Abstract
Polychaetes in the Southern Ocean are often thought to have wide distribution ranges on a horizontal and vertical scale. Here, this theory is tested for specimens commonly identified as the widely distributed glycerid Glycera kerguelensis using two molecular markers, the mitochondrial cytochrome oxidase c subunit I (COI) and the nuclear 28S rDNA. Identical morphospecies of three “populations” from three different habitats and two depth zones (abyssal plain 5,300 m, continental slope 2,000 m, sea mountain plateau 2,000 m) are compared. High genetic distances suggest the existence of three clades representing distinct species, identifying the investigated specimens as a complex comprising cryptic species with vertically restricted distribution. Two clades were found in sympatry on the Atka Bay slope in 2,000 m depth, one of these also found in similar depth on the plateau of the sea mountain Maud Rise. The third clade was limited to the abyssal plains (5,300 m) indicating the strong role of depth in the distribution of clades, possibly in conjunction with prevailing current systems. Evolution of the different clades is suggested to have resulted from a single emergence event with the origin of clades lying in the abyss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biodiversity and zoogeography of Southern Ocean (SO) benthic taxa has been a focus of polar marine biology for several years now. Summarizing the general picture, the shelf as well as the deep-sea benthos is species rich when compared to Arctic waters (Brandt et al. 2007a). A high degree in endemism is observed in taxa which have been focus of taxonomic attention, e.g., peracarids, molluscs, pycnogonids, and echinoderms (Brandt et al. 2007b; Munilla and Soler Membrives 2008). Concerning the evolution of species, the oceanographic isolation of the SO and its unique environment are believed to enhance species radiation and endemism (Hunter and Halanych 2008). Also, speciation is often discussed in context of the recurrent large-scale glaciation events on Milankovitch timescales, during which species went extinct or took refuge in ice-free shelf regions or the deep sea (submergence) (Thatje et al. 2005, 2008; Rogers 2007; Wilson et al. 2009). Nevertheless, an observation often made by taxonomists of all taxa is the occurrence of circum-Antarctic and eurybathic distribution patterns for many species (Brey et al. 1996; Arntz et al. 2005; Soler i Membrives et al. 2009) explained by re-invasion of the shelf from one or very few refugia and deeper waters during interglacial phases, the occurrence of strong current systems facilitating gene flow, and a lack of distribution barriers (e.g., a continuous shelf and no thermocline on a vertical scale). For the deep sea, as for the shelf, it seems that the SO works as a biodiversity pump (Clarke and Crame 1989; Ellingsen et al. 2007 and references cited therein), and species are believed to be able to invade more northward deep-sea basins via the Antarctic bottom water (e.g., Schüller and Ebbe 2007; Strugnell et al. 2008). However, first molecular studies on several benthic taxa from the SO shelf have already shown that we find distinct genetic patterns challenging the observation of wide distribution ranges of species and the idea of re-invasion of the Antarctic shelf from single refugia. Many populations seem to be not only geographically but also reproductively isolated from each other despite the lack of clear morphological differences in specimens (Held 2003; Held and Wägele 2005; Linse et al. 2007; Hunter and Halanych 2008; Krabbe et al. 2009) giving rise to the idea that cryptic speciation and smaller realized distribution ranges than originally believed might be a common phenomenon in SO shelf species. Also, current estimates on SO biodiversity and species distribution based purely on morpho-taxonomic studies have to be re-evaluated, given that SO biodiversity might be largely underestimated while the distribution ranges of some species in a horizontal and vertical scale might be overestimated. A thoughtful and efficient collaboration of taxonomists and molecular biologists is necessary to shed more light on these questions (Sands et al. 2008). However, the requirements for phylogeographic studies are rarely met in the SO as suitable sample sizes (numerous specimens of one species) and spatial sampling schemes are limited. Especially for the deep sea, sample sizes are small and the preservation of specimens for molecular studies has proven difficult. As a consequence, very few phylogeographic studies for SO deep-sea species are available to date and most shelf studies concentrated on isopods, molluscs, echinoderms, fishes, and recently pycnogonids (Held 2000, 2003; Held and Wägele 2005; Janko et al. 2007; Linse et al. 2007; Mahon et al. 2008; Wilson et al. 2007, 2009; Krabbe et al. 2009). Despite their high abundance and species richness in the macrobenthos, no phylogeographic studies have been done for polychaetes in the SO. However, they present a particularly interesting case for such studies given that their life history traits are very variable (brooders to free spawners) (Wilson 1991; Rouse and Pleijel 2006), and especially many deep-sea species are reported to have wide horizontal and/or vertical distribution ranges (Schüller and Ebbe 2007; Paterson et al. 2009).
This presented study is the first molecular analysis on specimens of a presumed eurybathic species with wide distribution ranges in the Southern Ocean (Glycera kerguelensis McIntosh 1885). Specimens of G. kerguelensis are commonly reported in high abundances in samples from large depth ranges in the SO (e.g., McIntosh 1885; Hartman 1964, 1967, 1978; Hartmann-Schröder and Rosenfeldt 1988, 1990, 1992). Therefore, the species represents a suitable model for phylogeographic studies. Several markers such as mitochondrial genes (e.g., cox3-trnQ-nad6, 16S or COI), RAPD-PCR or AFLPs have proven to be useful tools to distinguish species in polychaetes where morphological characters fail (e.g., von Soosten et al. 1998; Bleidorn et al. 2006; Barroso et al. 2010). In the past decade, studies on the molecular taxonomy and phylogeny of polychaetes with wide distribution ranges have become frequent. For a wide variety of polychaetes species, it is shown that distribution ranges are smaller than former morphological studies suggest and that cryptic speciation is often closely linked to geographic distances (von Soosten et al. 1998; Schmidt and Westheide 1999, 2000; Bleidorn et al. 2006; Wiklund et al. 2009; Barroso et al. 2010). But also sympatric occurrence of different genetic lineages of the same morphospecies without hybridization are reported, as e.g., for Hediste diversicolor (O.F. Müller, 1776) in the Baltic Sea (Audzijonyte et al. 2008). However, studies mainly concentrate on geographically separated populations from temperate shallow waters. This study presents the first analysis on SO polychaetes. The studied specimens originate from samples of different depth zones (bathyal and abyssal) and topological features (continental slope, sea mountain plateau, and abyssal plain) in the Eastern Weddell Sea thus representing samples from three different ecological realms on a relatively small spatial scale (<500 Nm). Under consideration of the reported distribution of G. kerguelensis and the finding of numerous cryptic species in other benthic taxa, morphological and molecular methods are used to question the identity of the species and to investigate if the gene flow between populations is continuous between the sampled regions or if yet invisible barriers such as water masses, physiological requirements in different depths, or environmental differences (e.g., sediment, food availability) work to separate already different species.
