Abstract
The Antarctic marine environment is extreme in its low temperatures and short periods of primary productivity. Invertebrates must therefore adapt to maximise reproductive output where low temperature and limited food slow larval development. Brooding is a common reproductive trait in Antarctic marine bivalves; larval development occurs within the mantle cavity, and larvae are released as fully developed young. Lissarca miliaris is a small, short-lived, shallow-water brooding bivalve of circum-Antarctic distribution and found most abundant in the sub-Antarctic Magellan Region and islands of the Scotia Arc. Here, an unusual hermaphrodite reproductive trait is described for L. miliaris from King George Island (62°14′S, 58°38′W) and Signy Island (60°42′S, 45°36′W), Antarctica, using histological and dissection techniques. Specimens demonstrate simultaneous and sequential hermaphrodite traits; male and female gonads develop simultaneously, but the production of oocytes is reduced while testes are ripe. Functional females are more abundant in specimens above 3 mm shell length, although male reproductive tissue persists and functional males are found in all size classes. The number of previtellogenic oocytes produced by far exceeds the number of oocytes extruded and brooded, which may indicate an ancestral link to a planktotrophic past. Hermaphroditism in L. miliaris maximises reproductive efficiency in a short-lived species, in which the female’s capacity to brood its young is limited, and demonstrates a specialised adaptation to a cold stenothermal and food-limited environment prevailing in the Southern Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brooding is a common and successful reproductive trait in many Antarctic and deep-sea invertebrates representing an extreme of parental trade-offs between quantity and quality, with lowest mortality and lowest fecundity (Vance 1973; Ripley and Caswell 2008). In some invertebrate groups from the Antarctic, such as echinoids and bivalves, the predominance of brooding traits is striking (for review see Poulin et al. 2002; Thatje et al. 2005), but the suggestion that this has been driven by the current cold stenothermal and stable environmental constraints is often questioned (Strathmann 1978; Poulin et al. 2002; Pearse and Lockhart 2004; Thatje et al. 2005; Pearse et al. 2008). It has been discussed that the phyletic constraints on the reproduction and extinction of many planktotrophic traits during previous glacial periods have driven the evolution of entirely brooding clades of taxa (Strathmann 1978; Emlet et al. 1985; Gallardo and Penchaszadeh 2001; Thatje et al. 2003; Pearse and Lockhart 2004; Pearse et al. 2008). Southern Ocean brooding species may have become isolated in ice-free shelf refuges on multiple occasions during glacial periods, forming fragmented and isolated populations, preventing gene flow and facilitating speciation of brooding clades over time (Thatje et al. 2005, 2008; Pearse et al. 2008; Thatje 2012).
Reproduction in polar and deep-sea benthic invertebrates has always been a subject of contrasting opinions and controversy, but there are still few detailed descriptions on the reproductive ecology of many benthic species, especially in the Antarctic. There is a well-documented mismatch between the long developmental times of indirect planktotrophic larvae and the short period of primary production in Polar Regions, potentially selecting for non-feeding larval forms (Thorson 1950; Mileikovsky 1971). Vance (1973) stated that selection must favour the reproductive trait with the greatest efficiency. Lecithotrophy and direct development have higher investment per egg with lower fecundity compared with planktotrophy. This is balanced by lower dependence on external food sources and settlement sites (Strathmann 1974). Recent studies have challenged the early views on the dominance of direct development in the Antarctic after the description of species with successful planktotrophic larval development in the Southern Ocean (Arntz and Gili 2001; Poulin et al. 2002; Raupach et al. 2010; but see Thatje 2012) and the belief that the isolation of the Southern Ocean has driven evolution and endemism in many taxa (Arntz et al. 1997; Clarke and Johnston 2003; Pearse and Lockhart 2004; Thatje et al. 2005).
