Abstract
Bryophytes from submerged habitats are particularly difficult to identify because developmental plasticity obscures their characteristic features. A deep-water moss population of uncertain identity was found isolated at a depth of 31 m within the perennially ice-covered Lake Vanda, Wright Valley, Antarctica. Through phylogenetic analysis of the chloroplast region, ribosomal subunit 4 gene, nuclear ribosomal DNA region, and the internal transcribed spacer region, the Lake Vanda moss was identified as the cosmopolitan species Bryum pseudotriquetrum and resolved to a clade containing exclusively specimens from Antarctica, specifically those from the neighbouring Taylor Valley and Granite Harbour. The close genetic similarity of the Lake Vanda population to other populations of B. pseudotriquetrum in Southern Victoria Land suggests that colonisation was likely to have been from local sources, and colonisation likely occurred at least 80–100 years ago, given the position of mosses in the deeper of two convection cells in the lake. Light and scanning electron microscopy of in vitro cultured specimens revealed adaptations to permanent submersion, including very thin cell walls, which may increase CO2 absorption under water. The production of rhizoidal knots in contaminated, low-nutrient media, but not in axenic cultures, might result from interactions between the moss and organisms in the microbial mat from which it was isolated. The absence of mosses around the lake margin or elsewhere in Wright Valley highlights the importance of freshwater ecosystems as refugia for biodiversity in Antarctica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctica is renowned as being one of the harshest environments on Earth, and the continent supports only two species of native flowering plants. What is often less widely known is that this seemingly inhospitable and barren land is occupied by a considerable diversity of bryophytes (mosses and liverworts). Bryophytes are one of the most common divisions of plants on the Antarctic continent, with 111 moss species belonging to 17 families in 55 genera and 27 liverwort species belonging to 12 families in 19 genera (Bednarek-Ochyra et al. 2000; Ochyra et al. 2008; Ellis et al. 2014). The remainder of the Antarctic flora is composed of lichens, another cryptogram group, but which outnumbers the bryophytes at around 300–400 species (Kappen 2000). Liverworts, which are more restricted by water regime than mosses and lichens, are only represented by one species in continental Antarctica (Bednarek-Ochyra et al. 2000).
Bryophytes are integral to many ecosystems, providing habitats and food for invertebrates as well as contributing to the carbon cycling of polar terrestrial ecosystems through the accumulation and release of organic matter (Richardson 1981). Bryophytes are mainly restricted to areas with a regular supply of water, for example soils wetted by rain or melting snow. They are thus most diverse within the maritime Antarctic islands and coastal zones of the Antarctic Peninsula, which are considerably milder and wetter than the lichen-dominated continent (Ochyra et al. 2008). Within these regions, fellfield areas (scree and glacial debris rendered unstable by winds and cryoturbic action) and geothermal sites are the most suitable habitats for bryophytes. However, there are several moss species that grow in coastal regions of continental Antarctica, and a few even withstand the extreme conditions of inland sites (Ochyra et al. 2008). A few species of mosses are also known from deep-water habitats (>0.5 m), such as B. pseudotriquetrum growing at water depths of up to 81 m in Radok Lake, Amery Oasis, East Antarctica (Wagner and Seppelt 2006) and Pohlia wilsonii from Schirmacher Oasis of coastal continental Antarctica (Tewari and Pant 1996).
A population of Bryum also grows in cyanobacteria-based microbial mats from a depth of 28 m in Lake Vanda, Wright Valley, Southern Victoria Land (77o32′S, 161o35′E) and represents the southernmost record of a moss from a perennial liquid deep-water habitat. No other populations were found in the lakes of the neighbouring Taylor Valley, nor in the littoral regions of lakes of Taylor and Wright Valleys (Kaspar et al. 1982).
The initial identification by Kaspar et al. (1982) referred to the moss as Bryum cf. algens Card., based on morphological characters obtained by light microscopy only. The species has since been synonymised by Ochyra (1998) as the cosmopolitan B. pseudotriquetrum (Hedw.) P. Gaertnn., B. Mey. & Scherb. However, to date, this assignment has not been confirmed using molecular phylogenies. Identifying submerged mosses in particular can be troublesome due to their plastic morphology. Molecular techniques, however, are now making it easier to determine the identity of ambiguous mosses. As well as the record from Radok lake mentioned above, this particular species is also known from depths of 36 m in Bangor’s Oasis, East Antarctica and from shallower depths of 2–9 m in pools near Syowa Station, East Ongul Island and pools within Ablation Valley, Alexander Island, Antarctica (Savich-Lyubitskaya and Smironova 1959; Light and Heywood 1975; Kanda and Iwatsuki 1989).
The aim of the present study was to verify the species assignment of the Bryum populations in Lake Vanda as Bryum pseudotriquetrum for the first time by way of phylogenetic analysis. In addition, possible morphological adaptations to the aquatic environment were evaluated by means of in vitro cultivation as well as light and scanning electron microscopy.