Materials and methods
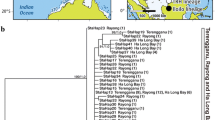
All taxonomic studies conducted during this project were done at the Ruhr-University in Bochum. The molecular analysis was carried out at the Canadian Centre of Biodiversity at the University of Guelph and is embedded in the iBOL (international Barcode of Life), CAML (Census of the Antarctic Marine Life) and CeDAMar (Census of the Diversity of Abyssal Marine Life) programs. Specimens of G. cf kerguelensis were collected at three different stations of the expedition ANDEEP-SYSTCO (ANT XXIV-2 with RV Polarstern) to the Atlantic sector of the SO in 2007/08 (Table 1, Fig. 1). The stations were positioned on the Atka Bay continental slope (station 17, ~2,100 m), on the Weddell abyssal plain (station 33, ~5,337 m), and on the plateau of Maud Rise (station 39, ~2,150 m), a seamount in the far eastern sector of the Weddell Sea. Samples were taken with an epibenthic sledge (EBS) or an Agassiz-Trawl (AGT) and immediately fixed in 98% pre-cooled ethanol after retrieval. The EBS was constructed of two nets (epi- and supranet, mesh size: 500 μm) with an opening of 1 × 0.5 m and a cot-end of 300-μm mesh size. The deployed AGT was equipped with an inlay-net of 500-μm mesh size. Effective trawling times (Table 1) resulted from about 10 min of active trawling with a ship speed of 1 kn over ground and subsequent heaving with a winch speed of 0.5 m/s until the gear left the bottom (ship speed: 0 kn over ground). Sorting of specimens was carried out at the earliest after 48 h and samples were subsequently stored in 98% ethanol at 1–4°C until further use. Specimens of G. cf kerguelensis were closely analyzed for their taxonomic characters. Identification took place under a dissecting microscope. Additionally, permanent slides (after Platt and Warwick 1988) of the parapodia, ailerons, jaws, and pharyngeal papillae were made for observation under higher magnification of a light microscope. Aileron base shapes were hereby only documented and taken into account when they could be observed at approximately the same angle in the slides (Table 2).
DNA was extracted from posterior segments of all 38 specimens via CTAB method. The anterior parts are kept in single vials as voucher and will be stored at the Zoological Museum (University of Hamburg) in the long term together with the permanent slides (Table 2). The specimens of Gk46 were destroyed during analyses. For PCR of mitochondrial cytochrome oxidase subunit I, the primers LCO 1490 [5′-GGTCAACAAATCATAAAGATATTGG-3′] and HCO 2198 [5′-TAAACTTCAGGGTGACCAAAAAATCA-3′] (Folmer et al. 1994) tailed with M13 [M13-tailF: 5′-TGTAAAAGGACGGCCAGT-3′; M13-tailR: 5′-CAGGAAACAGCTATGAC-3′] were used. When first PCR failed (Gk12, Gk14, Gk20, Gk43, Gk45, Gk55), modified versions of these (PolyLCO [5′-GAYTATWTTCAACAAATCATAAAGATATTGG-3′] and PolyHCO [5′-TAMACTTCWGGGTGACCAAARAATCA-3′] (Carr and Steinke pers. comm.)) were used. For 12.5 μl reactions, 2 μl template DNA was mixed with 0.06 μl (=0.5 U) Platinum-taq (Invitrogen), 6.25 μl trehalose (10%), 1.25 μl 10× PCR buffer, 0.625 μl Mg++ (50 mM), and 0.125 μl of each primer 10 μM), 0.0625 μl dNTPs (10 mM), and 2 μl water (ddH2O). The PCR temperature profile was: initial denaturation at 94°C, 1 min; 5 cycles of denaturation at 94°C, 30 s; annealing at 45–50°C, 40 s; elongation at 72°C, 60 s, 30–35 cycles of denaturation at 94°C, 30 s; annealing at 51–54°C, 40 s; elongation at 72°C, 60 s; final elongation at 72°C for 10 min. Bidirectional sequencing was carried out with M13F and R primers or the PolyLCO and PolyHCO primers (see above) on a 3730×l DNA Analyzer (Applied Biosystems) automated sequencer. All protocols and further details on extraction, PCR, and sequencing are accessible via the homepage of the Barcode of Life (www.barcodinglife.org). Also, nuclear 28S rDNA sequences were analyzed for eleven specimens representing the clades found during COI-analyses. For PCR amplification, the primers Po28F1 [5′-TAAGCGGAGGAAAAGAAAC-3′ (Struck et al. 2006)] and Po28R4 [5′-GTTCACCATCTTTCGGGTCCCAAC-3′ (Passamaneck et al. 2004)] were used. For 25 μl reactions, 1 μl template DNA was mixed with 0.1 μl (=0.5 U) Eurotaq (Biocat), 2.5 μl Eurotaq PCR buffer, 1 μl Mg++ (=2 mM), 2.5 μl dNTPs (=0.2 mM), and 0.125 μl of each primer (=0.5 pmol/μl) deluded in 17.65 μl water HPLC. The PCR temperature profile was: initial denaturation at 94°C, 3 min; 37 cycles of denaturation at 94°C, 30 s; annealing at 48°C, 30 s; elongation at 72°C, 120 s; final elongation at 72°C for 8 min. PCR products were purified with innuPREP PCRpure-Kit (Analytik Jena) and sequenced at Qiagen (Hilden, Germany). Sequences of COI and 28S were aligned separately with MUSCLE (Edgar 2004) implemented in Geneious 4.7.5 (Biomatters 2005–2008).
The COI alignment was cropped to a length of 592 bps. Corrected genetic distances between sequences and clades (substitution models: Kimura-2-Parameter for comparison with literature data, GTR+G was suggested as most suitable for presented data) were determined with PAUP* (Swofford 2002), and haplotype diversity was calculated after Nei (1987). In addition, a haplotype network was drawn using TCS 1.21 (fixed 50 steps connection limit, 95% (10 steps connection limit), and 90% (15 steps connection limit)) (Clement et al. 2000). For Bayesian tree reconstruction, the best substitution model was estimated using MrModeltest (Nylander 2004) embedded in the PAUP* plugin of Geneious and jModeltest 0.1 (Posada 2008). Trees were calculated with PAUP* (maximum likelihood) and MrBayes (GTR+G, 1,100,000 generations, 4 chains at a temperature at 0.2 (default setting), burn-in after reaching convergence at 50,000 generations), each unrooted. A second MrBayes-tree was calculated for final analyses using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001) (GTR+G, 10,000,000 generations, 4 independent runs with 4 heated and 3 cold chains, burn-in after reaching convergence at 50,000 generations) adding outgroups. The alignment was cropped to 556 bps, limited by sequence length available for published outgroups. As outgroups, COI sequences of Glycera sp. from British Columbian shallow waters (BCPOL390_08BAMPOL0361_Glycera (Carr unpublished)), Glycera nicobarica Grube, 1868 (Genbank: EU683436 (Neave et al. unpublished)), Glycera southeastatlantica Böggemann, 2009 (Genbank: GQ426630 (Böggemann 2009)), Glycera tridactyla Schmarda, 1861 (Genbank: AY583700 (Colgan et al. 2006)), and the goniadid species Goniada emerita Audouin and Milne-Edwards, 1833 (Genbank: EU835659 (Neave et al. unpublished)) were used. The tree was rooted with G. emerita. Additionally, a maximum parsimony tree was calculated with PAUP* to achieve bootstrap values (100 replicates, outgroup: G. emerita).
For correlation of genetic distances to geographic position and depths, a Mantel test was performed.
For analyses of 28S rDNA sequences, the alignment was cropped to 616 bps. Pairwise distances (Kimura-2-Parameter, GTR+G) within and between clades were calculated with PAUP*. For tree reconstruction outgroups of 28S rDNA sequence of Glycera dibranchiata Ehlers, 1868 (Genbank: AY995207.1 (Struck et al. 2006)), Glycera americana Leidy, 1855 (Genbank: EU418864 (Struck et al. 2008)), and the goniadid species Goniada brunnea Treadwell, 1906 (Genbank: DQ790037 (Struck et al. 2007)) were added. Phylogenetic reconstruction was estimated using MrBayes 3.1.2 GTR+G, (10,000,000 generations, 4 independent runs with 4 heated and 3 cold chains, burn-in after reaching convergence at 50,000 generations) and PAUP* (maximum parsimony, bootstrap number of replicates = 100, outgroup: G. brunnea).