Philobryidae are a notably speciose and increasingly studied family of bivalves, typical of the Southern Ocean and often occurring in high abundances in shallow bays and the continental shelf (Tevesz 1977; Arntz et al. 1997; Linse 2004). A characteristic important to the success of this group is the ability to brood young to juvenile stages, facilitated by the reduced or absent anterior adductor muscle and strongly constructed modified filibranch ctenidia (Tevesz 1977; Morton 1978; Prezant et al. 1992; Brey and Hain 1992; Hain and Arnaud 1992; Higgs et al. 2009). The philobryid Lissarca miliaris (Philippi, 1845) is a small ubiquitous bivalve (<6 mm shell length) often found attached to macroalgae by byssal threads between the intertidal and 270 m (Dell 1964). With a life span of up to 6 years, it is relatively short-lived for a cold-water invertebrate and broods up to 70 young in clusters within the mantle cavity for 17–18 months (Richardson 1979). Populations are known to occur along the Western Antarctic Peninsula, Scotia Sea islands, and in the Ross Sea, as well as reaching into the sub-Antarctic Magellan Region, Falkland and Kerguelen Islands (Huber 2010, www.scarmarbin.be). They are most commonly found in large numbers and dense aggregations in shallow bays (<30 m). The brooding characteristics of L. miliaris were described by Richardson (1979) from a population at Signy Island, but no study has described reproduction from gametogenesis. Only few studies have comprehensively described reproduction in any Antarctic philobryid species (Morton 1978; Brey and Hain 1992; Higgs et al. 2009). Philobryids are normally considered to be dioecious (Morton 1978; Richardson 1979; Brey and Hain 1992; Higgs et al. 2009) although a small percentage of Lissarca notorcadensis within certain size classes possess both testes and ovaries, indicating protandric hermaphroditism in a small proportion of a population (Prezant et al. 1992). Studies on the reproductive ecology of benthic invertebrates from the Southern Ocean are important for our knowledge of reproductive trade-offs in Polar Regions. This study describes the reproductive biology in L. miliaris and discusses the reproductive trade-offs associated with hermaphroditic traits within the species.
Materials and methods
Sample collection
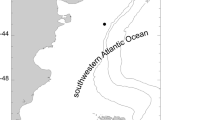
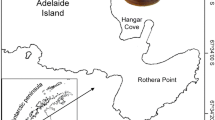
Specimens of L. miliaris were collected by divers at 10 m depth in Potter Cove, King George Island (Fig. 1a, c), Scotia Sea (62°14′S, 58°38′W) in December 1993 and January 1994 and fixed in 4 % buffered formalin. A total of 461 specimens were hand-collected from the intertidal of Shallow Bay, Signy Island (Fig. 1a, b), Scotia Sea (60°42′S, 45°36′W) in April 2002 and fixed in 96 % ethanol during the R/V Polarstern LAMPOS (ANT XIX/5) expedition (Arntz and Brey 2003).
Histology
Fifty-three individuals from King George Island (Potter Cove) and nineteen specimens from Signy Island (Shallow Bay) between 2.0 and 5.0 mm shell length were used for histological analysis. Maximum shell length, height and width were measured using a stereomicroscope. Whole animals were processed after decalcification (hydrochloric acid). Tissue was processed as described by Higgs et al. (2009) and embedded into wax blocks. Sections of 7 μm were mounted on slides and stained with haemotoxylin and counter-stained in eosin. Reproductive features were captured with a Nikon D5000 mounted on a stereomicroscope and analysed using SigmaScan Pro4 software; Feret diameters used to measure oocyte sizes.
Dissection
A total of 306 specimens from King George Island were selected for dissection. Bivalves were opened and the gonads examined. Separate sexes were easily identified in specimens above 2.5 mm shell length. The vitellogenic eggs of 90 females from 2.5 to 5.8 mm shell length were manually removed from the gonads and counted; 44 of these had oocytes photographed with a Leica Microsystem camera and Feret diameter calculated.
Sex ratios
Sex ratios were determined from specimens collected at King George Island. Histology was used to determine functional sex of specimens below 3 mm; males determined by mature ripe testis and females from the presence of previtellogenic oocytes and no testis. Above 3 mm shell length, specimens were dissected to observe functional sex. Hermaphrodite percentage in the population was determined by using histology data of all size classes and is observed as functional males with previtellogenic oocytes developing in a small portion of gonad.
Results
Lissarca miliaris demonstrate traits of both simultaneous and sequential hermaphroditism—sequential by a change in sex that can occur once within their lifetime and simultaneous by the occurrence of both male and female tissue present throughout the life of some specimens. Gametogenesis is observed from 2.00 mm shell length with oogenesis occurring in two small portions of gonad beside the posterior adductor muscle from 2.15 mm. Developing testis is observed from 2 mm as gonad extending from the posterior adductor muscle to the antero-dorsal half on both the left and right sides. Up to and over 200 previtellogenic oocytes develop in both males and females and vary in size between 19 and 50 μm (Figs. 2a, 5a). They appear densely packed or loosely arranged and often exceed the maximum number of eggs that become vitellogenic and brooded.
