surface (Fig. 8).
Depth profiles of stable isotope ratios of total organic carbon (δ13C) and total nitrogen (δ15N) in the Sk4C-02 sediment core from Lake Skallen Oike. Depths between surface-135 cm (light green), 135–152.5 cm (light brown) and 152.5–365.7 cm (light blue) are freshwater lake, saline lake and marine sediments, respectively
Discussion
Geochemical features and sources of organic components
The morphological features of the algae and cyanobacteria were mostly decomposed and identification of their species was impossible except for diatoms and desmids in the sediment core (Ohtani S pers. commun.), as was the case in previous studies of sediment cores from Lakes Ô-ike and Namazu-ike in the same region (Matsumoto et al. 2006).
Long-chain n-alkanes and n-alkanoic acids are widely distributed in lacustrine environments, and are used as biomarkers of vascular plants. These long-chain compounds are found in lake sediment samples from the Syowa Station area and the McMurdo Dry Valleys in Antarctica in spite of the absence of vascular plants (Matsumoto 1993; Matsumoto et al. 2006). Long-chain n-alkanes and n-alkanoic acids were relatively abundant in the upper unit of the core (approximately 150 cm-surface) compared with those in the lower unit (approximately 150 cm-bottom) (Fig. 6). Although the source organisms of these long-chain compounds are not yet clear, certain green algae are candidate source organisms as suggested by the abundance of lutein which is a biomarker of green algae in Lake Skallen Oike as discussed below. These long-chain compounds are probably derived from green algae (e.g. Comarium clepsydra and/or Oedegonium spp.). Branched (iso- and anteiso) alkanoic acids found in all depths of the core are originated from bacteria (Reddy et al. 2000, 2003).
Stenols and stanols are widely distributed in lacustrine and marine environments including Antarctica. Chlolest-5-en-3β-ol is a typical sterol of phytoplankton and microalgae (Matsumoto et al. 1982, 2006; Volkman et al. 1998). 24-Methylcholest-5-en-3β-ol is often abundant in diatoms (Volkman et al. 1998; Matsumoto et al. 2003). Although 24-ethylcholest-5-en-3β-ol is commonly used as a biomarker of vascular plants, it is known that many kinds of microalgae synthesize this sterol, and it is widely distributed in Antarctic lakes and soils often as the most predominant sterol (Matsumoto et al. 1982, 2006; Volkman et al. 1998). Stanols are found in microalgae (dinoflagellates, diatoms and raphidphytes, Volkman et al. 1998) and are also formed by bacterial reduction of stenols (Nishimura 1982). Sterols dominated with 24-methylcholest-5-en-3β-ol found in the bottom-150 cm of the sediment core are, therefore, mainly originated from diatoms, while predominance of 24-ethylcholest-5-en-3β-ol in the upper unit are probably derived from green algae and cyanobacteria (Matsumoto et al. 1982). Stanols may be derived from microalgae in the lake in addition to microbial reduction of stenols.
No one has reported photosynthetic pigments in the Syowa Station area. Photosynthetic pigments are useful marker of biological production and assemblage. Since there are significant differences in carotenoid composition according to algal taxa, sedimentary carotenoids give us direct information about the algal assemblage in the lake at the time of deposition (Leavitt and Hodgson 2001). In Sk4C-02 sediment core, all-trans lutein, all-trans zeaxanthin, and all-trans β,β-carotene were detected as major intact carotenoids (Fig. 7). Besides, 9-cis diatoxanthin and 9-cis alloxanthin were found in the sediment core. It is likely that all-trans alloxanthin and diatoxanthin from living algae were converted to their 9-cis isomers during diagenesis, and preserved in the sediment as their major isomers (Tani et al. 2009).
Photosynthetic pigments found in a sediment core from Pup Lagoon in the Larsemann Hills, East Antarctica, and their taxonomic affinities are summarized by Verleyen et al. (2004b). Chlorophyll a, pheophytin a and pyropheophytin a are ubiquitous pigments distributed in all photosynthetic plants (Verleyen et al. 2004b; Tani et al. 2009) and are markers of primary production in the lake and catchment. Fucoxanthin and diatoxanthin are distributed in Bacillariophyta (diatoms), Dinophyta (dinoflagellates), Chrysophyta (golden-brown algae), brown algae and Haptophycae, and are possible markers of these organisms (Chihara 1997; Jeffrey et al. 1997; Leavitt and Hodgson 2001; Tani et al. 2009). Presently, the dominant photosynthetic organisms in Lake Skallen Oike are green algae, diatoms and cyanobacteria, and other organisms are sparse (Ohtani S pers. commun.). Thus, it is most likely that fucoxanthin and diatoxanthin are derived from green algae, diatoms and cyanobacteria in the lake, particularly in the upper part above 152.5 cm. In the lower part it is possible, however, that some of the fucoxanthin is derived from brown algae and other marine algae found in the marine environment (e.g. prymnesiophytes, raphidophytes). Zeaxanthin is commonly distributed in Antarctic cyanobacteria, Chlorophyta and mosses (Verleyen et al. 2004b; Hodgson et al. 2005, 2006). Few or no mosses are found in the lake (Imura et al. 2003). Thus zeaxanthin is most likely derived from green algae and cyanobacteria in the lake. Trans-lutein is pigment of Chlorophyta, red algae, Charophyceae and vascular plants (Chihara 1997; Verleyen et al. 2004b; Tani et al. 2009), but no vascular plants are distributed in the Syowa Station area and no red algae and Charophyceae are found in the lacustrine environments. We therefore interpret lutein as being exclusively derived from the dominant green algae in the lake (including Comarium clepsydra and Oedegonium spp. Ohtani S pers. commun.). Pheophorbides and pyropheophorbide are common chlorophyll derivatives found in general algal detritus and zooplankton fecal pellets, respectively (Verleyen et al. 2004b). 9-cis Alloxanthin is an indicator of Cryptophyta since alloxanthin is specific to Cryptophyta (Jeffrey et al. 1997; Leavitt and Hodgson 2001). Interestingly, the occurrence of bacteriochlorophyll d and chlorobactene at sediment depths of 199.0 and 139.2 cm with the maximum at 139.2 cm reflects the presence of green sulfur bacteria (Pfennig 1967; Borrego and Garcia-Gil. 1994; Spuier et al. 2002).