Maps were designed with GebcoCE 1.0. Drawings of G. cf kerguelensis were digitalized with help of a Wacom pen tablet Intuos 3 and Adobe Illustrator CS3.
Results
Morphology
The distribution and taxonomic characters of the 38 specimens fitted well to the commonly used descriptions of Glycera kerguelensis (e.g., Hartman 1978; Hartmann-Schröder and Rosenfeldt 1988) justifying the identification of the specimens as such from a morphological point of view. No consistent morphological differences were found between the specimens who are therefore classified as morphologically the same species.
The specimens (Fig. 2) were all incomplete with part of their posterior segments missing, and non-ovigerous. Their sizes were therefore documented by (1) the distance of the prostomium tip to the middle of the sixth chaetigerous segment (fifth segment with biramous parapodia, present in all specimens sampled), (2) the width of the sixth chaetigerous segment between the parapodia, and (3) the width of the sixth chaetigerous segment including the parapodial lobes whenever a dorsal observation of the specimens was possible. For few specimens, the entire length and according number of chaetigers was documented. Length of the specimens to the sixth chaetiger (1) ranged from 0.47 to 1.66 mm, the width between the parapodia of the sixth chaetiger (2) ranged from 0.24 to 0.82 mm, and the measured width including the parapodial lobes (3) lay between 0.5 and 1.58 mm. The most complete specimens were 5.42 mm (26 chaetigers) to 12 mm (37 chaetigers) long. For comparison, Böggemann (2009) recorded South-East Atlantic deep-sea specimens of Glycera capitata Ørsted, 1842 with a maximum length of 20 mm for 43 chaetigers, the width of the sixth chaetiger (2) in his drawings resembled 0.47 mm. The great size ranges in the samples were independent from the sampling sites and lay well in the range documented by Böggemann (2009) for the deep sea in the closely related species indicating that the sampled specimens did not represent juveniles. The prostomium was conical, annulated with 8–11 rings, and bore four apical appendages. Chaetigerous segments were triannulated and gradually increasing in length toward median and posterior body. The parapodia were biramous except for the first, bearing a slender almost triangular prechaetal lobe that distinctly exceeded the neuropodial lobe. Postchaetal lobes were shorter and wider than prechaetal lobes. Two stout aciculae were visible under light microscope (Fig. 2a). The parapodia bore one to three simple chaetae dorsally in the notopodium; the remaining chaetae were all composite with three differently sized blades (Fig. 2b). Shifted to a dorso-lateral position above the parapodia a spherical to oval dorsal cirrus was present per chaetiger. The pharynx bore two types of papillae ((1) digitiform, (2) stouter, oval shaped, distally flattened in lateral view, Fig. 2c) and was distally equipped with a cross of four dark jaws. The accessory ailerons were characterized by a slightly concave base (Fig. 2d).
Phylogenetic and phylogeographic analyses
COI sequences of the same 38 specimens from the three different sites and depths in the Weddell Sea were achieved. Of these, seven specimens originated from the Weddell Sea abyss (St. 33, ~5,337 m), 10 from the Atka Bay slope (St. 17, ~2,100 m), and 21 specimens from Maud Rise, a sea mountain in the eastern part of the Weddell Sea (St. 39, ~2,150 m) (Fig. 1). Sequence lengths varied between 592 and 660 bps resulting in an alignment of 592 bps and average base frequencies of A 25.49%, C 23.55%, G 16.28%, and T 34.7%. In the alignment, 172 sites were variable, and 167 of them parsimony informative (details on sequence lengths and base content are given in Table 3). General topologies of the maximum likelihood, maximum parsimony, and Bayesian trees were identical.
Analysis of sequences resulted in a total of 22 different haplotypes (A-U, Online Resource ESMTab 1), 16 of them being unique. Two haplotypes were found in two specimens each (Q and T), one in three specimens (J), and one in five specimens (B). Each of these four haplotypes was limited to one location only. Haplotype P was found in ten specimens, two of them from station 17 (Atka Bay slope) and eight from station 39 (Maud Rise). The haplotypes clustered in three well-supported clades (posterior probabilities of 0.93–1.0 in the Bayesian tree, Fig. 3). Despite the high connection limits of 95% (10 steps) to up to 50 substitution steps, a single network was not achieved under statistical parsimony, rather three disconnected networks (Fig. 4).
Bayesian tree of COI mtDNA sequences (556 bps) of G. cf kerguelensis from the expedition ANDEEP-SYSTCO (GTR+G, 10,000,000 generations, burn-in 50,000 generations), outgroups: Glycera sp. (BCPOL390_08BAMPOL0361_Glycera; Carr unpublished), Glycera nicobarica (Genbank: EU683436, Neave et al. unpublished), Glycera southeastatlantica (Genbank: GQ426630, Böggemann 2009), Glycera tridactyla (Genbank: AY583700, Colgan et al. 2006 Goniada emerita (Genbank: EU835659, Neave et al. unpublished). Branch labels indicate posterior probabilities and bootstrap value (maximum parsimony, in []) if they are above 0.7/70. Labels of taxa correspond to station, specimens id, and Genbank acc. #, respectively
Haplotype network calculated with TCS 1.21 (fixed 50 steps connection limit), Haplotype letters correspond to Table 3, numbers in [] behind haplotypes indicate haplotype frequency per station if greater 1. aa amino acid, bp base pairs, St. station
-
Clade I: Specimens from the Atka Bay slope and Maud Rise (St. 17 and 39, ~2,050–2,190 m, 13 haplotypes [J–U]—9 unique).
-
Clade II: Specimens from the Atka Bay slope (St. 17, ~2,050–2,190 m, 5 haplotypes [D–H]—all unique).
-
Clade III: Specimens from the abyssal Weddell Sea (St. 33, ~5,337 m, 3 haplotypes [A–C]—2 unique).
Clade III found in the Weddell Sea abyss is suggested as the most basal of the three clades in the Bayesian tree based on COI. The three clades form a monophyletic group next to the yet unidentified Glycera species from British Columbian shelf waters. Pairwise genetic distances (GTR+G) within clades ranged from 0 to 2.0% (Table 4). The highest mean distance was found in clade I with a mean distance of 0.6%, the lowest in clade III (0.1%). Between clades, genetic distances ranged from 25.6% (minimum genetic distance between clades I and II) to 45.8% (maximum genetic distance between clades I and III). The absence of genetic distances between 2 and 25% results in a large barcoding gap. Between the three clades of G. cf kergulensis and the well-defined Glycera species recovered from Genbank genetic distances ranged from 31.9 to 66.2% (GTR+G), respectively, 22.4–28.2% (K2P), genetic distances to Goniada emerita ranged from 39.2 to 52.5% (GTR+G), respectively, 25.4 to 29.4% (K2P), questioning the identification of Glycera tridactyla (AY583700, distances GTR+G to clade I-III: 56.8–66.2%). A more detailed summary of genetic distances (GTR+G and K2P) and haplotype diversity is given in Table 4 and ESMTab 1. Haplotype diversity (h) was highest in clade II with only unique haplotypes (h = 1), followed by clade I (h = 0.846). Clade III showed the lowest haplotype diversity (h = 0.524) due to the abundance (71% of all specimens) of haplotype B. High branch support values (Fig. 3) in the Bayesian tree analyses as well as the great genetic distances strongly suggest that the three different clades represent three different species.