Lissarca miliaris. a, b King George Island January 1994, c, d Signy Island April 2002. a Section through the posterior part of the body of functional male (3.6 mm shell length) showing histology of previtellogenic oocytes in a small portion of gonad next to posterior adductor muscle; b transverse section through dorsal portion of gonad in a functional female (4.2 mm shell length) showing histology of vitellogenic oocytes; c transverse section of entire brooding female (4.2 mm shell length) showing histology of brooded ova in mantle cavity; d transverse section of mantle cavity of brooding female (3.1-mm shell length) showing histology section of advanced brooded veligers. pvo Previtellogenic oocyte, m muscle, vo vitellogenic oocyte, ct ctenidia, vel veliger. Wax sections, 7 μm thick, stained with haemotoxylin and eosin. Scales 100 μm
Lissarca miliaris, King George Island January 1994. a Micrograph image showing previtellogenic oocytes developing next to posterior adductor muscle and testis in functional male (4.2 mm shell length); b micrograph showing dorsal view of vitellogenic oocytes lined up in dorsal portion of gonad (4.9 mm shell length); c micrograph showing brooded vitellogenic oocytes in mantle cavity held in place by maternal tissue and thin chords (4.7 mm shell length); d micrograph of cluster of vitellogenic oocytes brooded in the mantle cavity (4.7 mm shell length). m Muscle, pvo previtellogenic oocytes, t testis, vo vitellogenic oocytes, ch thin chords, mt maternal tissue. Scales 1 mm
Vitellogenesis occurs from 2.9 mm length; <35 oocytes from the site of oogenesis develop on each side in dorsal parts of gonad extending from the posterior adductor muscle to the anterior-dorsal half (Fig. 2b). Oocytes are lined up in tightly packed rows in the dorsal parts of the gonad and measure between 200 and 500 μm Feret diameter at King George Island (mean = 368 μm, SD = 44, n = 396) depending on degree of vitellogenic accumulation (Fig. 3b). The observed number of eggs per female increased with shell length from 2.9 mm (r 2 = 0.451, F 1,89 = 72.34, p < 0.001, King George Island) but also showed large variation within size groups (Fig. 4). Between 15 and 69 eggs were found in females of 2.9 to 5.0 mm shell length. Fully developed but unfertilised eggs are extruded into the mantle cavity and are initially held together in two clusters on each side (Fig. 2c) by an unidentified maternal tissue (Fig. 3c, d). This tissue breaks down, probably after fertilisation but the clusters remain loosely held together by thin chords resembling thin strands of byssus (Fig. 3c). Embryos develop larval shells and the large prodissoconch (Signy Island mean length = 699 μm, SD = 33, n = 47) often displays a distinct growth disturbance mark. Fully developed but pre-release shelled veliger was only found in specimens from Signy Island and were loose in the mantle cavity (Fig. 2d) with a mean shell length of 0.715 mm (SD = 0.04, n = 13). The smallest released shelled young from King George Island had a shell length of 0.775 mm.
Hermaphroditism is most commonly observed as functional males with ripe testis and previtellogenic oocytes developing in the small portions of gonad beside the posterior adductor muscle (Figs. 3a, 5a–d). In functional females, hermaphroditism is observed above 2.9 mm shell length with broods or large vitellogenic oocytes and reduced testis at the edges of the dorsal portion of the gonad (Fig. 5e). Sex ratio data from specimens at King George Island demonstrates an increase in functional females from 2.5 mm and a corresponding decrease in male abundance (Fig. 6). The proportion of functional males with previtellogenic oocytes increases in stages from 15 to 17 % between 2 and 2.99 mm, 33–37 % between 3 and 3.99 mm and 83–100 % between 4 and 4.99 mm. The changing ratios, supported by evidence of hermaphrodite stages, suggest that the dominant sex of the species changes with age but both male and female portions of gonad persist. Testis appears to be the dominant functional gonad up to 3 mm shell length, but previtellogenic oocytes are also developing simultaneously. During vitellogenesis, the testis becomes inactive and reduced while the oocytes develop in ovaries that form dorsally, in the position that testis had previously occupied. Brooding females with partly shelled veligers were found to have large (250–500 μm) vitellogenic oocytes developing in the dorsal parts of the gonad, and there is no evidence that suggests the male portions of gonad become active at larger sizes once reduced. No differences in reproductive trait were observed between the two populations studied, and despite the temporal difference between collections, no differences in the hermaphrodite stage were observed between sites.