Sedimentary sequences and the timing of the transition from marine to lacustrine environments
Holocene relative sea level changes in the Vestfold Hills and Larsemann Hills, East Antarctica, have been obtained by dating the lacustrine-marine and marine-lacustrine transitions recorded in lake sediment cores of isolation basins which were formally connected to the sea. In the Larsemann Hills relative sea level rose to a high stand of approximately 8 m above present between ca. 7,570–7,270 and 7,250–6,950 cal BP, and in the Vestfold Hills relative sea level rose to a maximum of approximately 9 m above present at 6,200 14C year BP (ca. 7,005–7,133 cal BP) (Zwartz et al. 1998; Verleyen et al. 2004b, 2005). Little comparable isolation-based evidence is, however, available to constrain the relative sea level history of coastal environments of the Soya Kaigan in East Antarctica during the Holocene. The age of the core top was not large (150 cal BP) and thus the reservoir effect was considered small (Fig. 4). Presently only meltwater derived from snow is supplied to the lake from its catchment, and no glacial meltwater (which may contain old carbon) has been supplied in the last 2,000 years (Seto et al. 2002). The age of the core bottom (378.0 cm) was measured as 7,030 ± 59 cal BP. The mean sedimentation rate in Lake Skallen Oike was calculated to be 0.55 mm a−1, and may have been higher in the upper unit above 152.5 cm.
In the lower unit the clayish mud with laminations (378.0–152.5 cm) containing glacial sediments and echinoid spines is interpreted as being formed in a marine environment. The evidence for this is discussed below.
The TOC/TS weight ratio near the bottom (364.6 cm) of the core was 0.4 and increased gradually to 1.2 at a depth of 152.5 cm, and then increased markedly from this depth to the surface to a value of approximately 5 (Fig. 5). This suggests strongly a change from marine to lacustrine sediments in the core, since the TOC/TS ratios of marine sediments are generally much lower than those of freshwater sediments (Berner and Raiswell 1984; Sampei et al. 1997a, 1997b).
Generally, Antarctic marine sediments influenced by phytoplankton have δ13C values lower than −22‰ (Boutton 1991). In the Sk4C-02 sediment core, the mean δ13C value between the depths of 350.8 cm and 139.2 cm was −20.2‰, which is slightly higher than that of present Antarctic marine sediments (Fig. 8). It is also higher than the δ13C values of −23 to −24‰ recorded in the sediments of Moutonnée Lake in Antarctica between 7,500–8,000 cal BP (Smith et al. 2007). The higher δ13C values in the marine sediments of Moutonnée Lake were the result of terrestrial organic matter inputs, since Smith et al. (2006) reported that the δ13C values of benthic cyanobacteria collected from melt water streams in the catchment of Moutonnée Lake ranged from −17 to −19‰. In the Sk4C-02 sediment core, zeaxanthin was detected notably between the depths of 251.9 and 231.2 cm (Fig. 7), suggesting an enhanced input and retention of organic matter originated from terrestrial green algae and cyanobacteria in the sediments. This is a plausible mechanism as δ13C values indeed increase towards the end of the marine zone, implying that when the marine bay became shallower and formed a coastal lagoon as a result of isostatic uplift, this was accompanied with an enhanced influence of terrestrial organic matter.
δ15N values between the depths of 350.8 cm and 150.7 cm were about 5‰ (Fig. 8), which is within the range of diatom-bound δ15N in Holocene sediment cores from the Antarctic Ocean (Robinson and Sigman 2008). The δ15N value of 5.5‰ at a depth of 150.7 cm decreased abruptly to 1.3‰ at 120.8 cm and maintained lower values (0.41–1.7‰). These lower δ15N values reflect the onset of lacustrine conditions in Lake Skallen Oike as discussed below.
Diatoms are very useful marker of salinity changes and have been used to identify marine to lacustrine transitions in Antarctic lakes from the Larsemann Hills (Verleyen et al. 2004b; Hodgson et al. 2006) and changing lake water salinity in the Vestfold Hills (Roberts and McMinn 1998). Diatoms in the Lake Skallen Oike sediment core showed marked changes from marine species (Diploneis subcincta, etc.) at a depth of 153.0 cm, brackish-water species (Craspedostauros laevissima) at a depth of 141.5 cm, and freshwater species (Amphora cf. veneta) at a depth of 72.5 cm in the core. The freshwater green alga Comarium clepsydra was abundant at a depth of 72.5 cm (Ohtani S pers. commun.).