Inspecting the differences between the three clades in further detail, a maximum of 15 nucleotide substitutions are found within clades (Clade 1, haplotypes I and J, Fig. 4). Between clades 97 (minimum number between clades I and II) and 131 (maximum number between clades I and III), nucleotide substitutions are observed. Although they mainly concern the 3rd codon position (145 substitutions, 1st base: 24 substitutions, 2nd base: 3 substitutions), they account for a total of 9 different amino acid substitutions. Details on extent and position of amino acid differences between the clades are summarized in Online Resource ESMTab 2. Five amino acid substitutions have a possible functional consequence in that amino acids are substituted by amino acids of different side chain polarity and charge. Three of these “functional” changes concern clade III, one further is an autapomorphy to haplotype A of clade III, and the fifth concerns clade II. The changes have consequences for the predicted secondary structure of the cytochrome c oxidase protein subunit I in that helix lengths and number and position of turns vary between different clades.
The haplotype and clade distribution positively correlates with geographic distances and depths (Mantel test with 10000 permutations, P < 0.0001 in both cases). Geographic position and depths of stations are not independent of each other, and correlation to depths (r(AB) = 0.764, not exceeded by any permutation) is slightly stronger than to distance (r(AB) = 0.335, exceeded thrice).
The separation of the three clades is supported by analyses of 28S rDNA sequences (Table 5). All three clades are recovered in the Bayesian tree (Fig. 5), supported by posterior probabilities of 1.0 (clade II and III) and 0.91 (clade I). General topology of the maximum parsimony tree was identical. As in the COI analysis, clade III forms the most basal clade in the 28S tree. The lower posterior probability of clade I is caused by one nucleotide substitution in Gk45 forming the only within clade substitution in the data set. Between clades, mean pairwise distances ranged from 2.68 to 6.01% (GTR+G) opposed to 0–0.05% within clades (Table 6). The overall pairwise identity was 97.6% with 580 identical sites (94%) and 36 variable sites, all of them parsimony informative. Mean base frequencies were as follows: A 18.2%, C 26.9%, G 33.7%, T 21.2%.
Bayesian tree of 28S rDNA sequences (616 bps) of G. cf kerguelensis from the expedition ANDEEP-SYSTCO (GTR+G, 10,000,000 generations, burn-in 50,000 generations), outgroups: Glycera dibranchiata (Genbank: AY995207.1, Struck et al. 2006), Glycera americana (Genbank: EU418864, Struck et al. 2008), Goniada brunnea (Genbank: DQ790037, Struck et al. 2007). Branch labels indicate posterior probabilities and bootstrap value (maximum parsimony, in []) if they are above 0.7/70. Labels of taxa correspond to clade, specimens id, and Genbank acc. #, respectively
Discussion
Taxonomic status, ecology, and morphology of G. kerguelensis
The species G. kerguelensis has been recorded for the complete Atlantic sector of the Southern Ocean, Kerguelen Island and the waters around the Antarctic Peninsula (e.g., McIntosh 1885; Hartman 1964, 1967, 1978; Hartmann-Schröder and Rosenfeldt 1988, 1990, 1992) in depths from few hundred to over 4,000 m. Based on morphological analyses, Böggemann (2002) questioned the validity of the species and made it a synonym of Glycera capitata. However, Hartmann-Schröder (1986) had previously found that the SO specimens have distinctly longer dorsal prechaetal lobes than specimens of the European species supporting the status of G. kerguelensis as a valid species. The presented morphological analyses substantiate these differences in the parapodial shape. Also, the aileron base of the observed specimens is slightly concave as opposed to straight ones recorded for G. capitata by Böggemann (2002). From a morphological point of view, all specimens included in the presented study belong to G. kerguelensis as clearly defined variations in their morphology among them and compared to published descriptions (e.g., Hartman 1978) were missing. Differences in total length or morphometric relations followed no pattern and could be assigned to age of specimens and preservation artifacts. Comparison of the found size ranges to findings of G. capitata from the deep Southeast Atlantic (Böggemann 2009) suggest that analyzed specimens were not juveniles and found morphological characters truly resemble species characters. The genetic differentiation of specimens into three well-defined clades, however, puts doubt behind the assumption that found specimens really belong to the species G. kerguelensis whose type locality are the shallow waters of Kerguelen Island (McIntosh 1885). The specimens are therefore herein referred to as G. cf kerguelensis. If any or which of the three clades represents specimens of the originally described G. kerguelensis is only determinable based on comparison with genetic data from the type locality. Also, morphological analyses with higher magnification such as scanning electron microscopy (SEM) might uncover morphological differences between the clades themselves and compared to the original G. kerguelensis that then allow an unambiguous differentiation and description of the clades without the need of molecular references.
As for all deep-sea polychaetes in the Southern Ocean, nothing definite is known about feeding behavior and reproduction of the species. From shallow waters, we know that species of Glycera Savigny, 1818 build semi-permanent tubes in the sediment and lurk for their prey (e.g., small crustaceans, other polychaetes) which they then immobilize and kill with help of venom from gland associated with the jaw apparatus (Ockelmann and Vahl 1970; Fauchald and Jumars 1979; Manaranche et al. 1980; Böggemann 2005). Their ability to swim is used for escape responses or during epitocy for spawning (Röder and Röder 1974). Böggemann (2002) presumed that an occasional upwards swimming of atok specimens can result in a horizontal drift with water currents. Shallow water species of Glycera are described to develop long natatory chaetae during epitoky. Spawning is synchronized and the gametes are released into the water by rapture of the body walls. Larvae are planktotrophic and active (Simpson 1962; Strathmann 1987; Shanks 2001). Both the feeding behavior and the presumed drift of atok specimens are also easily imaginable for deep-sea glycerids. However, free-living larvae have not been recorded for SO deep-sea polychaetes and only scarce information is available on deep-sea polychaete reproduction in general (Blake and Narayanaswamy 2004). Since sampling methods currently applied are not fit for catching bottom-near plankton, reproduction and dispersal via free-living larvae, either lecitotrophic as proposed for deep-sea benthos in general or planktotrophic, cannot be ruled out for SO deep-sea polychaetes. The analyzed specimens were all atok and non-ovigerous giving no indication about their possible reproduction mode.
Phylogeny and distribution
The great differences of the genetic distances between and within the three clades strongly suggest three independently evolving lineages. Thus, despite the lack of morphological features supporting the genetic findings, it is proposed that the three clades represent three different, possibly cryptic species. The apparent lack of distinguishing morphological characters might be evidence for recent separation of species that has not yet been accompanied by respective morphological changes. Another possibility is that clades are defined by physiology rather than morphology. If clades inhabit comparable ecological niches at different stations, equal selective pressures might work to prevent phenotype divergence.
The three stations sampled are positioned in a triangle with stations 33 and 39 closest to each other (distance st. 17–33: ~480 Nm [~889 km], st. 17–39: ~370 Nm [~685 km], st. 33–39: ~192 Nm [~356 km]). Specimens from clade I were found at stations 17 and 39 both situated in about 2,000 m depth, while not at station 33 in over 5,330 m depth which is closer to Maud Rise than the Atka Bay slope. Clade III in contrast was only found in the abyssal Weddell Sea (St. 33). Depth has been identified as a possible driving force in deep-sea invertebrate speciation before (France and Kocher 1996; Chase et al. 1998; Quattro et al. 2001; Zardus et al. 2006) and explanations are sought in depth-related historical patterns, environmental gradients, and physiological adaptations especially concerning hydrostatic sensibility of enzymes, proteins, and larval development (Chase et al. 1998; Zardus et al. 2006). The three clades differ not only in their COI nucleotide sequence but also show substantial changes in the COI amino acid sequences. Especially haplotypes of clade III show a minimum of three amino acid replacements with different polarity and charge giving a possible indication for a manifestation of species’ separation in physiology.