Materials and methods
Study site
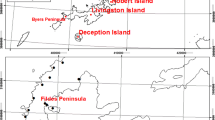
Lake Vanda is positioned at 77o31′47″S 161o34′32″E (Fig. 1), has an area of 7.8 km2 and is covered by layer of ice between 3.5 and 4 m thick. Around 15% of photosynthetically active radiation (PAR) passes through the 3.5-m thick ice cover unhindered (Howard-Williams et al. 1998). The lake occupies a closed basin, with an inflow but no outflow, hence the level of the lake is set by the balance between ablation from the ice surface and the inflow of meltwater. In recent years, the lake has been rising in level and in 2010 was just over 75 m deep; when the moss was first discovered in 1980, the lake was 10-m shallower (Hawes et al. 2013). The lake has an unusual physical structure with increasing conductivity and temperature with depth (Castendyk et al. 2016). It is divided into three main layers, two convection layers from 4- to 24-m depth (temperature and conductivity 4.5 °C and 960 µS cm−1) and from 28- to 45-m depth (6.5 °C and 1620 µS cm−1), transitioning below this to a near-continuously increasing density gradient below 45 m, culminating in a hypersaline brine at >20 °C in the third and deepest layer (Mikucki et al. 2010).
The lake floor is covered to at least 50 m with microbial mats that are composed primarily of cyanobacteria from the genera Phormidium, Leptolyngbya and Pseudanabaena (Hawes et al. 2013; Zhang et al. 2015). Growing in association with the microbial mats are moss populations, but only at depths greater than 28 m in the lower convection cell. A careful examination of the lake shore and areas in and around the river flowing into Lake Vanda did not reveal any mosses.
Sampling
Samples of the microbial mats were collected from a 1 m2 area at a depth of 31 m (corresponding to the middle convection cell of the lake) by a diver in December 2013. At the time of sampling, that water depth had a temperature of 6.7 °C with a pH of 8.5 and a conductivity of 1620 µS/cm. Incident irradiance was measured to be ~100 µmol photons/m2/s with a LI-COR Biosciences LI-192 cosine corrected underwater quantum detector attached to a LI-1400 data logger, and water nitrate-N concentration was 30 µg L−1 and dissolved reactive phosphorous (DRP) concentration was 1 µg L−1 (Hawes unpublished data). Approximately 50 moss gametophytes were dissected from the microbial mats within 48 h of collection and kept at approximately 10 °C until return to the Natural History Museum, London. The moss samples were imaged under the light microscope and used for initial in vitro cultivation within 6 weeks of collection.
In vitro cultivation
Moss gametophytes were rinsed in distilled water and placed directly onto 10% Parker’s nutrient medium (Klekowski 1969) solidified with 1% Phytagel, following the method by Duckett et al. (2004). After 6 weeks, once the gametophytes had regenerated and produced extensive protonemal colonies bearing new shoots, subculturing was carried out on a variety of media such as 100 and 1% Parker’s medium, known to stimulate production of protonemal gemmae and rhizoidal tubers, respectively (Duckett et al. 2004); BCD medium supplemented with 1 mM CaCl2, 5 mM ammonium tartrate and solidified by 0.8% agarose (Nishiyama et al. 2000), as it promotes faster regeneration than other growth media for bulking up of tissue for phylogenetic analyses. A single plate of the moss was used in subculturing to make 15 replicates on 100% Parker’s medium, 15 replicates on 1% Parker’s medium and 35 replicates on BCD medium. Cultures were maintained in a growth cabinet at 18 °C with a 14/10 h day/night regime and a daytime irradiance of 100 mmol photons m−2 s−1. The ambient CO2 was kept at ~440 ppm. Cultures were assessed visually on a weekly basis for regeneration and contamination. When axenic fragments were found in otherwise contaminated cultures, these were removed and placed onto fresh medium. Voucher specimens of the moss were deposited with the Natural History Museum, London.
Light and scanning electron microscopy (SEM)
Regenerated tissues (leaves, protonemata and rhizoids) at various stages of development were mounted in water and photographed under a Zeiss Axio Microscope (Zeiss, Germany) equipped with a MRc digital camera. For SEM, cultures were processed following the protocol of Duckett and Ligrone (1995). Briefly, tissues were fixed in 3% glutaraldehyde at room temperature for 24 h. After several rinses in distilled water, specimens were dehydrated over 24 h in a graded (10–100%) ethanol series; critical-point dried using CO2 as transfusion fluid; sputter coated with 20 nm of palladium-gold; and observed with a FEI Quanta scanning electron microscope (FEI, Hillsboro, Oregon, USA) operating at 10 kV.
DNA extraction
DNA was extracted from a single axenic culture of the Lake Vanda moss using the Hexadecyltrimethyl-ammonium bromide (CTAB) method. In addition, DNA was extracted from herbarium specimens (Natural History Museum) of several Bryum species collected from Antarctica and for which neither nrITS or rps4 sequences are currently available in GenBank, including: B. archangelicum Bruch & Schimp., B. pseudotriquetrum (Hedw.) P. Gaertnn., B. Mei. & Scherb, B. argenteum Hedw., B. pallescens Schwägr, B. orbiculatifolium Cardot & Broth. and B. dichotomum Hedw. (Table 1).
For DNA extraction, approximately 5 mm2 of material from the Lake Vanda culture and from each of 22 herbarium specimens was ground in an acid-washed mortar and pestle with approximately the same volume of sterile sand until a fine powder had been achieved. The powder was added to a 1.5-ml Eppendorf tube containing 0.5 ml of CTAB buffer (2% CTAB, 100 mM Tris (pH 8.0), 20 mM EDTA (pH 8.0), 1.4 M Sodium Chloride, and 1% PVP), 50 μl of 10% Sarkosyl solution in 100 mM Tris (pH 8.0) and 10 μl of Proteinase K 40 (units/ml). Samples were vortexed and placed in a 60 °C water bath heated for 30 min. Afterwards, 0.5 ml of SEVAC (Iso Amyl alcohol (24:1)) was added and mixed by vortexing. The tubes were centrifuged for 3 min at 13,000 rpm, and the resulting upper layer was then carefully transferred and the step was repeated. After adding 400 μl of Isopropanol, samples were centrifuged for 3 min and the supernatant was discarded. The extracted DNA was washed by adding 0.5 ml 70% ethanol and centrifuged for 3 min. The supernatant was removed, and the final DNA pellet air-dried for 10 min. The DNA was re-suspended in 30 μl of sterile PCR-water.