These multi-proxy analyses revealed, therefore, that substantial environmental changes associated with the transition from marine to lacustrine environments occurred at a depth of 152.5 cm (ca. 3,590 cal BP) in the Lake Skallen Oike sediment core. The ongoing retreat of glaciers during the mid-Holocenec hypsithermal (MHH, 4,700–2,700 cal BP, Bentley and Hodgson 2009), and ongoing isostatic uplift of Soya Kaigan accounts for most of this change (Verleyen et al. 2004b; Hodgson et al. 2006). The contribution from global eustatic sea level is considered negligible because it has been estimated that global sea level fall was 0.7 ± 0.1 m between 4,000 and 2,500 years BP (Goodwin 1998). The Holocene marine limit along the Soya Kaigan is estimated to have been approximately 18 m asl based on analyses of raised beach deposits (Miura et al. 1998c). The linear average isostatic crustal uplifting rate of the lake catchment was calculated as 2.8 mm a−1.
Three distinct zones can therefore be recognized on the basis of sedimentary facies, elemental data, pigment and stable isotope ratios of δ13C and δ15N in the Lake Skallen Oike sediment core (Figs. 3, 5, 6, 7, 8). Below, we discuss separately a coastal marine period (378.0–152.5 cm), a transition period (152.5–135 cm) and a lacustrine period (135 cm-surface) of the lake. Table 1 summarises TOC, TN and TS contents and their ratios for these periods in the lake’s history.
Coastal marine conditions (378.0–152.5 cm, ca. 7,030–3,590 cal BP)
The lower marine clayish mud sediments contain three glaciomarine sediment layers at depths of 262–258, 251 and 229–227 cm formed at ca. 5,260–5,200, 5,100 and 4,760–4,730 cal BP, respectively (Figs. 3, 4). These glacial sediments were probably deposited during the advance and retreat of local glaciers due to climatic warming, with the terminus of the glaciers being very close to the marine basin where the sediments were deposited. Palaeoclimate evidence from around Antarctica suggests that there was a period of warming, the MHH, between 4,700 to 2,700 cal BP (Bentley and Hodgson 2009), although individual paleoclimate records show a strong influence of local and regional climates on the precise timing and magnitude of this event (Ingólfsson et al. 1998; Verleyen et al. 2004b; Bentley and Hodgson 2009). Although no detailed information on paleoclimate is currently available for the Soya Kaigan, it is possible that this area was in a transition period from cool climate to the MHH at the time these glacial sediments were deposited. Under this scenario the glacial sediments may have been formed by repeated glacial retreats and advances and subsequent changes in coastal marine depositional environments during this period. At this time the basin would have been a marine inlet or lagoon with a calculated sill depth of approximately −5 to −7 m.
Although organic components are largely decomposed in the sediments, the TOC and TN contents provide markers of biological production (Matsumoto et al. 2003). TOC and TN contents in the marine zone (364.6–153.0 cm) ranging from 0.24 to 2.79% with a mean of 1.35 ± 0.74% and from 0.05 to 0.37% with a mean of 0.18 ± 0.09%, respectively, are relatively low, and thus the biological production was low as compared with the transition and lacustrine zones (Table 1). Since chlorophyll a is ubiquitous in all phytoplankton taxa, the total concentration of these chlorophyll a derivatives (Chl-a) in sediments may be an indicator of total primary production in the lake at the time of deposition (Soma et al. 2003). Chl-a concentrations showed two maxima at depths around 140 cm-surface and 230 cm (Fig. 7). The former was much higher than the latter. No peaks of TOC and TN contents were, however, found at 230 cm sediment depth suggesting that stability of Chl-a in the marine zone was rather low.
Fucoxanthin, cis-diatoxanthin and zeaxanthin were detected between depths of 350.8 and 153.0 cm, which in marine environments can be derived from diatoms, prymnesiophytes, brown seaweeds, raphidophytes, dinoflagellates, prochlorophytes, coccoid cyanobacteria, green algae and chrysophytes (Jeffrey et al. 1997; Verleyen et al. 2004b). However, the relatively high abundance of 24-methylcholest-5-en-3β-ol suggests a predominance of diatoms (Volkman et al. 1998; Matsumoto et al. 2003) in this marine sediment zone (Fig. 6). Cis-alloxanthin (Cryptophyta) was distributed at depths of 350.8–153.0 cm with a small peak at 199.0 cm (ca. 4,300 cal BP), together with small peak of bacteriochlorophyll d and chlorobactene, which are typical pigments of green sulfur bacteria as discussed below (Fig. 7). This provides evidence of anoxic bottom water forming in the lagoon or inlet, possibly brought about by seasonal ice cover and/or seasonal damming of the sill, as has been found in similar sites in the Larsemann Hills (Hodgson et al. 2009). Cryptophyta are found both in seawater and freshwater and are commonly found in stratified water conditions. Thus the marine zone of Lake Skallen Oike is characterized by low biological production with a predominance of diatoms.