Lissarca miliaris, Signy Island April 2002. a, b Transverse section through posterior part of body in functional male (3.5 mm shell length) showing; a previtellogenic oocytes developing in two areas of gonad beside the posterior adductor muscle; b previtellogenic oocytes developing beside the posterior adductor muscle next to mature testis. c, d Two dorsal–ventral sections from same specimen (3.1 mm shell length) demonstrating (c) testis reduced in size and low density in spent male (d) oocytes from portion of gonad by posterior adductor muscle spreading into portion of gonad the testis occupies; e Dorsal–ventral section showing functional female (4.1 mm shell length), which contained shelled brood (not seen) with vitellogenic oocytes and reduced testis. m Muscle, pvo previtellogenic oocytes, t testis, vo vitellogenic oocytes. Wax sections, 7 μm thick, stained with haemotoxylin and eosin. Scales 100 μm unless stated
Discussion
Brooding and gametogenesis
The Antarctic shallow-water environments are dynamic and diverse habitats, disturbed by ice scouring, cold temperatures and high concentrations of short-lived primary production (Gutt 2001; Thatje et al. 2005; Barnes and Conlan 2007). In such limiting conditions, a diverse range of benthic fauna have evolved traits that not only increase the survival of young but also maximise reproductive output. The brooding traits of L. miliaris are not unusual for philobryids although there are distinct differences to the described traits of L. notorcadensis (Brey and Hain 1992) and Adacnarca nitens (Higgs et al. 2009). L. miliaris are typically abundant in shallow water, commonly in high abundances in the intertidal and sub-tidal shallow bays in the sub-Antarctic (Dell 1964; Richardson 1979), whereas the previously studied L. notorcadensis were found between 80 and 1,108 m in the Weddell Sea and A. nitens at 84 m in the Ross Sea (Brey and Hain 1992; Higgs et al. 2009). Different environmental constraints associated with these depths and latitudes may influence reproductive cycles, investment of energy into offspring, and brooding characteristics.
Despite a smaller shell size, L. miliaris have larger broods than L. notorcadensis, but this is balanced by smaller young—L. miliaris producing young up to 0.77 mm shell length compared with 1.25 mm in L. notorcadensis (Table 1). Both species have seasonal reproduction, but L. miliaris have a shorter 18-month reproductive cycle compared with the 24-month cycle of L. notorcadensis (Richardson 1979; Brey and Hain 1992). In contrast, A. nitens are asynchronous producing few eggs at a time in small numbers continuously through the year (Higgs et al. 2009). Availability of food is the single most likely factor explaining these differences with L. miliaris in this study being exposed to the high concentrations of phytoplankton during the summer months at Signy Island (Clarke et al. 1988) and Potter Cove, King George Island (Klöser et al. 1994; Schloss et al. 2002; Tatian et al. 2002) while their deeper relatives may only be exposed to sinking phytodetritus or low concentrations of phytoplankton during blooms.
Egg size of L. miliaris are similar to L. notorcadensis and A. nitens, developing up to 500 μm and as described in A. nitens, the eggs appear to have a high yolk content, demonstrating a high investment of energy into each egg (Higgs et al. 2009). It is not clear how fertilisation occurs although it is likely that the unidentified maternal tissue (Fig. 3c, d) may play a role in holding the ova in position or in fertilisation.
A previously unknown trait observed in L. miliaris is the large number of previtellogenic oocytes that develop in a small portion of gonad beside the posterior adductor muscle which far exceed the number of young an individual can brood, perhaps in the lifetime of the animal. Brooding traits are considered to have evolved from more primitive planktotrophic traits as a consequence to environmental conditions and once lost, these traits are not likely to be reacquired (Strathmann 1985). These previtellogenic eggs are akin in size (19–50 μm), number (200+) and arrangement to planktotrophic species and may be indicative of a planktotrophic past of an ancestor species.
Hermaphroditism
Hermaphroditism is not a rare reproductive trait in Southern Ocean molluscs and has been described in the bivalves Laternula elliptica (Bosch and Pearse 1988), Thracia meridonalis (Sartori and Domaneschi 2005) and the brooding bivalves Mysella charcoti and M. narchii (Passos and Domaneschi 2009), all demonstrating simultaneous hermaphroditism. Sequential protandric hermaphroditim is also common in molluscs where an organism functions initially as a male and then female at a later stage (Heller 1993) and is potentially advantageous for small brooding species, which are limited by space available to brood their young (Ghiselin 1969; Heath 1977; Wright 1988; Heller 1993). The occurrence of different types of hermaphrodite traits within populations is known to persist in species of Crassostrea (Heller 1993). This group of oysters is mostly single sex but also demonstrates protandric and simultaneous hermaphrodite traits, the cues of which are genetically or environmentally driven (Coe 1943; Guo et al. 1998; Ruiz-Verdugo et al. 2000).