Polymerase chain reaction (PCR)
The nucleotide sequence of one chloroplast DNA region, the ribosomal subunit 4 gene (rps4), and one nuclear ribosomal DNA region, the nuclear internal transcribed spacer region (nrITS), were amplified by PCR for each moss sample. The primers used were RPS4F (5′-ATGTCCCGTTATCGAGGACCT-3′) and TRNS (5′-TACCGAGGGTTCGAATC-3′), and AB101F (5′- ACGAATTCATGGTCCGGTGAAGTGTTCG-3′) and AB102R (5′-TAGAATTCCCCGGTTCGCTCGCCGTTAC-3′), respectively (Nadot et al. 1994; Sun et al. 1994). The PCR was performed in 25 μl reaction volumes containing 0.5 μl of Bioline taq polymerase (5 U/μl), 2.5 μl of 10 × buffer, 1.25 μl MgCl (50 mM), 1–2 μl of DNA, 1 μl (10 μM) of each primer, 0.5 μl of dNTPs (25 mM) and sterile PCR-water. The thermocycling regime was run for 30–40 cycles and PCR conditions were 94 °C for 30 s, 52 °C (up to 54 °C for ITS primers) for 30 s and 72 °C for 1 min for both primer sets. Successful amplifications were identified by gel electrophoresis using 1% of agarose. Samples displaying double bands were excised and purified using a QIAGEN MinElute Gel Extraction Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Sequencing was carried out using the same primers as used for the amplicon generation by PCR at the Natural History Museum sequencing facility using Applied Biosystems 3730xl DNA analyser (Applied Biosystems, Foster City, CA).
Sequence alignment and phylogenetic analysis
Phylogenetic analyses were performed for both rps4 and nrITS. To add to the specimens sequenced in this study, sequences for other species were sourced from GenBank (Tables 2 and 3). Species of Bryaceae were chosen based on works by Cox and Hedderson (2003) and Pedersen et al. (2006). In total, this material covered seven sections of Bryum and 11 genera. Sequences were first aligned by the Opal 2.1.0 algorithm using the Opalescent package and manually corrected in Mesquite (Wheeler and Kececioglu 2007). Maximum likelihood (ML) and Bayesian Markov Chain Monte Carlo inference (BI) analytical methods were implemented for both rps4 and nrITS. For both regions, jModelTest 2 (Guindon and Gascuel 2003; Darriba et al. 2012) was used via the CIPRES Science Gateway v.3.1 (Miller et al., 2010) to ascertain the best-fitting substitution model as indicated by the corrected Akaike Information Criteria (AICc). For both regions, a general time-reversible model with gamma rates was selected (GTR+G). Funaria hygrometrica was used as an outgroup in all analyses. The ML analyses were performed using RAxML-HPC2 (v.8.1.24; Stamatakis, 2014) on XSEDE through the CIPRES Science Gateway. A rapid bootstrapping analysis was performed for the maximum number of 1000 bootstraps (BS). A majority rule consensus tree was calculated from the bootstraps using Consense (Felsenstein 1993). The BI analyses were performed using BEAST (v.1.8.2; Drummond and Rambaut 2007) on XEDE through the CIPRES Science Gateway. A file containing all the parameters to be used was created for each gene using BEAUti (v.1.8.2; Drummond et al. 2012). A lognormal relaxed clock was used, and the tree model used was a Yule speciation process. The following parameters for substitution rate and gamma shape estimated by jModelTest 2 for ITS were used in the analysis: Ra(AC) = 1.0976, Rb(AG) = 1.6088, Rc(AT) = 1.1220, Rd(CG) = 1.1772, Re(CT) = 1.6927, Rf(GT) = 1, gamma shape = 0.6764. The following parameters for substitution rate and gamma shape estimated by jModelTest 2 for rps4 were used in the analysis: Ra(AC) = 0.962, Rb(AG) = 3.389, Rc(AT) = 0.243, Rd(CG) = 1.227, Re(CT) = 3.389, Rf(GT) = 1, gamma shape = 0.58. Markov chain Monte Carlo analyses were run for 50,000,000 generations each, sampling parameter values every 5000 generations. Distribution and convergence of the parameter were checked via TRACER (v1.6). The burn-in (1000 trees) was discarded and then TreeAnnotator (Drummond and Rambaut 2007) was used to find the maximum clade credibility tree from the posterior distribution of trees using median ages. The final trees of both ML and BI analyses were edited using FigTree v1.4.2 (Rambaut 2009). Sequences are available under the NCBI accession numbers.