Transition period from saline lake to freshwater lake (152.5–135 cm, ca. 3,590–3,290 cal BP)
TOC and TN contents in the black sediment layer (150.7–134.6 cm) range from 2.62 to 6.34% with a mean of 3.91 ± 1.48% and from 0.27 to 0.56% with a mean of 0.35 ± 0.11%, respectively, are considerably higher than those in marine zone (Table 1). This may be due to an increase in biological production (Table 2).
The peak of bacteriochlorophyll d and chlorobactene at depths of 153.0-approximately 135 cm (ca. 3,780–3,360 cal BP) suggests the presence of green sulfur bacteria (Pfennig 1967; Borrego and Garcia-Gil. 1994; Spuier et al. 2002). Green sulfur bacteria require sulfide as an electron donor for photosynthesis. We therefore interpret the presence of green sulfur bacteria as indicating the presence of a stratified water column with a chemocline and an anoxic (sulfidic) layer at the bottom of photic zone. This is consistent with Lake Skallen Oike being isolated from the sea and becoming stratified as the isolated marine water was overlain by freshwater supplied from meltwater to the lake surface. Interestingly, the presence of bacteriochlorophyll d and chlorobactene from green sulfur bacteria is also consistent with the presence of cis-alloxanthin from the Cryptophyta which are known to tolerate moderate salinity and stratified water conditions. Spuier et al. (2002) also detected bacteriochlorophylls c and d in an Antarctic lake sediment core from Kirisjes Pond in the Larsemann Hills, and interpreted them as an indicator of photic zone anoxia between 6,285–6,525 year BP.
Lacustrine sediments (134.6 cm-surface, ca. 3,290 cal BP-present)
Very high TOC and TN contents ranging from 4.71 to 23.3% with a mean of 11.7 ± 3.3% and from 0.49 to 2.43% with a mean of 1.13 ± 0.32%, respectively, were found in the lacustrine zone, which also contained morphologically recognizable algal debris (Table 1). These TOC and TN contents are 3.0 and 3.2 times higher than those of transition zone (152.5–135 cm), and 8.7 and 6.3 times higher than those of marine sediment zone (bottom-153.0 cm), respectively (Table 1). These profiles suggest that biological production increased dramatically due to the transition from a marine to a lacustrine environment. Similarly after isolation from the sea, a significant increase in organic matter was observed in a sediment core from Pup Lagoon, a coastal lake in the Larsemann Hills, East Antarctica (Verleyen et al. 2004b), and Lake Reid in the same area (Hodgson et al. 2005). Laminated microbial mats in the lacustrine sediments of Pup Lagoon are dominated by cyanobacteria, with diatoms and green algae as sub-dominants (Verleyen et al. 2004b). In Lake Skallen Oike TOC and TN contents fluctuated widely over short periods of time, especially between 150.7 cm depth and the surface, reflecting short term changes (2.3 cm: 42 years) in biological production and/or lake conditions (Fig. 5).
At present, the bottom of Lake Skallen Oike is covered with microbial mats composed mainly of green algae (e.g. Cosmarium clepsydra) together with smaller amounts cyanobacteria and diatoms (Ohtani S pers. commun.). Floating pancake-like algal aggregates composed mainly of Oedegonium spp. and cyanobacteria were found in early austral summer. Because dissolved inorganic nitrogen (DIN) contents in the water column are less than 0.5 μmol l−1, and are below detection limits at water depths of > 3 m at present Lake Skallen Oike (Fukui et al. 1985), phytoplankton other than nitrogen fixing cyanobacteria would not grow so fast in the water column. The results of photosynthetic pigments, however, indicate a low density of cyanobacteria in Lake Skallen Oike (Fig. 7).
Nutrients to sustain vigorous primary production in microbial mats are supplied presumably from interstitial water beneath the mat, because no DIN was detected in the lake bottom water (Fukui et al. 1985). High δ13C values (−9.87 to −15.58‰) and low δ15N values (0.41–1.7‰; Fig. 8) in the lake can be explained as follows. Ammonium produced by the decomposition of organic matter is depleted in δ15N by 5 to 10‰ (Feigin et al. 1974; Freyer and Aly 1975). When the nitrogen source contained abundant ammonium, significant depletion of δ15N occurs in plant tissues (Yoneyama et al. 2001). On the other hand, microbial mats in the lake bottom are likely diffusion-limited resulting in a reduced fractionation by primary producers. This is the case for Lake Fryxell in the McMurdo Dry Valleys of Antarctica where δ13C values of 31 benthic organic matter (moat microbial mats) samples ranged from −7.7 to −2.7‰ with a mean of −4.6‰ (Lawson et al. 2004). High δ13C values and low δ15N values in the lacustrine sediments, therefore, likely result from the primary production conditions in the benthic microbial mats.
Supposing the primary producers in Antarctica, both terrestrial and aquatic, are mostly C3 plants, high δ13C would denote a reduced fractionation of δ13C during photosynthesis, which often occurs in aquatic environments but is rare for terrestrial plants. TOC, photosynthetic pigments and isotope ratios results strongly suggest that the dominant primary producers changed from pelagic diatoms to green sulfur bacteria, and to benthic green algae during the transition from a marine inlet to a stratified saline lake with a chemocline, and finally to a lacustrine environment. These changes enabled high organic production by utilizing the interstitial ammonium and induced the decrease of sediment δ15N values at the depths of 120.8 cm and thereafter in the Sk4C-02 sediment core. Further studies on algal distributions in Lake Skallen Oike are required.