Sequential protandric hermaphroditism was described in L. notorcadensis when a 5 % of a population from the Scotia Sea was found to possess both testes and ovaries while the proportion of males was highest in the smallest size classes (Prezant et al. 1992). Hermaphroditism in L. miliaris is different, however, as it demonstrates both simultaneous and sequential traits. The long retention of male gonads in mature females and development of primary oocytes in mature males are evidence of simultaneous gonad development, but the reduction of one of the gonads is indicative of sequential hermaphroditism. Sex-ratio data in this study support the change of sex from male to female and the proportion of hermaphrodites in the entire population is 30 %, higher than the 5 % observed in L. notorcadensis (Prezant et al. 1992). The higher percentage of females in the 2.00–2.49 mm size class may relate to the difficulty of determining immature testis from other tissue types and the development of previtellogenic oocytes may occur before the formation of determinable testis.
The occurrence of reduced testes in brooding L. miliaris could also be evidence of the rarer alternating sex hermaphrodite trait where specimens change sex more than once in their lifetime. This is commonly found in temperate and tropical Ostrea spp., which change sex seasonally (Asif 1979; Heller 1993) and is supported in this study by the observation of reduced testis in late brooding females, indicating a possible male reproductive stage whilst brooding young. Reduced testis at this stage of reproduction is curious as its position is in the portion of gonad where vitellogenesis of oocytes occurs. Figure 5e, which shows the undeveloped testis in the dorsal portion of gonad and vitellogenic oocytes, may be in a transitional stage where the oocytes are being extruded into the mantle cavity and the testes are re-developing; however, only regular sampling of L. miliaris would confirm this reproductive stage. Alternating sexuality would enable females to allocate energy to male gonad production and remain sexually active during long periods of brooding. In a short-lived species with a long period between gametogenesis and larval release, alternating sexuality would maximise the reproductive output of L. miliaris that may otherwise only reproduce up to 3 times within their life span. Ripe testis has not, however, been observed in a brooding L. miliaris, and larger brooding females have been observed with small vitellogenic oocytes. It is not possible to accurately determine the percentage of functional females with testis in L. miliaris as reduced testes are not easily distinguished from surrounding tissue.
Lissarca miliaris may have evolved hermaphrodite brooding traits in response to a number of different constraints including small adult size and isolation. Small size is commonly associated with brooding as larger animals can often produce more young than they can brood (Strathmann and Strathmann 1982; Strathmann et al. 1984). In the Antarctic context, brooding is likely to be so high due to speciation within brooding clades and the possible extinction of non-brooding species during glacial periods (for discussion see Poulin et al. 2002; Thatje et al. 2005). Hermaphroditism can also be linked to small sizes, however, as the female’s limited capacity to brood may select for sequential protandric hermaphroditism (Ghiselin 1969; Heath 1977; Strathmann and Strathmann 1982; Wright 1988) and the retention of unused gonad may represent an intermediate stage of development. Isolation and small population size may also select for hermaphrodite traits as this may increase the chances of successful reproduction (Ghiselin 1969; Heller 1993).
Evolutionary consequences
The limited capability for dispersal in brooding species can be overcome by rafting, and the unique oceanographic conditions offered by the Antarctic circumpolar current allow for connectivity between separated island groups (Highsmith 1985; Martel and Chia 1991; Smith 2002; Nikula et al. 2010). It has to be recognised, however, that genetic diversity in brooders appears high, frequently resulting in cryptic speciation and species clusters of circum-Antarctic distribution (Held 2003; Held and Wagele 2005; Cope and Linse 2006; Linse et al. 2007; Hunter and Halanych 2008; Leese et al. 2008; for discussion see Thatje 2012). The distribution of L. miliaris in the Scotia Sea is likely to be the consequence of dispersal via macroalgal rafting (Highsmith 1985; Higgs et al. 2009) or juvenile byssus drifting (Sigurdsson et al. 1976; Beaumont and Barnes 1992). Hermaphroditism may have facilitated founding populations and population expansion following habitat contraction during glacial periods (Thatje et al. 2008, Thatje 2012) as small populations in ice-free refugia would be likely to have both sexes for reproduction. Future molecular studies into the origins of L. miliaris may provide evidence of the Scotia Sea Islands acting as stepping-stones into the Antarctic from the Magellan region during recolonisation events (Arntz 1999).