Results
Culturing and microscopy
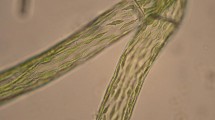
Wild, sterile gametophytes of the Lake Vanda moss growing within the microbial mats were yellow-green in colour and etiolated (Fig. 2a). The elongated stems, ca. 12 mm long, had consistently small leaves (ca. 0.5 mm in length) evenly arranged along their length with interleaf distances of up to 0.4 mm, and numerous rhizoidal filaments (Fig. 2b).
a Wild, sterile gametophytes of the Lake Vanda moss growing within the microbial mats (scale = 7 mm); b etiolated gametophyte dissected from microbial mat (scale = 3 mm); c in vitro moss culture grown on high medium (scale = 3 mm); d in vitro moss culture grown on low medium showing copious rhizoid production (scale = 6 mm); e SEM image of regenerated tissues gave rise to vivid green dense cushions with numerous shoots, extensive protonemata and high leaf densities towards the top of the gametophytes (scale = 2 mm); f protonemata with chloronemal and caulonemal filaments by light microscopy (scale = 100 μm); g SEM image of thin cell walls with chloroplasts casting ‘shadows’ through the leaf lamina (ghost plastids); h light microscopy image of brown-pigmented, highly branched rhizoids (scale = 50 μm); i brown rhizoids packed with lipid droplets (scale = 20 μm); and j SEM image of fine rhizoids forming a knot (scale = 20 μm)
The wild gametophytes regenerated in vitro within 6 weeks of culturing and produced extensive protonemal colonies with young shoots. Subculturing using both gametophore and protonemal fragments as inocula was successful on high- and low-nutrient media (Fig. 2c, d). On high-nutrient media (100% Parker and BCD), regenerating tissues gave rise to vivid green dense cushions with numerous shoots and extensive protonemata (chloronemal and caulonemal filaments) after 3 weeks (Fig. 2c, f). Leaves were arranged along the stems rather distant below and more crowded and larger higher up (basal leaves: 0.31–0.33 mm long and 0.1–0.14 mm wide; upper leaves: 0.9–1.2 mm long and 0.35–0.4 mm wide). Overall leaf morphology was typical of B. pseudotriquetrum; however, leaves of both wild and cultured specimens had very thin cell walls with chloroplasts casting ‘shadows’ through the leaf lamina (ghost plastids) when viewed under the SEM (Fig. 2g).
Cultures grown on the low-nutrient medium consisted of smaller, less dense cushions (Fig. 2d). Shoot and leaf morphology were the same as in the high-nutrient cultures; however, the filamentous system consisted almost entirely of brown-pigmented, highly branched rhizoids packed with lipid droplets and with highly papillose cell walls (Fig. 2h, i).
A peculiarity of low-nutrient cultures contaminated with cyanobacteria and/or fungi was that the rhizoids traced a highly undulating course on the surface of the medium and their finer ramifications often became intertwined, forming characteristic knots. These knots were neither observed in axenic cultures nor in high-nutrient contaminated cultures (Fig. 2h–j). However, neither protonemal gemmae nor rhizoidal tubers were seen on any of the Lake Vanda B. pseudotriquetrum cultures, similar to northern hemisphere collections.
Phylogenetic analyses
The phylogenetic position of the Lake Vanda moss was confirmed to be in the genus Bryum by means of ML phylogenetic analysis of the nrITS and rps4 genes. Analysis using nrITS generated a phylogenetic tree that grouped the Vanda Bryum pseudotriquetrum with Antarctic B. pseudotriquetrum with moderate support (BS ≥ 50; Fig. 3). This clade was nested within a clade composed of mainly B. pseudotriquetrum (Antarctic and non-Antarctic sequences) and B. archangelicum (BS ≥ 50). The remaining Antarctic B. pseudotriquetrum specimens sequenced in this study were part of this larger clade.
Maximum Likelihood analysis of Bryum species based on ITS region sequences (Ln-likelihood = −12,519). The lake Vanda Bryum is marked in bold. Sequences contributed by the current study are denoted by an asterisk. Specimens that originate from Antarctica are underlined. An open circle at the base of a node indicates a BS value of ≥50 and those with ≥70 are indicated by a cross. The scale bar represents 0.2 substitutions per site
The BI analysis for ITS provided a tree which had much higher posterior confidence values than the ML analyses with most clades receiving posterior probabilities (PP) higher than 0.95 (Fig. 4). In the BI analysis, the Vanda Bryum was nested inside a cluster containing all the Antarctic and the one Australian B. pseudotriquetrum sequences (PP ≥ 0.95). Within this, the Vanda Bryum was most similar to moss specimens also originating from Southern Victoria Land, neighbouring Taylor Valley and Granite Harbour (PP ≥ 0.95). This clade was nested within a clade, which encompassed the remainder of the B. pseudotriquetrum sequences and among them, all the B. archangelicum sequences (PP ≥ 90).
Bayesian Inference analysis of Bryum species based on ITS region sequences. The lake Vanda Bryum is is marked in bold. Sequences contributed by the current study are denoted by an asterisk. Specimens that originate from Antarctica are underlined. An open circle at the base of a node indicates a PP of ≥90 and those with ≥95 are indicated by a cross. The scale bar represents 0.6 substitutions per site
The rps4 ML analysis placed the Vanda Bryum alongside B. archangelicum; however, this relationship received very poor support (BS < 50; Online Resource 1). The sections of Bryum themselves were found to be polyphyletic. In general, the relationships within the tree had low bootstrap values. A BI analysis of rps4 was performed and on this tree, the Vanda Bryum was placed beside B. pallescens but was not significantly supported (PP < 0.90; Online Resource 2) and also confirmed polyphyly in this Bryum section.