Conclusions
Sedimentary facies, multi-proxy analysis of organic components, stable isotope ratios of organic carbon and nitrogen and AMS dating of carbon 14C of a sediment core from Lake Skallen Oike (Sk4C-02) from the Soya Kaigan in East Antarctica were studied to determine the geochemical features and sources of organic components, changes in biological production and species composition and changes from marine to lacustrine environments associated with relative sea level change. The results are summarized as follows:
-
(1)
The Sk4C-02 sediment core was composed of clayish mud (silt and clay) containing laminae, glacial sediments and echinoid spines formed in a marine environment between 378.0–152.5 cm. This was overlain by organic sediments containing algal mats formed during a marine to lacustrine transition (152.5–135 cm) and in lacustrine environments between 135 cm-surface.
-
(2)
The ages of surface sediments (0–2.3 cm) and the bottom sediments (378.0 cm) were 150 (AD1950-1640) and 7,030 ± 59 cal BP, respectively. The mean sedimentation rate was estimated to be 0.55 mm/year.
-
(3)
Multi-proxy analyses revealed that drastic environmental changes associated with the transition from marine to lacustrine environments occurred at a depth of 152.5 cm (ca. 3,590 cal BP). The ongoing retreat of glaciers during the MHH, and ongoing isostatic uplift are the main reasons for this isolation, whereas eustatic sea level change is believed to have played only a minor role. The mean local isostatic uplift rate was calculated to be 2.8 mm/year.
-
(4)
The coastal marine period in Lake Skallen Oike is characterized by low biological production with the predominance of diatoms. Three glacial sediment layers at ca. 5,260–5,200 (262–258 cm), 5,100 (251 cm) and 4,760–4,730 cal BP (229–227 cm) were probably formed by the nearby retreats and advances of glaciers due to climatic changes.
-
(5)
The transition zone from a marine inlet to a freshwater lake (153.0-approximately 135 cm, ca. 3,590–3,290 cal BP) was characterized by stratified conditions with marine water overlain by freshwater, and a chemocline developed together with an anoxic (sulfidic) layer in the bottom of photic zone. Green sulfur bacteria and Cryptophyta were the major photosynthetic organisms during the transition with Cryptophyta tolerating the moderate salinity and stratified water conditions.
-
(6)
The lacustrine zone (approximately 135 cm-surface, ca. 3,290 cal BP-present) is characterized by high biological production by green algae (e.g. Comarium clepsydra and Oedegonium spp.) with some contribution from cyanobacteria and diatoms. Biological production during this period was 8.7 times higher than that of coastal marine period.
References
Bentley MJ, Hodgson DA (2009) Antarctic Ice Sheet and climate history since the last Glacial Maximum. Pages News 17(1):28–29
Berkman PA, Andrews JT, Björck S, Colhoun EA, Emslie SD, Goodwin ID, Hall BL, Hart CP, Hirakawa K, Igarashi A, Ingolfsson O, López-Martínez J, Lyons WB, Mabin MCG, Quilty PG, Taviani M, Yoshida Y (1998) Circum-Antarctic coastal environmental shifts during the Lake Quaternary reflected by emerged marine deposits. Antarct Sci 10:345–362
Berner RA, Raiswell R (1984) C/S method for distinguishing freshwater from marine sedimentary rocks. Geology 12:365–368
Borrego CM, Garcia-Gil LJ (1994) Speciation of bacteriochlorophyll homologues from green photosynthetic sulfur bacteria by reversed phase HPLC. Photosynth Res 41:157–163
Boutton T (1991) Stable carbon isotope ratios of natural materials: II atmospheric, terrestrial, marine, and freshwater Environments. In: Brian CDCaF (ed) Carbon isotope techniques, Academic Press, San Diego, 274 p
Britton G, Liaaen-Jensen S, Pfander H (eds) (2004) Carotenoid handbook. Birkhauser Verlag, Basel, p 563
Chihara M (ed) (1997) Biology of algal diversity. Uchida Rokakuho Publishing Co. Ltd., Tokyo, 386 p, (in Japanese)
Feigin AD, Shearer GB, Kohl DH, Commoner B (1974) Variations of the natural nitrogen-15 abundance in nitrate mineralized during incubation of several Illinois soils. Soil Sci Soc Am Proc 38:90–95
Freyer HD, Aly AIM (1975) Nitrogen-15 studies on identifying fertilizer excess in environmental systems. In: Isotope ratios as pollutant source and behavior indicators. IAEA, Int. At. Energy Agency, Vienna, pp 21–23
Fukui F, Torii T, Okabe S (1985) Vertical distribution of nutrients and DOC in lake waters near Showa Station, Antarctica. Antarct Rec 86:28–35