Lissarca miliaris demonstrates a specialised reproductive trait in the Southern Ocean and provides more evidence to the hypothesis that brooding in small bivalves may provide an evolutionary advantage over other Antarctic fauna (Dell 1972; Poulin et al. 2002; Thatje et al. 2005, 2008; Higgs et al. 2009). The occurrence of hermaphroditism in this species is a further adaptation that has enabled the species to maximise their reproductive output and perhaps to colonise islands on both sides of the polar front. Philobryid bivalves represent many species of small benthic Antarctic fauna by demonstrating common traits (small size, brooding, and variable Antarctic/sub-Antarctic distributions), and this study highlights the need for further investigation into brooding traits and the processes that may have driven unique characteristics. Lissarca spp. also make a good model species for future studies of population genetics, potentially revealing in detail the origins and radiation processes of the philobryid bivalves and increasing our understanding on the processes that have driven the evolution of Antarctic benthic diversity.
Summary
Lissarca miliaris show an unusual reproductive trait that may function to maximise reproduction in a cold stenothermal and food-limited environment and are distinct from other Antarctic bivalves by;
-
A transitional hermaphrodite phase in L. miliaris from male to female occurring throughout every size class, and functional males are found in the largest size class.
-
Male reproductive tissue persisting long after changing sex and observed in late brooding females, 18–24 months after the change in sex.
-
The production of a large number of previtellogenic oocytes in functional males, where the number of oocytes observed exceeds the number of young an individual is likely to brood in a lifetime.
References
Aldea C, Olabarria C, Troncoso JS (2008) Bathymetric zonation and diversity gradient of gastropods and bivalves in West Antarctica from the South Shetland Islands to the Bellingshausen Sea. Deep Sea Res I 55:350–368
Arntz WE (1999) Magellan-Antarctic: ecosystems that drifted apart. Summary review. Sci Mar 63:503–511
Arntz WE, Brey T (2003) The expedition ANTARKTIS XIX/5 (LAMPOS) of RV Polarstern in 2002. Rep Polar Mar Res 462:1–120
Arntz WE, Gili JM (2001) A case for tolerance in marine ecology: let us not put out the baby with the bathwater. Sci Mar 65:283–299
Arntz WE, Gutt J, Klages M (1997) Antarctic marine biodiversity: an overview. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 3–14
Asif M (1979) Hermaphroditism and sex reversal in the four common oviparous species of oysters from the coast of Karachi. Hydrobiologia 66:49–55
Barnes DKA, Conlan KE (2007) Disturbance, colonization and development of Antarctic benthic communities. Phil Trans R Soc B Biol Sci 362:11–38
Beaumont AR, Barnes DA (1992) Aspects of veliger larval growth and byssus drifting of the spat of Pectin maximus and Aequipectin (Chlamys) opercularis. J Mar Sci 49:417–423
Bosch I, Pearse JS (1988) Seasonal pelagic development and juvenile recruitment of the bivalve Laternula elliptica in McMurdo Sound, Antarctica. Am Zool 28:A89
Brey T, Hain S (1992) Growth, reproduction and production of Lissarca notorcadensis (Bivalvia: Philobryidae) in the Weddell Sea, Antarctica. Mar Ecol Prog Ser 82:219–226
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Oceanogr Mar Biol 41:47–114
Clarke A, Holmes LJ, White MG (1988) The annual cycle of temperature, chlorophyll and major nutrients at Signy Island, South Orkney Islands, 1969–82. Br Antarct Surv Bull 80:65–86
Coe WR (1943) Sexual differentiation in mollusks. I. Pelecypods. Q Rev Biol 18:154–164
Cope T, Linse K (2006) Morphological differences in Lissarca notorcadensis Melvill and Standen, 1907 from the Scotia, Weddell and Ross Seas. Deep Sea Res II 53:903–911
Dell RK (1964) Antarctic and sub-Antarctic Mollusca: Amphineura, Scaphapoda and Bivalvia. Discov Rep 33:93–250
Dell RK (1972) Antarctic benthos. In: Russell FS, Yonge M (eds) Advances in marine biology, vol X. Academic, London, pp 1–216
Emlet RB, McEdward LR, Strathmann RR (1985) Echinoderm larval ecology viewed from the egg. In: Lawrence J (ed) Echinoderm studies, vol 1. Balkema, Rotterdam, pp 55–136