As stated earlier, only very little is known about distribution of polychaetes in deep waters. We can only assume that deep-sea glycerids develop through free-living larvae, as do their shallow water relatives. The different water layers structuring the Weddell Sea therefore play a major role in dispersal of species as larval dispersal might be restricted on a vertical scale by the boundary layers of different water masses. The two sampled depth realms of around 2,000 m (st. 17 and 39) and below 5,000 m (st. 33) are isolated from each other by antipodal water currents. The Weddell abyss is characterized by the northwards flowing Antarctic Bottom Water (Knox and Lowry 1977) resulting in a down-slope connection with the Atka Bay slope but entire isolation from the Maud Rise plateau. Maud Rise and the Atka Bay slope share a unidirectional connection through the southward flowing North Atlantic Deep Water/Circumpolar Deep Water (Foldvik and Gammelsrød 1988) which could serve as a possible pathway for clade I-larvae to disperse from Maud Rise to the Atka Bay slope on a short ecological time scale (Fig. 6). Nevertheless, considering the high genetic diversity and Maud Rise’s hydrography, it comes as a surprise that clade I found at Maud Rise was also found elsewhere. Maud Rise represents a special feature in the Weddell Sea. An anticyclonic westward current system has developed around the sea mountain partially isolating the water masses within (Bersch et al. 1992; Schröder and Fahrbach 1999). Although this warm water current is still in exchange with the surrounding water masses, a quasi stationary anomaly of cold water masses characterizes the plateau of Maud Rise (Bersch et al. 1992) giving reason to expect an elevated degree of isolation in the faunal composition and populations there.
The slopes connecting station 33 with stations 17 and 39 have not been sampled. Therefore, it cannot be ruled out that along-slope gene flow existed or still exists and clade III and clades I and/or II represent parapatric clades. Based on the presented data, the clades’ origin is suggested to lie in the abyssal Weddell Sea. Both, the COI and 28S analyses suggest clade III as the basal group indicating that at least one emergence event occurred in the past originating from the deep sea (Fig. 6). The number and location of emergence events remains uncertain. The presented analyses suggest one event as clades I and II form a monophyletic group sharing a common ancestor. However, the presented dataset is too small to rule out the possibility of several independent emergence events. Emergence and submergence events, especially behind the background of the evolution of the Antarctic shelf benthos after the last glacial maximum, have long been thought to cause a major connection between the Antarctic shelf and the Antarctic deep sea that strongly shaped the recent diversity of both realms. Strugnell et al. (2008) presented detailed evidence for a Southern Ocean shallow water origin of deep-sea octopuses distributed even beyond the polar front while Mincks et al. (2009) found molecular evidence for a deep-sea origin of the shallow water spionid Auriospio foodbancsia Mincks et al. 2009. To further investigate the historical and present direction of gene flow in Southern Ocean deep-sea polychaetes and related evolutionary processes, numerous requirements have yet to be fulfilled. The question most desirable to be answered is certainly that of the reproduction and dispersal of Polychaeta in the deep sea. Also, the great genetic distances frequently found between clades and species in polychaetes have to be further analyzed. Hebert et al. (2003) showed that COI sequences offer a reliable tool to identify lepidopteran species due to a clear gap between intra- and interspecific divergences. Genetic distances defining species, especially in vertebrates and insects, are expected to be found around 2–3% and higher (Avise and Walker 1999; Hebert et al. 2003). Dawson and Jacobs (2001), based on experience from various marine invertebrates, consider genetic distances in COI of 10–20% as a benchmark for distinct species. Distances in COI between well-defined polychaete species are commonly reported to lie above 10% (Wiklund et al. 2009), often even above 20% (p-distance, K2P, HKY) (Barroso et al. 2010 and cited therein; Schulze et al. 2000; Glover et al. 2005a; Martin et al. 2008; Virgilo et al. 2009), which is distinctly higher than in other invertebrates and especially mammals. Brett (2006) studied 16 morphologically identified polychaete species from New Zealand and found that interspecific distances in polychaete COI sequences ranged from 13.8 to 36.8% while intraspecific distances lay between 0 and 5%. Further molecular studies on polychaete COI showed that genetic differences between closely related species or lineages of morphospecies regularly lie between 6 and 22% while within species/clades they seldom exceed 3% (Schulze et al. 2000; Hurtado et al. 2004; Glover et al. 2005a; Braby et al. 2007; Martin et al. 2008; Nygren et al. 2009; Wiklund et al. 2009) giving substance to the suggestion of Barroso et al. (2010) that COI evolves more quickly in polychaetes than in other benthic invertebrates studied to date. Glover et al. (2005b) found an unusual ration in unsynonymous to synonymous substitution sites in COI sequences between two distinct clades of the whale fall inhabiting polynoid Bathykurilia guaymasensis Pettibone, 1989 giving rise to the assumption that COI evolution might also underlie positive selection processes in some case which could heavily influence the rates of divergence. Sequences of deep-sea Glycera and other polychaete species from the deep Southeast Atlantic Ocean (Böggemann 2009) resulted in comparable distances to shallow water polychaetes. Also, Glover et al. (2005a) and Braby et al. (2007) found interspecific distances between shallow and deep-water Osedax Rouse et al., 2004 species to lie between 8 and 25% with the highest interspecific distances between the deep-water species Osedax rubiplumus Rouse et al., 2004 and Osedax “spiral”, indicating that evolution times in deep-sea polychaetes might be comparable to that in shallow water species.
The presented data fall well within this bimodal distribution of inter- and intraspecific divergences without intermediates. Additionally, the level of genetic divergence between the three clades found equals, and in some cases even exceeds that known between COI sequences of undisputed polychaete species. They also overlap with the range of distances between Glycera species and the species Goniada emerita that belongs to the sister family Goniadidae, underlining Barroso et al.’s (2010) hypothesis that divergence of COI in polychaetes is supposedly higher on an evolutionary time scale than expected from experiences with other animal taxa. Held (2003) proposed the criterion of bimodal distribution of divergence to be a prerequisite for the detection of cryptic species. He also stated that the genetic differentiation must be persistent in sympatry. Clades I and II fulfill this requirement at station 17. It is thus strongly suggested that the three different clades represent cryptic, or considering the lack of application of SEM in morphological investigations, at least pseudocryptic, species. Also, the analyses of the nuclear, and generally more conserved 28S rDNA also in polychaetes (e.g., in Chrysopetalidae (Wiklund et al. 2009)), strongly supports the separation of the three clades found. While within clade divergence was basically undetected apart from one substitution in clade I, among clades mean divergences between 2.5 and 5.4% were found. However, since the sample set was very small in the nuclear analyses, the lack of intermediates might be misleading here. More data from polychaetes are imperatively necessary to better evaluate intra- and interspecific divergence in the COI sequences of Polychaeta and truly judge about their variability and thus usefulness to ultimately distinguish species rather than mere populations.