Goodwin ID (1998) Did changes in Antarctic ice volume influence late Holocene sea-level lowering? Quat Sci Rev 17:319–332
Hodgson DA, Smol JP (2008) High latitude paleolimnology. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers—Limnology of Arctic and Antarctic aquatic ecosystems. Oxford University Press, Oxford, UK, pp 43–64
Hodgson DA, Verleyen E, Squier AH, Sabbe K, Squier AH, Keely BJ, Leng MJ, Saunders M, Vyverman K (2005) Late Quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core. Quat Res 64:83–99
Hodgson DA, Verleyen E, Squier AH, Sabbe K, Keely BJ, Saunders M, Vyverman K (2006) Interglacial environments of coastal east Antarctica: comparison of MIS1 (Holocene) and MIS 5e (Last Interglacial) lake-sediment records. Quat Sci Rev 25:179–197
Hodgson DA, Verleyen E, Vyverman W, Sabbe K, Leng MJ, Pickering M, Keely BJ (2009) A geological constraint on relative sea level in Marine Isotope Stage 3 in the Larsemann Hills, Lambert Glacier region, East Antarctica (31, 366–33, 228 cal yr BP). Quat Sci Rev 28:2689–2696
Hughen KA, Baillie MGL, Bard E, Bayliss A, Beck JW, Bertrand CJH, Blackwell PG, Buck CE, Burr GS, Cutler KB, Damon PE, Edwards RL, Fairbanks RG, Friedrich M, Guilderson TP, Kromer B, McCormac FG, Manning S, Bronk Ramsey C, Reimer PJ, Reimer RW, Remmele S, Southon JR, Stuiver M, Talamo S, Taylor FW, van der Plicht J, Weyhenmeyer CE (2004) Marine04 marine radiocarbon age calibration, 0–26 cal kyr BP. Radiocarbon 46:1059–1086
Imura S, Bando T, Seto K, Ohtani S, Kudoh S, Kanda H (2003) Distribution of aquatic mosses in the Soya Coast region, East Antarctica. Polar Biosci 16:1–10
Ingólfsson Ó, Hjort C, Berkman PA, Björck S, Colhoun E, Goodwin ID, Hall B, Hirakawa K, Melles M, Möller P, Prentice ML (1998) Antarctic glacial history since the Last Glacial Maximum: an overview of the record on land. Antarct Sci 10:326–344
Jeffrey SW, Mantoura RFC, Wright SW (eds) (1997) Phytoplankton pigments in oceanography. UNESCO Publishing, Paris 661
Kashiwaya K, Ochiai S, Sakai H, Kawai T (2001) Orbit-related long-term climate cycles revealed in a 12-Myr continental record from Lake Baikal. Nature 410:71–74
Lawson J, Doran PT, Kenig F, Des Marais DJ, Priscu JC (2004) Stable carbon and nitrogen isotopic composition of benthic and pelagic organic matter in lakes of the McMurdo Dry Valleys, Antarctica. Aquatic Geochem 10:269–301
Leavitt PR, Hodgson DA (2001) Sedimentary pigments. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Volume 3: Terrestrial, algal, and siliceous indicators. Kluwer, Dordrecht, pp 296–325
Matsumoto GI (1993) Geochemical features of the McMurdo Dry Valley lakes. In: Green W, Friedmann EI (eds) Physical and biogeochemical processes in Antarctic Lakes (Antarct ResSer 59). Amer Geophys Union, Wash DC, pp 95–118
Matsumoto GI, Watanuki K (1992) Geochemical features of organic components in extremely acid Crater Lake (Yugama) of Kusatsu-Shirane Volcano in Japan. Geochem J 26:117–136
Matsumoto G, Torii T, Hanya T (1979) Distribution of organic constituents in lake waters and sediments of the McMurdo Sound region in the Antarctic. Mem Natl Inst Polar Res Spec Issue 13:103–120
Matsumoto G, Torii T, Hanya T (1982) High abundance of algal 24-ethylcholesterol in Antarctic lake sediment. Nature 299:52–54
Matsumoto GI, Fujimura C, Minoura K, Takamatsu N, Takemura T, Hayashi S, Shichi K, Kawai T (2003) Estimation of paleoenvironmental changes in the Eurasian continental interior during the last 12 million years derived from organic components in sediment cores (BDP96&98) from Lake Baikal. In: Kashiwaya K (ed) Long continental records from Lake Baikal. Springer-Verlag, Tokyo, pp 75–94
Matsumoto GI, Komori K, Enomoto A, Takemura T, Imura S, Ohyama Y, Kanda H (2006) Environmental changes in Syowa Station area of Antarctica during the last 2,300 years inferred from organic components in sediments cores. Polar Biosci 19:51–62
Minagawa M, Winter DA, Kaplan IR (1984) Comparison of Kjeldahl and combustion methods for measurement of nitrogen isotope ratios in organic matter. Anal Chem 56:1859–1861
Miura H, Mamoku H, Igarashi A, Moriwaki K (1998a) Late Quaternary raised beach deposits and radiocarbon dates of marine fossils around Lützow-Holm Bay: Special map series of National Institute of Polar Research, National Institute Polar Research, Tokyo, pp 1–46
Miura H, Moriwaki K, Maemoku H, Hirakawa K (1998b) Fluctuations of the East Antarctic ice-sheet margin since the last glaciations from stratigraphy of raised beach deposits along the Soya Coast. Annals Glaciaol 27:297–301