Gallardo CS, Penchaszadeh PE (2001) Hatching mode and latitude in marine gastropods: revisiting Thorson’s paradigm in the southern hemisphere. Mar Biol 138:547–552
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Guo XM, Hedgecock D, Hershberger WK, Cooper K, Allen SK (1998) Genetic determinants of protandric sex in the Pacific oyster, Crassostrea gigas. Evolution 52:394–402
Gutt J (2001) On the direct impact of ice on marine benthic communities, a review. Polar Biol 24:553–564
Hain S, Arnaud PM (1992) Notes on the reproduction of high-Antarctic molluscs from the Weddell Sea. Polar Biol 12:303–312
Heath DJ (1977) Simultaneous hermaphroditism; cost and benefit. J Theor Biol 64:363–373
Held C (2003) Molecular evidence for cryptic speciation within the widespread Antarctic crustacean Ceratoserolis trilobitoides (Crustacea, Isopoda). In: Huiskes AH, Gieskes WW, Rozema J, Schorno RM, van der Vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys, Leiden, pp 135–139
Held C, Wagele JW (2005) Cryptic speciation in the giant Antarctic isopod Glyptonotus antarcticus (Isopoda: Valvifera: Chaetiliidae). Sci Mar 69:175–181
Heller J (1993) Hermaphroditism in molluscs. Biol J Linn Soc 48:19–42
Higgs ND, Reed AJ, Hooke R, Honey DJ, Heilmayer O, Thatje S (2009) Growth and reproduction in the Antarctic brooding bivalve Adacnarca nitens (Philobryidae) from the Ross Sea. Mar Biol 156:1073–1081
Highsmith RC (1985) Floating and algal rafting as potential dispersal mechanisms in brooding invertebrates. Mar Ecol Prog Ser 25:169–179
Huber M (2010) Lissarca miliaris (Philippi, 1845). In: De Broyer C, Clarke A, Koubbi P, Pakhomov E, Scott F, Vanden Berghe E, Danis B (eds) The SCAR-MarBIN register of Antarctic marine species (RAMS). http://www.scarmarbin.be/rams.php?p=taxdetails&id=197240 on 2011-11-10
Hunter RL, Halanych KM (2008) Evaluating connectivity in the brooding brittle star Astrotoma agassizii across the Drake Passage in the Southern Ocean. J Hered 99:137–148
Klöser H, Ferreyra G, Schloss I, Mercuri G, Laturnus F, Curtosi A (1994) Hydrography of Potter Cove, a small fjord-like inlet on King George Island (South Shetland). Estuar Coast Shelf Sci 38:523–537
Leese F, Kop A, Wagele JW, Held C (2008) Cryptic speciation in a benthic isopod from Patagonian and Falkland Island waters and the impact of glaciations on its population structure. Front Zool 5:1–15
Linse K (2004) Scotia Arc deep-water bivalves: composition, distribution and relationship to the Antarctic shelf fauna. Deep Sea Res II 51:1827–1837
Linse K, Cope T, Lörz A-N, Sands CJ (2007) Is the Scotia Sea a center of Antarctic marine diversification? Some evidence of cryptic speciation in the circum-Antarctic bivalve Lissarca notorcadensis (Arcoidea: Philobryidae). Polar Biol 30:1059–1068
Martel A, Chia FS (1991) Drifting and dispersal of small bivalves and gastropods with direct development. J Exp Mar Biol Ecol 150:131–147
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10:193–213
Morton B (1978) Biology and functional-morphology of Philobrya munita (Bivalvia Philobryidae). J Zool 185:173–196
Nikula R, Fraser CI, Spencer HG, Waters JM (2010) Circumpolar dispersal by rafting in two subantarctic kelp-dwelling crustaceans. Mar Ecol Prog Ser 405:221–230
Passos FD, Domaneschi O (2009) The anatomical characters related to the brooding behavior of two Antarctic species of Mysella Angas, 1877 (Bivalvia, Galeommatoidea, Lasaeidae), with direct and indirect evidences of ovoviviparity. Polar Biol 32:271–280
Pearse JS, Lockhart SJ (2004) Reproduction in cold water: paradigm changes in the 20th century and a role for cidaroid sea urchins. Deep Sea Res II 51:1533–1549
Pearse JS, Mooi R, Lockhart SJ, Brandt A (2008) Brooding and species diversity in the Southern Ocean: selection for brooders or speciation within brooding clades? In: Krupnik I, Lang MA, Miller SE (eds) Smithsonian at the poles: contributions to international polar year science. Proceedings of the Smithsonian at the Poles Symposium, Smithsonian Institution Scholarly Press, Washington, DC, pp 181–196