Discussion
This study is the first to confirm, by means of molecular analyses, previous determinations of the Lake Vanda moss as B. pseudotriquetrum (Kaspar et al. 1982; Ochyra et al. 2008). Our analyses provide further insights into the phylogenetic position of the Lake Vanda population, resolving it to a clade of B. pseudotriquetrum containing exclusively specimens from Antarctica, specifically those from the neighbouring Taylor Valley and Granite Harbour. This clade was contained within a larger clade of B. pseudotriquetrum sequences, all but one originating from Antarctica. Furthermore, our phylogenetic tree shows that the clade containing all the Antarctic B. pseudotriquetrum sequences are grouped separately from the clade containing both B. archangelicum and non-Antarctic B. pseudotriquetrum.
Bryum pseudotriquetrum is one of the most widespread mosses in Antarctica. It is found on the South Sandwich, South Orkney and South Shetland Islands, as well as the Antarctic Peninsula. On the continent itself, it is present on the Maud, Enderby, Wilkes and Ross sectors (Ochyra et al. 2008). Although it is usually considered a terrestrial species, there are at least twelve separate reports of B. pseudotriquetrum residing in aquatic habitats in Antarctica, including the particularly deep depths of 34 m in Lake Progress and 81 m in Radok Lake (Li et al. 2009; Kurbatova and Andreev 2015). Conditions in Antarctic lakes are suboptimal for moss growth; however, they might be far more hospitable than the surrounding terrain. Indeed, mosses are not known to grow terrestrially anywhere in Wright Valley. Kaspar et al. (1982) proposed that the Lake Vanda B. pseudotriquetrum is unable to grow on the surrounding soil due to high salinity, freeze–thaw cycles, desiccation and cyclonic winds. Absence of submerged lake mosses from the vicinity has been reported for other lakes (Ignatov and Kurbatova 1990) suggesting that the aquatic form might fill a distinct niche (Gąbka et al. 2014). Thus Lake Vanda might serve as a reservoir of biodiversity for the region by providing a refuge from the more extreme conditions on the surface. Lakes in the region have also acted as local refugia over much longer timescales during glaciations. Copepods for example are known to have survived the glaciations in Lake Joyce (Karanovic et al. 2014). The idea of ice-free parts of the continent acting as refugia during glaciations is now widely accepted (Convey et al. 2009).
The close genetic similarity of the Lake Vanda population to other populations of B. pseudotriquetrum in Southern Victoria Land suggests that colonisation was likely to have been from local sources. The closest moss populations to Wright Valley are unconfirmed reports of mosses on the Asgard Range, which divides Wright Valley and Taylor Valley (Kaspar et al. 1982). It is very plausible that propagules from Taylor Valley and the Asgard Range travel through Wright Valley, and it is possible that they might have founded the Lake Vanda population. Wind is a major mechanism of dispersal in Antarctica for a range of organisms, including invertebrates, algae, lichens and mosses (Ellis-Evans and Walton 1990). A study by Skotnicki et al. (2001) recorded the dispersal of propagules of the moss Campylopus pyriformis from Mount Melbourne to Mount Erebus (a distance of 300 km) where it grows as protonemata.
Lake Vanda is multi-layered with two convectively mixed water layers (4–25 m and 27–45 m) separated by a steep density gradient and overlying a continuous density gradient from 45 m to the lake floor (Castendyk et al. 2016). Using a combination of water balance models and benthic microbial mat data, Castendyk et al. (2016) showed that the upper convection cell is a recent phenomenon, having formed over the last 80–100 years. B. pseudotriquetrum in Lake Vanda is restricted to the lower of the two convection cells. The absence of mixing between the two convection cells would have prevented movement of passive propagules from the lower cell to the upper, and only propagules entering the lake from outside could have colonised the upper convection cell (Castendyk et al. 2016). The absence of B. pseudotriquetrum from the upper, more recent cell, therefore suggests that the last successful colonisation event occurred prior to the formation of the upper convection cell, which is more than 80–100 years ago. The lower convecting layer is thought to have formed approximately 1000 years ago as freshwater began to flow into the lake after a prolonged period of dry-down and salinisation (Castendyk et al. 2016). The basal waters of the lake are hypersaline and dimly lit, and it is currently not possible to determine whether the lower depth limit of the moss is set by either irradiance or salinity.
Thus, the distribution of the moss is very much a product of the particular limnology of Lake Vanda, and if the lake were to change, the growth conditions of the moss would undoubtedly do likewise. For example, the lake has been rising in level for the past 100 years, which appears to be due to an increase in the number of summer days above freezing (Hawes et al. 2013). Increased inflow is causing the upper convection cell to expand, and it is unknown how the moss will be affected. Implications of an increased depth could range from extinction due to reduced light levels, since the moss cannot migrate to the upper convection cell, to potential expansion if the convection cells ultimately mix. Hence the Lake Vanda moss population may provide a useful model on how highly plastic mosses respond to environmental change such as lake level rise.