Miura H, Maemoku H, Seto K, Moriwaki K (1998c) Late Quaternary East Antarctic melting event in the Soya Coast region based on stratigraphy and oxygen isotopic ratio of fossil molluscs. Polar Geosci 11:260–274
Murayama H (1977) General characteristics o the Antarctic lakes near Syowa Station. Antarct Rec 58:43–62 (in Japanese with English abstract)
Murayama H, Hidaka H, Yoshida Y (1988) A preliminary report on some limnological investigations of lakes in the vicinity of Syowa Station by Japanese Antarctic Research Expedition in 1981 and 1985. Antarct Rec 32:25–37 (in Japanese with English abstract)
Nara F, Tani Y, Soma Y, Soma M, Naraoka H, Watanabe T, Horiuchi K, Kawai T, Oda T, Nakanura T (2005) Response of phytoplankton productivity to climate change recorded by sedimentary photosynthetic pigments and other biological indicators in Lake Hovsgol (Mongolia) for the last 23,000 years. Quat Internat 136:71–81
Nishimura M (1982) 5β-isomers of stanols and stanones as potential markers of sedimentary organic quality and depositional paleoenvironments. Geochim Cosmochim Acta 46:423–432
Ohzono M, Tabei T, Doi K, Shibuya K, Sagiya T (2006) Crustal movement of Antarctica and Syowa Station based on GPS measurements. Earth Planets Space 58:795–804
Pfennig N (1967) Photosynthetic bacteria. Ann Rev Microbiol 21:285–324
Porra PJ (2006) Spectrometric assays for plant, algal and bacterial chlorophylls. In: Grimm B, Prra RJ, Rudiger W, Scheer H (eds) Chlorophylls and bacteriochlorophylls, biochemistry, biophysics, functions and application. Springer, Dordrecht, pp 95–107
Raymo ME, Ruddiman WF (1992) Tectonic forcing of late Cenozoic climate. Nature 359:117–122
Reddy GSN, Aggarwal RK, Matsumoto GI, Shivaji S (2000) Arthrobacter flavus sp. nov., a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley, Antarctica. Int J Sys Evol Microbiol 50:1553–1561
Reddy GSN, Prakash JSS, Srinivas R, Matsumoto GI, Shivaji S (2003) Leifsonia rubra sp. nov. and Leifsonia aurea sp. nov., psychrophiles from a pond in Antarctica. Int J Syst Evol Microbiol 53:977–984
Reimer PJ, Baillie MGL, Bard E, Bayliss A, Beck JW, Bertrand CJH, Blackwell PG, Buck CE, Burr GS, Cutler KB, Damon PE, Edwards RL, Fairbanks RG, Friedrich M, Guilderson TP, Hogg AG, Hughen KA, Kromer B, McCormac G, Manning S, Ramsey CB, Reimer RW, Remmele S, Southon JR, Stuiver M, Talamo S, Taylor FW, van der Plicht J, Weyhenmeyer CE (2004) IntCal04 terrestrial radiocarbon age calibration, 0–26 cal kyr BP. Radiocarbon 46:1029–1058
Roberts D, McMinn A (1998) A weighted-averaging regression and calibration model for inferring lakewater salinity from fossil diatom assemblages in saline lakes of the Vestfold Hills: a new tool for interpreting Holocene lake histories in Antarctica. J Paleolimnol 19:99–113
Robinson RS, Sigman DM (2008) Nitrogen isotopic evidence for a poleward decrease in surface nitrate within the ice age Antarctic. Quat Sci Rev 27:1076–1090
Sampei Y, Kurakado Y, Shimizu A, Takayasu K, Ishida H (1997a) Distribution of organic carbon, nitrogen and sulfur in surface sediments of Lake Saroma and Lake Abashiri, Hokkaido, Japan. Res Org Geochem 12:51–60 (in Japanese with English abstract)
Sampei Y, Matsumoto E, Kamei T, Tokuoka T (1997b) Sulfur and organic carbon relationship in sediments from coastal brackish lakes in Shimane peninsula district, southwest Japan. Geochem J 31:245–262
Seto K, Imura S, Bando T, Kanda H (2002) Paleoenvironment of Holocene recorded in Antarctic lakes. Gekkan Chikyu 24:31–36 (in Japanese)
Shackleton NJ, Berger A, Peltier WR (1990) An alternative astronomical calibration of the lower Pleistocene timescale based on ODP site 677. Trans Royal Soc Edinburgh: Earth Science 81:251–261
Short DA, Mengel JG, Crowley TJ, Hyde WT, North GR (1991) Filtering of Milankovitch cycles by earth’s geography. Quat Res 35:157–173
Smith JA, Hodgson DA, Bentley MJ, Verleyen E, Leng MJ, Roberts SJ (2006) Limnology of two Antarctic epishelf lakes and their potential to record periods of ice shelf loss. J Paleolimnol 35:373–394
Smith JA, Bentley MJ, Hodgson DA, Roberts SJ, Leng MJ, Lloyd JM, Barrett MS, Bryant C, Sugden DE (2007) Oceanic and atmospheric forcing of early Holocene ice shelf retreat, George VI Ice Shelf, Antarctica Peninsula. Quat Sci Rev 26:500–516
Soma Y, Tanaka A, Soma M, Kawai T (1996) Photosynthetic pigments and perylene in the sediments of southern basin of Lake Baikal. Org Geochem 24:553–561
Soma Y, Soma M, Tanaka A, Kawai T (2001) 2.8 million years of phytoplankton history in Lake Baikal recorded by the residual photosynthetic pigments in its sediment core. Geochem J 35:377–383