Implications for SO deep-sea invertebrate diversity and distribution
Phylogeographic studies on Southern Ocean shelf species of various invertebrate taxa have shown that cryptic speciation might be a common phenomenon in the shallow Southern Ocean. Originally believed circum-Antarctic distribution patterns are challenged by high genetic divergences between distinct populations of e.g., Isopoda or Pycnogonida (Held 2003; Held and Wägele 2005; Raupach and Wägele 2006; Mahon et al. 2008). Linse et al. (2007) found circum-Antarctic distributions on the continental shelf for the bivalve Lissarca notorcadensis Melvill and Standen, 1907 possible but discovered a distinctly separated genetic lineage for this species around the islands in the Scotia Sea. Additionally, the Antarctic polar front has been demonstrated to act as a barrier hindering gene flow between the SO and the South American shelves in numerous species (Hunter and Halanych 2008; Thornhill et al. 2008; Krabbe et al. 2009). Most of these studies revealed that despite a rather local restriction of genetic populations/lineages, genetic divergences are seldom correlated with geographic distance. All in all, molecular approaches to species diversity show that Southern Ocean diversity might be largely underestimated while species’ distribution might be overestimated in several cases (Mincks et al. 2009). Due to great difficulties especially regarding suitable samples (quantity and quality), no comparable studies from the Southern Ocean deep sea exist to date. The presented study presents the first phylogeographic data on SO deep-sea polychaetes. The outcome of the analyses lives up to the expectations adopted from SO shelf studies in that populations from different regions show higher genetic divergence than morphology suggests. For polychaetes in general, many of the existing phylogeographic studies world-wide suggest that wide distribution ranges as suggested by taxonomy might not be always be supported by genetic data (von Soosten et al. 1998; Schmidt and Westheide 1999, 2000; Patti and Gambi 2001; Bleidorn et al. 2006; Rice et al. 2008; Wiklund et al. 2009) although some studies supporting cosmopolitan species also exist (Schmidt and Westheide 2000; Westheide et al. 2003). Also, the presented results are in line with the observation in molluscs and the amphipod Eurythenes gryllus (Lichtenstein, 1822) that genetic variation and divergence in the deep sea is greater on a vertical scale than on a horizontal scale with higher diversities in around 2000 m depths than in the lower bathyal and abyss (France and Kocher 1996; Chase et al. 1998; Quattro et al. 2001; Zardus et al. 2006). Despite the frequent lack of clear topographic structuring, the deep sea exhibits a yet undiscovered high genetic diversity. Cryptic speciation might be as common a phenomenon in deep waters as it is on the shelf, only that there divergent populations are separated by physical and environmental forces we have yet to uncover.
References
Arntz WE, Thatje S, Gerdes D, Gili JM, Gutt J, Jacob U, Montiel A, Orejas C, Teixidó N (2005) The Antarctic-Magellan connection: macrobenthos ecology on the shelf and upper slope, a progress report. Sci Mar 69(Suppl. 2):237–269
Audzijonyte A, Ovcarenko I, Bastrop R, Väinölä R (2008) Two cryptic species of the Hediste diversicolor group (Polychaeta, Nereididae) in the Baltic Sea, with mitochondrial signatures of different population histories. Mar Biol 155:599–612
Avise JC, Walker D (1999) Species realities and numbers in sexual vertebrates: perspectives from an asexually transmitted genome. Proc Nat Acad Sci USA 96:992–995
Barroso R, Klautau M, Solé-Cava AM, Paiva PC (2010) Eurythoe complanata (Polychaeta: Amphinomidae), the ‘cosmopolitan’ fireworm, consists of at least three cryptic species. Mar Biol 157:69–80
Bersch M, Becker GA, Frey H, Koltermann KP (1992) Topographic effects of the Maud Rise on the stratification and the circulation of the Weddell Gyre. Deep-Sea Res 39(2):303–331
Blake JA, Narayanaswamy BE (2004) Benthic infaunal communities across the Weddell Sea Basin and South Sandwich Slope, Antarctica. Deep-Sea Res II 51:1797–1815
Bleidorn C, Kruse I, Albrecht S, Bartolomaeus T (2006) Mitochondrial sequence data expose the putative cosmopolitan polychaete Scolopos armiger (Annelida, Orbiniidae) as a species complex. BMC Evol Biol 6(47):1–13
Böggemann M (2002) Revision of the Glyceridae Grube 1850 (Annelida: Polychaeta). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 555, Frankfurt am Main
Böggemann M (2005). Revision of the Goniadidae (Annelida, Polychaeta). Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg (Neue Folgen) 39, Frankfurt am Main
Böggemann M (2009) Polychaetes (Annelida) of the abyssal SE Atlantic. Org Divers Evol 9:251–428
Braby CE, Rouse GW, Johnson SB, Jones WJ, Vrijenhoek RC (2007) Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey Bay, California. Deep-Sea Res I 54:1773–1791
Brandt A, Brix S, Brökeland W, Choudhury M, Kaiser S, Malyutina M (2007a) Deep-sea isopod biodiversity, abundance, and endemism in the Atlantic sector of the Southern Ocean—results from the ANDEEP I–III expeditions. Deep-Sea Res II 54:1760–1775
Brandt A, De Broyer C, De Mesel I, Ellingsen KE, Gooday AJ, Hilbig B, Linse K, Thomson MRA, Tyler PA (2007b) The biodiversity of the deep Southern Ocean benthos. Philos Trans Roy Soc B 362:39–66
Brett CD (2006) Testing the effectiveness of the mtDNA Cytochrome c oxidase subunit 1 (COI) gene locus for indentifying species of Polychaete worm (Polychaeta: Annelida) in New Zealand. Master thesis, The University of Waikato, New Zealand
Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE (1996) Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarct Sci 8:3–6
Chase MR, Etter RJ, Rex MA, Quattro JM (1998) Bathymetric patterns of genetic variation in a deep-sea protobranch bivalve, Deminucula atacellana. Mar Biol 131:301–308
Clarke A, Crame JA (1989) The origin of the Southern Ocean marine fauna. In: Crame JA (ed) Origins and evolution of the Antarctic biota. Geological Society of London Special Publication 47, pp 253–268
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659. doi:10.1046/j.1365-294x.2000.01020.x
Colgan DJ, Hutchings PA, Braune M (2006) A multigene framework for polychaete phylogenetic studies. Org Divers Evol 6:220–235
Dawson MN, Jacobs DK (2001) Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biol Bull 200:92–96
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi:10.1093/nar/gkh340
Ellingsen KE, Brandt A, Ebbe B, Linse K (2007) Diversity and species distribution of polychaetes, isopods and bivalves in the Atlantic sector of the deep Southern Ocean. Pol Biol 30(10):1265–1273
Fauchald K, Jumars PA (1979) The diet of worms: a study of polychaete feeding guilds. Oceanography and Marine Biology—An Annual Review, vol 17, Aberdeen, pp 193–284
Foldvik A, Gammelsrød T (1988) Notes on Southern Ocean hydrography, sea-ice and bottom water formation. Palaeogeo Palaeoclim Palaeoecol 67:3–17
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299