Poulin E, Palma AT, Feral JP (2002) Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol Evol 17:218–222
Prezant RS, Showers M, Winstead RL, Cleveland C (1992) Reproductive ecology of the Antarctic bivalve Lissarca notorcadensis (Philobryidae). Am Malacol Bull 9:173–186
Raupach MJ, Thatje S, Dambach J, Rehm P, Misof B, Leese F (2010) Genetic homogeneity and circum-Antarctic distribution of two benthic shrimp species of the Southern Ocean, Chorismus antarcticus and Nematocarcinus lanceopes. Mar Biol 157:1783–1797
Richardson MG (1979) The ecology and reproduction of the brooding Antarctic bivalve Lissarca miliaris. Brit Antarct Surv B 49:91–115
Ripley BJ, Caswell H (2008) Contributions of growth, stasis, and reproduction to fitness in brooding and broadcast spawning marine bivalves. Popul Ecol 50:207–214
Ruiz-Verdugo CA, Ramirez JL, Allen SK, Ibarra AM (2000) Triploid catarina scallop (Argopecten ventricosus Sowerby II, 1842): growth, gametogenesis, and suppression of functional hermaphroditism. Aquaculture 186:13–32
Sartori AF, Domaneschi O (2005) The functional morphology of the Antarctic bivalve Thracia meridionalis Smith, 1885 (Anomalodesmata: Thraciidae). J Mollusc Stud 71:199–210
Schloss IR, Ferreyra GA, Ruiz-Pino D (2002) Phytoplankton biomass in Antarctic shelf zones: a conceptual model based on Potter Cove, King George Island. J Mar Syst 36:129–143
Sigurdsson JB, Titman CW, Davies PA (1976) The dispersal of young post-larval bivalve molluscs by byssus threads. Nature 262:386–387
Smith SDA (2002) Kelp rafts in the Southern Ocean. Glob Ecol Biogeogr 11:67–69
Strathmann RR (1974) Spread of sibling larvae of sedentary marine invertebrates. Am Nat 108:29–44
Strathmann RR (1978) The evolution and loss of feeding larval stages of marine invertebrates. Evolution 32:894–906
Strathmann RR (1985) Feeding and nonfeeding larval development and life-history evolution in marine invertebrates. Ann Rev Ecol Syst 16:339–361
Strathmann RR, Strathmann MF (1982) The relationship between adult size and brooding in marine-invertebrates. Am Nat 119:91–101
Strathmann RR, Strathmann MF, Emson RH (1984) Does limited brood capacity link adult size, brooding, and simultaneous hermaphroditism? A test with the starfish Asterina phylactica. Am Nat 123:796–818
Tatian M, Sahade R, Kowalke J, Kivatinitz SC, Esnal GB (2002) Food availability and gut contents in the ascidian Cnemidocarpa verrucosa at Potter Cove, Antarctica. Polar Biol 25:58–64
Tevesz MJS (1977) Taxonomy and ecology of the Philobryidae and Limopsidae (Molusca: Pelecypoda). Postilla 171:1–64
Thatje S (2012) Effects of capability for dispersal on the evolution of diversity in Antarctic benthos. Integr Comp Biol. doi:10.1093/icb/ics/ics105
Thatje S, Schnack-Schiel S, Arntz WE (2003) Developmental trade-offs in subantarctic meroplankton communities and the enigma of low decapod diversity in high southern latitudes. Mar Ecol Prog Ser 260:195–207
Thatje S, Hillenbrand CD, Larter R (2005) On the origin of Antarctic marine benthic community structure. Trends Ecol Evol 20:534–540
Thatje S, Hillenbrand CD, Mackensen A, Larter R (2008) Life hung by a thread: endurance of Antarctic fauna in glacial periods. Ecology 89:682–692
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45
Vance RR (1973) Reproductive strategies in marine benthic invertebrates. Am Nat 107:339–352
Wright WG (1988) Sex change in the mollusca. Trends Ecol Evol 3:137–140
Acknowledgments
Adam J. Reed was supported through an NERC PhD studentship. Thanks are due to captain and crew of R/V Polarstern for assistance at sea, Prof. Paul Tyler for helpful discussion and interpretation of the results and Huw Griffiths for help with maps. The authors would also like to thank the three anonymous reviewers for helping to improve this manuscript. This study is part of the British Antarctic Survey Polar Science for Planet Earth Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reed, A.J., Thatje, S. & Linse, K. An unusual hermaphrodite reproductive trait in the Antarctic brooding bivalve Lissarca miliaris (Philobryidae) from the Scotia Sea, Southern Ocean. Polar Biol 36, 1–11 (2013). https://doi.org/10.1007/s00300-012-1233-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1233-0