Turning to morphology, the main features of wild gametophytes of Bryum pseudotriquetrum from Lake Vanda are typical for deep-water mosses. In particular, the etiolated shoots with very small leaves (Wagner and Seppelt 2006; Ochyra et al. 2008; Li et al. 2009) are in line with previous descriptions of sub-aerial Antarctic B. pseudotriquetrum populations (Seppelt and Green 1998; Ochyra et al. 2008). Shoot etiolation and small leaves are a direct response to the limited light availability of deep-water habitats. An additional feature of the Lake Vanda B. pseudotriquetrum, as revealed by SEM, is the extremely thin leaf cell walls. These are a common plastic adaptation of aquatic and semi-emergent mosses (Vitt and Glime 1984; Ares et al. 2014), thought to facilitate carbon dioxide uptake (Mommer and Visser 2005) by submerged leaves. High levels of carbon dioxide in some water bodies have been found to play a particularly important role for the survival of deep-water mosses, countering other factors that constrain growth (Lovalvo et al. 2010). The chloroplast ‘shadows’ seen in SEM have also been noted in the similarly thin-walled cells of the thalloid liverwort Cyathodium growing on a dripping cliff face (Duckett and Ligrone 1995).
Another possible adaptation to the deep-water habitat of Lake Vanda is the extensive system of rhizoids with terminal knots produced by the Lake Vanda population when growing on low-nutrient contaminated medium, i.e. the in vitro conditions that more closely resemble its natural growth conditions. Rhizoids are known to show thigmotropism (Goode et al. 1992), exhibiting nutation until or when they contact a surface (Duckett 1994; Ares et al. 2014). Production of rhizoids rather than protonemal filaments in response to low-nutrient conditions has been reported before in other moss species (Duckett and Matcham 1995). However, the knotting behaviour observed exclusively on contaminated media might reflect developmental changes in response to other organisms in the cultures, including those that associate with Bryum in nature, i.e. the cyanobacteria forming the microbial mats in which Bryum grows. There are several reports of developmental changes and/or nutritional benefits to mosses in variously loose associations with microorganisms, both in culture (Spiess et al. 1971; Glime and Knoop 1986; Ares et al. 2014) and in the wild (Berg et al. 2013; Rousk et al. 2013; see Ares et al. 2014 for a discussion). It may be that the Bryum population from Lake Vanda, an oligotrophic lake, might somewhat benefit from its association with the cyanobacteria-based microbial mat in which the moss grows—whether through nutrient transfer or acquisition of nutrients from decomposition of the mats. Possible interactions between the Lake Vanda moss and the organisms forming the microbial mats certainly deserve further scrutiny.
The submerged habit of the Lake Vanda Bryum pseudotriquetrum is not unique to Antarctica, nor is it restricted to this species. Bryum pseudotriquetrum has been recorded from streams in the UK, Portugal and Canada (Watson 1919; Holmes 1985; Glime and Vitt 1986; Vieira et al. 2005) and from lakes and glaciofluvial springs in Sweden (Persson 1942; Hoffsten and Malmqvist 2000). Some other 85 moss species are known to grow in submerged habitats, some reaching depths comparable to B. pseudotriquetrum in Antarctica (Ignatov and Kurbatova 1990). Light and Smith (1976) found eight species growing at depths between 1 and 20 m in four lochs in the Scottish Highlands, and a large study of lakes in South Island, New Zealand, reported that 25% of the lakes contained deep-water mosses, including over half of the world’s records for mosses submerged at depths greater than 50 m and spanning 42 taxa in 11 families, including several species of Bryum (de Winton and Beever 2004).
Conclusion
Molecular analysis of the nrITS and rps4 regions confirmed the previous identification of the Lake Vanda moss population as Bryum pseudotriquetrum and further resolves it to a subcluster containing exclusively specimens from neighbouring regions in Antarctica. The close genetic similarity of the Lake Vanda population to other populations of B. pseudotriquetrum in Southern Victoria Land suggests that colonisation was likely to have been from local sources whilst the position of the moss in the lake places the last colonisation event at over 100 years ago. Very thin leaf cell walls in wild and cultured specimens, together with the production of copious rhizoidal knots on contaminated media, point to possible adaptations to the deep-water environment occupied by the Lake Vanda Bryum where it grows in close associations with cyanobacteria. How mosses cope with deep-water habitats and how they might be affected by climatic change deserves further scrutiny. The recording of B. pseudotriquetrum in Lake Vanda, Wright Valley, and its genetic similarity to other populations in the region, provides further support for the importance of freshwater ecosystems as refugia for biodiversity in Antarctica.