France SC, Kocher TD (1996) Geographic and bathymetric patterns of mitochondria116S rRNA sequence divergence among deep-sea amphipods, Eurythenes gryllus. Mar Biol 126:633–643
Glover AG, Källström B, Smith CR, Dahlgren TG (2005a) World-wide whale worms? A new species of Osedax from the shallow north Atlantic. Proc R Soc B 272:2587–2592
Glover AG, Goetze E, Dahlgren TG, Smith CR (2005b) Morphology, reproductive biology and genetic structure of the whale-fall and hydrothermal vent specialist, Bathykurila guaymasensis Pettibone, 1989 (Annelida: Polynoidae). Mar Eco 26:223–234
Hartman O (1964) Polychaeta Errantia of Antarctica. Antarct Res Ser 3:1–131
Hartman O (1967) Polychaetous annelids collected by the USNS ELTANIN and Staten Island cruises, chiefly from Antarctic Seas. Allan Hancock Monogr Mar Biol, 2:1–387, 51 pls
Hartman O (1978) Polychaeta from the Weddell Sea Quadrant, Antarctica. Paper 4. In: Biology of the Antarctic Seas VI, Antarct Res Ser 26:125–223
Hartmann-Schröder G (1986) Die Polychaeten der 56. Reise der “Meteor” zu den South Shetland-Inseln (Antarktis). Mitt Hambg Zool Mus Inst 83:71–100
Hartmann-Schröder G, Rosenfeldt P (1988) Die Polychaeten der „Polarstern”—Reise ANT III/2 in die Antarktis 1984. Teil 1: Euphrosinidae bis Chaetopteridae. Mitt Hambg Zool Mus Inst 85:25–72
Hartmann-Schröder G, Rosenfeldt P (1990) Die Polychaeten der „Walther Herwig”-Reise 68/1 nach Elephant Island (Antarktis) 1985. Teil 1: Aphroditidae bis Cirratulidae. Mitt Hambg Zool Mus Inst 87:89–122
Hartmann-Schröder G, Rosenfeldt P (1992) Die Polychaeten der „Polarstern”-Reise ANT V/1 in die Antarktis 1986. Teil 1: Euphrosinidae bis Iphitimidae. Mitt Hambg Zool Mus Inst 89:85–124
Hebert PDN, Cywinsky A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc Roy Soc London B. doi:10.1098/rspd.2002.2218
Held C (2000) Phylogeny and biogeography of Serolid Isopods (Crustacea, Isopoda; Serolidae) and the use of ribosomal expansion segments in molecular systematics. Mol Phylogen Evol 15(2):165–178
Held C (2003) Molecular evidence for cryptic speciation within the widespread Antarctic crustacean Ceratoserolis trilobitoides (Crustacea, Isopoda). In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der Vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys Publishers, Leiden, pp 135–139
Held C, Wägele JW (2005) Cryptic speciation in the giant Antarctic isopod Glyptonotus antarcticus (Isopoda: Valvifera: Chaetiliidae). Sci Mar 69:175–181
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinform 1:754–755. doi:10.1093/bioinformatics/17.8.754
Hunter RL, Halanych KM (2008) Evaluating connectivity in the brooding brittle star Astrotoma agassizii across the Drake Passage in the Southern Ocean. J Hered. doi:10.1093/jhered/esm119
Hurtado LA, Lutz RA, Vrijenhoek RC (2004) Distinct patterns of genetic differentiation among annelids of eastern Pacific hydrothermal vents. Mol Ecol 13:2603–2615
Janko K, Lecointre G, DeVries A, Couloux A, Cruaud C, Marshall C (2007) Did glacial advances during the Pleistocene influence differently the demographic histories of benthic and pelagic Antarctic shelf fishes?—Inferences from intraspecific mitochondrial and nuclear DNA sequence diversity. BMC Evol Biol 7:220. http://www.biomedcentral.com/1471-2148/7/220
Knox GA, Lowry JK (1977) A comparison between the benthos of the Southern Ocean and the North Polar Ocean with special reference to the Amphipoda and the Polychaeta. In: Dunbar MJ (ed) Polar Oceans. Polar Oceans Conference 1974, Montreal, pp 423–462
Krabbe K, Leese F, Mayer C, Tollrian R, Held C (2009) Cryptic mitochondrial lineages in the widespread pycnogonid Colossendeis megalonyx Hoek, 1881 from Antarctic and Subantarctic waters. Pol Biol. doi:10.1007/s00300-009-0703-5
Linse K, Cope T, Lörz AN, Sands C (2007) Is the Scotia Sea a centre of Antarctic marine diversification? Some evidence of cryptic speciation in the circum-Antarctic bivalve Lissarca notorcadensis (ArCOIdea: Philobryidae). Pol Biol 30(8):1059–1068
Mahon AR, Arango CP, Halanych KM (2008) Genetic diversity of Nymphon (Arthropoda: Pycnogonida: Nymphonidae) along the Antarctic Peninsula with a focus on Nymphon australe Hodgon 1902. Mar Biol 155:315–323
Manaranche R, Thieffry M, Israel M (1980) Effect of the venom of Glycera convoluta on the spontaneous quantal release of transmitter. J Cell Biol 85:446–458
Martin D, Gil J, Carreras-Carbonell J, Bhaud M (2008) Description of a new species of Mesochaetopterus (Annelida, Polychaeta, Chaetopteridae), with re-description of M. xerecus and an approach to the phylogeny of the family. Zool J Linn Soc London 152:201–225
McIntosh WC (1885) Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–1876. Chall Rep 12:1–554
Mincks SL, Dyal PL, Paterson GLJ, Smith CR, Glover AG (2009) A new species of Aurospio (Polychaeta, Spionidae) from the Antarctic shelf, with analysis of its ecology, reproductive biology and evolutionary history. Mar Eco 30:181–197
Munilla T, Soler Membrives A (2008) Check-list of the pycnogonids from Antarctic and sub-Antarctic waters: zoogeographic implications. Antarct Sci 21(2):99–111
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nygren A, Eklöf J, Pleijel F (2009) Arctic-boreal sibling species of Paranaitis (Polychaeta, Phyllodocidae). Mar Biol Res 5:315–327
Nylander JAA (2004) MRMODELTEST v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala
Ockelmann KW, Vahl O (1970) On the biology of the polychaete Glycera alba, especially its burrowing and feeding. Ophelia 8:275–294
Passamaneck YJ, Schander C, Halanych KM (2004) Investigation of molluscan phylogeny using large-subunit and small-subunit nuclear rRNA sequences. Mol Phylogen Evol 32:25–38
Paterson GLJ, Glover AG, Barrio Froján CRS, Whitaker M, Budaeva N, Chimonides J, Doner S (2009) A census of abyssal polychaetes. Deep-Sea Res II 56:1739–1746
Patti FP, Gambi MC (2001) Phylogeography of the invasive polychaete Sabella spallanzanii (Sabellidae) based on the nucleotide sequence of internal transcribed spacer 2 (ITS2) of nuclear rDNA. Mar Ecol Prog Ser 215:169–177
Platt HM, Warwick RM (1988) Freeliving marine nematodes. Part II. British Chromadorids. In: Kermack DM, Barnes RSK (eds) Synopsis of the British Fauna (new series), 38. E.J. Brill and Backhuys, Leiden, pp 14–21
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Quattro JM, Chase MR, Rex MA, Greig TW, Etter RJ (2001) Extreme mitochondrial DNA divergence within populations of the deep-sea gastropod Frigidoalvania brychia. Mar Ecol 139:1107–1113
Raupach MJ, Wägele JW (2006) Distinguishing cryptic species in Antarctic Asselota (Crustacea: Isopoda)—a preliminary study of mitochondrial DNA in Acanthaspidia drygalskii. Antarct Sci 18(2):191–198
Rice SA, Karl S, Rice KA (2008) The Polydora cornuta complex (Annelida: Polychaeta) contains populations that are reproductively isolated and genetically distinct. Invertebr Biol 127(1):45–64
Röder A, Röder H (1974) Dreidimensionale Schwimmbewegungen (Helikoidalbewegung) bei polychaeten Meereswürmern. Teil I Antriebsmechanismus und Bewegungsablauf. Nat Mus 104(12):372–378