Soma M, Soma Y, Tani Y, Itoh N, Kurihara K, Nara F, Tanaka A, Kawai T (2003) Residual photosynthetic pigments in the sediment of Lake Baikal as indicators of phytoplankton history. In: Kashiwaya K (ed) Long continental records from Lake Baikal. Springer-Verlag, Tokyo, pp 137–160
Soma Y, Tani Y, Soma M, Mitake H, Kurihara R, Hashimoto S, Watanabe T, Nakamura T (2007) Sedimentary steryl chlorin esters (SCEs) and other photosynthetic pigments as indicator of paleolimnological change over the last 28,000 years from the Buguldeika Saddle of Lake Baikal. J Paleolimnol 37:163–175
Spuier AH, Hodgson DA, Keely BJ (2002) Sedimentary pigments as makers for environmental change in an Antarctic lake. Org Geochem 33:1655–1665
Tani Y, Kurihara K, Nara F, Itoh N, Soma M, Soma Y, Tanaka A, Yoneda M, Hirota MY, Shibata Y (2002) Temporal changes in the phytoplankton community of the southern basin of Lake Baikal over the last 24,000 years recorded by photosynthetic pigments in a sediment core. Org Geochem 33:1621–1634
Tani Y, Matsumoto GI, Soma M, Soma Y, Hashimoto S, Kawai T (2009) Photosynthetic pigments in sediment core HDP-04 from Lake Hovsgol, Mongolia, and their implication for changes in algal productivity and lake environment for the last 1 Ma. Quat Internat 205:74–83
Verleyen E, Hodgson DA, Vyverman W, Roberts D, McMinn A, Vanhouttedz K, Sabbe K (2003) Modelling diatom responses to climate induced fluctuations in the moisture balance in continental Antarctic lakes. J Paleolimnol 30:195–215
Verleyen E, Hodgson DA, Sabbe K, Vyverman W (2004a) Late Quaternary deglaciation and climate history of the Larsemann Hills (East Antarctica). J Quat Sci 19:361–375
Verleyen E, Hodgson DA, Sabbe K, Vanhoutte K, Vyverman W (2004b) Coastal oceanographic conditions in the Prydz Bay region (East Antarctica) during the Holocene recorded in an isolation basin. Holocene 14:246–257
Verleyen E, Hodgson DA, Milne GA, Sabbe K, Vyverman W (2005) Relative sea-level history from the Lambert Glacier region, East Antarctica, and its relation to deglaciation and Holocene glacier readvance. Quat Res 63:45–52
Volkman JK, Barrett SM, Blackburn SI, Mansour MP, Sikes EL, Gelin F (1998) Microbial biomarkers: a review of recent research developments. Org Geochem 29:1163–1179
Watanabe T, Nakamura T, Watanabe Nara F, Kakegawa T, Horiuchi K, Senda R, Oda T, Nishimura M, Matsumoto GI, Kawai T (2009) High-time resolution AMS 14C data sets for Lake Baikal and Lake Hovsgol sediment cores: changes in radiocarbon age and sedimentation rates during the transition from the last glacial to the Holocene. Quat Internat 205:12–20
Yamamoto S, Yoshioka H, Ishiwatari R (2007) Pyrolysis- and chemical degradation-GC/MS analyses of environmental kerogen and humic substances and their application to geochemistry. Bunseki Kagaku 56:71–91 (in Japanese with English abstract)
Yoneyama T, Matsumaru T, Usu K, Engelaar WMHG (2001) Discrimination of nitrogen isotopes during absorption of ammonium and nitrate at different nitrogen concentrations by rice (Oryza sativa L.) plants. Plant Cell Environ 24:133–139
Yoshida Y, Moriwaki K (1979) Some considerations on elevated coastal features and their dates around Syowa Station, Antarctica. Mem Natl Inst Polar Res Spec Issue 13:220–226
Zwartz D, Bird M, Stone J, Lambeck K (1998) Holocene sea-level change and ice-sheet history in the Vestfold Hills, East Antarctica. Earth Planet Sci Let 155:131–145
Acknowledgments
The authors appreciate Japanese Antarctic Research Expedition (JARE) 45th and 46th members, especially Drs Sato T, Uemura T and Kudoh S for their kind support in sampling of sediment cores, Antarctica. We thank Prof Ohtani S of Shimane University and Dr Kudoh S of National Institute of Polar Research (Japan) for their useful information on the distribution of algae and cyanobacteria in Lake Skallen Oike. We thank especially associate editor Dr Hodgson D A for useful comments and improving the manuscript, and anonymous reviewers, Editors-in-Chief Dr Whitmore Th J for their critical reviewing and useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, G.I., Tani, Y., Seto, K. et al. Holocene paleolimnological changes in Lake Skallen Oike in the Syowa Station area of Antarctica inferred from organic components in a sediment core (Sk4C-02). J Paleolimnol 44, 677–693 (2010). https://doi.org/10.1007/s10933-010-9448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-010-9448-y