References
Ares A, Duckett JG, Pressel S (2014) Asexual development and protonemal development in vitro in Fontinalis antipyretica Hedw. J Bryol 36:122–133
Bednarek-Ochyra H, Váňa J, Ochyra R, Lewis Smith RI (2000) The liverwort flora of Antarctica. Polish Academy of Sciences, Krakow
Berg A, Danielsson Å, Svensson BH (2013) Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil 362:271–278
Castendyk DN, Obryk MK, Leidman SZ, Gooseff M, Hawes I (2016) Lake Vanda: a sentinel for climate change in the McMurdo Sound regions of Antarctica. Glob Planet Change 144:213–227
Convey P, Stevens MI, Hodgson DA, Smellie JL, Hillenbrand C-D, Barnes DKA, Clarke A, Pugh PJA, Linse K, Cary SC (2009) Exploring biological constraints on the glacial history of Antarctica. Quat Sci Rev 28:3035–3048
Cox CJ, Hedderson TAJ (1999) Phylogenetic relationships among the ciliate arthrodontous mosses: evidence from chloroplast and nuclear DNA sequences. Plant Syst Evol 215:119–139
Cox CJ, Hedderson TAJ (2003) Phylogenetic relationships within the moss family Bryaceae based on chloroplast DNA evidence. J Bryol 25:31–40
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
de Winton MD, Beever JE (2004) Deep-water bryophyte records from New Zealand lakes. New Zeal J Mar Fresh Res 38:329–340
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Duckett JG (1994) Studies of protonemal morphogenesis in mosses VI. The foliar rhizoids of Calliergon stramineum (Brid.) Kindb. function as organs of attachment. J Bryol 18:239–252
Duckett JG, Ligrone R (1995) Cyathodium Kunze, (Cyathodiaceae, Marchantiales), a tropical liverwort genus and family new to Europe, in Southern Italy. J Bryol 28:88–96
Duckett JG, Matcham HW (1995) Studies of protonemal morphogenesis in mosses VII. The perennial rhizoids and gemmiferous protonema of Dicranella heteromalla (Hedw.) Schimp. J Bryol 18:407–424
Duckett JG, Burch J, Fletcher PW, Matcham HW, Read DJ, Russell AJ, Pressel S (2004) In vitro cultivation of bryophytes: a review of practicalities, problems, progress and promise. J Bryol 26:3–20
Ellis LT et al (2014) Bryological Notes new national and regional bryophyte records 41. Bryol 36:306–324
Ellis-Evans JC, Walton D (1990) The process of colonisation in Antarctic terrestrial and freshwater ecosystems. Polar Biol 3:151–163
Felsenstein J (1993) Phylip (phylogeny inference package), version 3.5c. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Gąbka M, Rusińska A, Barylski J (2014) Floristic studies on freshwater algae of Lake Gondwana, Northern Victoria Land (Antarctica). Hydrobiologia 316:81–90
Glime JM, Knoop BC (1986) Spore germination and protonema development of Fontinalis squamosa. J Hattori Bot Lab 61:487–497
Glime JM, Vitt DH (1986) A comparison of bryophyte species diversity and niche structure of montane streams and banks. Can J Bot 65:1824–1837
Goode JA, Duckett JG, Stead AJ (1992) Towards an understanding of developmental interrelationships between chloronema, caulonema, rhizoids and plates in mosses; a comparative study. Cryptogam Bot 3:50–59
Guerra J, Jiménez-Martínez JF, Cano MJ, Jiménez-Fernández JA (2011) A contribution to the phylogenetic study of Mielichhoferiaceae-Mniaceae (Bryophyta) based on molecular sequence data. Nova Hedwigia 93:47–56
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704
Hawes I, Sumner DY, Andersen DT, Jungblut AD, Mackey TJ (2013) Timescales of growth response of microbial mats to environmental change in an ice-covered Antarctic lake. Biol 2:151–176
Hills SKK, Stevens MI, Gemmil CEC (2010) Molecular support for Pleistocene persistence of the continental Antarctic moss Bryum argenteum. Antarct Sci 22:721–726
Hoffsten P, Malmqvist B (2000) The macroinvertebrate fauna and hydrogeology of springs in central Sweden. Hydrobiol 436:91–104
Holmes NTH (1985) Vegetation of the River Dee. In: Jenkins D (ed) The biology and management of the River Dee. The Lavenham Press Ltd, Suffolk, pp 42–55
Holyoak DT, Hedenãs L (2006) Morphological and molecular studies of the intergrading taxa Bryum neodamense and B. pseudotriquetrum (Bryopsida: Bryaceae). J Bryol 28:299–311
Howard-Williams C, Schwarz A-M, Hawes I (1998) Optical properties of the McMurdo Dry Valley Lakes, Antarctica. In: Priscu JC (ed) Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union, Washington, pp 189–205
Ignatov MS, Kurbatova NB (1990) A review of deep water bryophytes with new records from USSR. Hikobia 10:393–401
Kanda H, Iwatsuki Z (1989) Two aquatic mosses in the lakes near Syowa Station, Continental Antarctica. Hikobia 10:293–297
Kappen L (2000) Some aspects of the great success of lichens in Antarctica. Antarct Sci 12:314–324
Karanovic T, Gibson JAE, Hawes I, Andersen DT, Stevens MI (2014) Diacyclops (Copepoda: Cyclopoida) in continental Antarctica, including three new species. Antarct Sci 26:250–260
Kaspar M, Simmons GM Jr, Parker BC, Seaburg KG, Wharton RA Jr, Lewis Smith RI (1982) Bryum Hedw. Collected from Lake Vanda, Antarctica. Bryologist 85:424–430
Kato K, Arikawa T, Imura S, Kanda H (2013) Molecular identification and phylogeny of an aquatic moss species in Antarctic lakes. Polar Biol 36:1557–1568
Klekowski EJ Jr (1969) Reproductive biology of the Pteridophyta. II. A study of the Blechnaceae. J Linn Soc Bot 62:361–377
Kurbatova LE, Andreev MP (2015) Bryophytes of the Larsemann Hills (Princess Elizabeth Land, Antarctica). Nov Sist Nizsh Rast 49:360–368