Rogers AD (2007) Evolution and biodiversity of Antarctic organisms: a molecular perspective. Philos Trans Roy Soc B 362:2191–2214
Rouse G, Pleijel F (eds) (2006) Reproductive biology and phylogeny of Annelida. Science Publishers, Enfeld, NH, USA
Sands CJ, Convey P, Linse K, McInnes SJ (2008) Assessing meiofaunal variation among individuals utilizing morphological and molecular approaches: an example using the Tardigrada. BMC Ecol 8:7. doi:10.1186/1472-6785-8-7
Schmidt H, Westheide W (1999) Genetic relationships (RAPD-PCR) between geographically separated populations of the “cosmopolitan” interstitial polychaete Hesionides gohari (Hesionidae) and the evolutionary origin of the freshwater species Hesionides riegerorum. Biol Bull 196:216–226
Schmidt H, Westheide W (2000) Are the meiofaunal polychaetes Hesionides arenaria and Stygocapitella subterranea true cosmopolitan species?—results of RAPD-PCR investigations. Zool Scr 29:17–27
Schröder M, Fahrbach E (1999) On the structure and the transport of the Eastern Weddell Gyre. Deep-Sea Res 46:501–527
Schüller M, Ebbe B (2007) Global distributional patterns of selected deep-sea polychaeta (Annelida) from the Southern Ocean. Deep-Sea Res II 54(16–17):1737–1751
Schulze SR, Rice SA, Simon JL, Karl SA (2000) Evolution of poecilogony and the biogeography of North American populations of the polychaete Streblospio. Evolution 54:1247–1259
Shanks AL (2001) An identification guide to the larval marine invertebrates of the Pacific Northwest. Oregon State University Press, Corvallis, OR
Simpson M (1962) Reproduction of polychaete Glycera dibranchiata at Solomons, Maryland. Biol Bull 123:396–411
Soler i Membrives A, Turpaeva E, Munilla T (2009) Pycnogonids of the Eastern Weddell Sea (Antarctica), with remarks on their bathymetric distribution. Pol Biol. doi:10.1007/s00300-009-0635-0
Strathmann MF (1987) Reproduction and development of marine invertebrates of the northern pacific coast: data and methods for the study of eggs, embryos, and larvae. University of Washington Press, Seattle, WA
Struck TH, Purschke G, Halanych KM (2006) Phylogeny of Eunicida (Annelida) and exploring data congruence using a partition addition bootstrap alteration (PABA) approach. Syst Biol 55(1):1–20
Struck TH, Schult N, Kusen T, Hickman E, Bleidorn C, McHugh D, Halanych KM (2007) Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evol Biol 7(57). doi:10.1186/1471-2148-7-57
Struck TH, Nesnidal MP, Purschke G, Halanych KM (2008) Detecting possibly saturated positions in 18S and 28S sequences and their influence on phylogenetic reconstruction of Annelida (Lophotrochozoa). Mol Phylogen Evol 48(2):628–645
Strugnell J, Rogers AD, Prodöhl PA, Collins MA, Allcock AL (2008) The thermohaline expressway: the Southern Ocean as a centre of origin for deep-sea octopuses. Cladistics 24:853–860
Swofford DL (2002) PAUP* Phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, MA
Thatje S, Hillenbrand CD, Larter R (2005) On the origin of Antarctic marine benthic community structure. Trends Ecol Evol 20(10):534–540
Thatje S, Hillenbrand CD, Mackensen A, Larter R (2008) Life hung by a thread: endurance of Antarctic fauna in glacial periods. Ecology 89(3):682–692
Thornhill DJ, Mahon AR, Norenburg JL, Halanych KM (2008) Open-ocean barriers to dispersal: a test case with the Antarctic Polar Front and the ribbon worm Parborlasia corrugatus (Nemertea: Lineidae). Mar Ecol 17:5194–5197
Virgilo M, Fauvelot C, Constantini F, Abbiati M, Backeljau T (2009) Phylogeography of the common ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distribution. Mol Ecol 18:1980–1994
von Soosten C, Schmidt H, Westheide W (1998) Genetic variability and relationships among geographically widely separated populations of Petitia amphophthalma (Polychaeta: Syllidae). Results from RAPD-PCR investigations. Mar Biol 131:659–669
Westheide W, Haβ-Cordes E, Krabusch M, Müller M (2003) Ctenodrilus serratus (Polychaeta: Ctenodrilidae) is a truly amphi-atlantic meiofauna species—evidence from molecular data. Mar Biol 142:637–642
Wiklund H, Glover AG, Johannessen PJ, Dahlgren TG (2009) Cryptic speciation at organic-rich marine habitats: a new bacteriovore annelid from whale-fall and fish farms in the North-East Atlantic. Zool J Linn Soc 155:774–785
Wilson WH (1991) Sexual reproductive modes in polychaetes: classification and diversity. Bull Mar Sci 48:500–516
Wilson NG, Hunter RL, Lockhart SJ, Halanych KM (2007) Multiple lineages and absence of panmixia in the “circumpolar” crinoid Promachocrinus kerguelensis from the Atlantic sector of Antarctica. Mar Biol 152:895–904
Wilson NG, Schrödl M, Halanych KM (2009) Ocean barriers and glaciation: evidence for explosive radiation of mitochondrial lineages in the Antarctic sea slug Doris kerguelenensis (Mollusca, Nudibranchia). Mol Ecol 18:965–984
Zardus JD, Etter RJ, Chase MR, Rex MA, Boyle EE (2006) Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939). Mol Ecol 15:639–651
Acknowledgments
I am greatly indebted to the scientists and laboratory technicians at the International Barcode of Life, University of Guelph, whose help ensured the achievement of the barcoding. Special thanks go to Christy Carr and Dirk Steinke for their close collaboration and constant effort. Also, I would like to thank the Boehringer Ingelheim Fonds, Rachel Grant, and Katrin Linse as representatives of CAML, as well as CeDAMar, for giving me the opportunity to take the samples and later barcode them in collaboration with iBOL. Many thanks go to Florian Leese, Christoph Mayer, and Chester Sands for their help with the sequence analyses and fruitful discussions. Sequencing was carried out at the Biodiversity Institute of Ontario and supported by Genome Canada through the Ontario Genomics Institute. This study was further financed by help of CeDAMar (Census for the Diversity of Abyssal Marine Life), CAML (Census of Antarctic Marine Life), the Boehringer Ingelheim Fonds, and the German Science Foundation (SCHU 2443/2-1+2). This manuscript is contribution number 58 to the Census of Antarctic Marine Life and number 145 to ANDEEP.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
300_2010_913_MOESM1_ESM.pdf
ESMTab 1 Pairwise genetic distances among COI sequences of G. cf kerguelensis (592bps)—right upper half: Kimura-2-Parameter model, in percent, left lower half: GTR+G in percent. Supplementary material 1 (PDF 26 kb)
300_2010_913_MOESM2_ESM.pdf
ESMTab 2 Changes in COI amino acid sequences between clades of G. cf kerguelensis (background: gray—polar, white—non-polar; fond: bold—acid, regular—neutral, abbreviations: *—functional changes, A—alanine, E—glutamic acid, I—isoleucine, L—leucine, M—methionine, S—serine, V—valine). Supplementary material 2 (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Schüller, M. Evidence for a role of bathymetry and emergence in speciation in the genus Glycera (Glyceridae, Polychaeta) from the deep Eastern Weddell Sea. Polar Biol 34, 549–564 (2011). https://doi.org/10.1007/s00300-010-0913-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0913-x