Li S, Ochyra R, Wu P, Seppelt R, Cai M, Wang H, Li C (2009) Drepanocladus longifolius (Amblystegiaceae), an addition to the moss flora of King George Island, South Shetland Islands, with a review of Antarctic benthic mosses. Polar Biol 32:1415–1425
Light JJ, Heywood RB (1975) Is the vegetation of continental Antarctica predominantly aquatic? Nature 256:199–200
Light JJ, Lewis Smith RI (1976) Deep-water bryophytes from the highest Scottish lochs. J Bryol 9:55–62
Lovalvo D, Clingenpeel SR, McGinnis S, Macur RE, Varley JD, Inskeep WP, Glime J, Nealson K, McDermott TR (2010) A geothermal-linked biological oasis in Yellowstone Lake, Yellowstone National Park, Wyoming. Geobiology 8:327–336
Mikucki J, Lyons BW, Hawes I, Lanoil BD, Doran PT (2010) Saline lakes and ponds in the McMurdo Dry Valleys: ecological analogs to Martian paleolake environments. In: Doran PT, Lyons WB, McKnight DM (eds) Life in Antarctic deserts and other cold dry environments: astrobiological analogs. Cambridge University Press, Cambridge, pp 160–194
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE). New Orleans, pp 1–8, 14 November 2010
Mommer L, Visser EJW (2005) Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Ann Bot 96:581–589
Nadot A, Bajon R, Lejeune B (1994) The chloroplast gene rps4 as a tool for the study of Poaceae phylogeny. Plant Syst Evol 191:27–38
Nishiyama T, Hiwatashi Y, Sakakibara K, Kato M, Hasebe M (2000) Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res 7:9–17
Ochyra R (1998) The moss flora of King George Island. Polish Academy of Sciences, W. Scafer Institue of Botany, Kracow
Ochyra R, Lewis-Smith RI, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Pedersen N, Russell SJ, Newton AE, Ansell SW (2006) A novel molecular protocol for the rapid extraction of DNA from bryophytes and the utility of direct amplification of DNA from a single dwarf male. Bryologist 109:257–264
Persson H (1942) Bryophytes from the bottom of some lakes in North Sweden. Bot Notiser Lunds Bot Foren 1942:308–324
Rambaut A (2009). FigTree v1.4.2. http://tree.bio.ed.ac.uk/software/figtree/
Richardson DHS (1981) The biology of mosses. Blackwell Scientific Publications, Oxford
Rousk K, Jones DL, DeLuca T (2013) Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front Microbiol 4:1–10
Savich-Lyubitskaya LI, Smironova ZN (1959) Novyy vid roda Bryum Hedw. Iz oazisa Bangera. Inf Byull Sov 7:34–39
Seppelt RD, Green TGA (1998) A bryophyte flora for Southern Victoria Land, Antarctica. New Zeal J Bot 36:617–635
Shaw AJ, Cox CJ, Goffinet B (2005) Global patterns of moss diversity: taxonomic and molecular inferences. Taxon 54:337–352
Skotnicki ML, Selkirk PM, Broady P, Adam KD, Ninham JA (2001) Dispersal of the moss Campylopus pyriformis on geothermal ground near the summits of Mount Erebus and Mount Melbourne, Victoria Land, Antarctica. Antarct Sci 13:280–285
Skotnicki ML, Selkirk PM, Boger SD (2012) New records of three moss species (Ptychostomum pseudotriquetrum, Schistidium antarctici and Coscinodon lawianus) from the southern Prince Charles Mountains, Mac.Robertson Land, Antartica. Polar Rec 48:394–400
Spiess LD, Lippincott B, Lippincott JA (1971) Development and gametophore initiation in the moss Pylaisiella selwynii as influenced by Agrobacterium tumefaciens. Am J Bot 58:726–731
Stamatakis A (2014) RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinform 10:1–2
Sun Y, Skinner DZ, Liang GH, Hulbert SH (1994) Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor Appl Genet 89:26–32
Tewari SD, Pant G (1996) Some moss collections from Dakshin Gangotri, Antarctica. Bryol Times 91:17
Vieira C, Sérgio C, Séneca A (2005) Threatened bryophytes occurrence in Portuguese stream habitat. Bol Soc Esp Briol 26–27:103–118
Vitt DH, Glime JM (1984) The structural adaptations of aquatic moss. Lindbergia 10:95–110
Wagner B, Seppelt R (2006) Deep-water occurrence of the moss Bryum pseudotriquetrum in Radok Lake, Amery Oasis, East Antarctica. Polar Biol 29:791–795
Watson W (1919) The bryophytes and lichens of fresh water. J Ecol 7:71–83
Wheeler TJ, Kececioglu JD (2007) Multiple alignments by aligning alignments. Bioinformatics 23:559–568
Zhang L, Jungblut AD, Hawes I, Andersen DT, Sumner SY, Mackey TJ (2015) Cyanobacterial diversity in benthic mats of the McMurdo Dry Valley lakes, Antarctica. Polar Biol 38:1097–1110
Zhang T, Zhang Y, Liu H, Wei Y, Li H, Su J, Zhao L, Yu L (2013) Diversity and cold adaptation of culturable endophytic fungi from bryophytes in the Fildes Region, King George Island, maritime Antarctica. FEMS Microbiol Lett 341:52–61
Acknowledgements
We thank Jeannine Marquardt for advising on phylogenetic analyses, Alex Aitken and Stephen Russell for their invaluable help in laboratory matters, Tomasz Goral for his assistance in SEM imaging, and Len Ellis for advice and help with locating specimens in the NHM bryophyte herbarium. Logistical support was provided to K081 by the New Zealand Antarctic Programme. We also thank three anonymous reviewers and the editor for their time and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rankin, A.H., Pressel, S., Duckett, J. et al. Characterisation of a deep-water moss from the perennially ice-covered Lake Vanda, Antarctica. Polar Biol 40, 2063–2076 (2017). https://doi.org/10.1007/s00300-017-2127-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2127-y