Abstract
Plants display an amazing ability to synthesize a vast array of secondary metabolites that are an inexhaustible source of phytochemicals, bioactive molecules some of which impact the human health. Phytochemicals present in medicinal herbs and spices have long been used as natural remedies against illness. Plant tissue culture represents an alternative to whole plants as a source of phytochemicals. This approach spares agricultural land that can be used for producing food and other raw materials, thus favoring standardized phytochemical production regardless of climatic adversities and political events. Over the past 20 years, different strategies have been developed to increase the synthesis and the extraction of phytochemicals from tissue culture often obtaining remarkable results. Moreover, the availability of genomics and metabolomics tools, along with improved recombinant methods related to the ability to overexpress, silence or disrupt one or more genes of the pathway of interest promise to open new exciting possibilities of metabolic engineering. This review provides a general framework of the cellular and molecular tools developed so far to enhance the yield of phytochemicals. Additionally, some emerging topics such as the culture of cambial meristemoid cells, the selection of plant cell following the expression of genes encoding human target proteins, and the bioextraction of phytochemicals from plant material have been addressed. Altogether, the herein described techniques and results are expected to improve metabolic engineering tools aiming at improving the production of phytochemicals of pharmaceutical and nutraceutical interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary metabolites of plants: chemical classes and physiological roles played in planta
Due to their mode of life, plants display a remarkable genetic complexity that underline their ability to synthesize a vast array of natural organic substances, known also as secondary metabolites, some of which are bioactive molecules able to influence human metabolism and health. Contrarily to primary metabolites, such as amino acids, nucleotides, organic acids, phytosterols, photosynthetic pigments, which are present in all plant organisms, secondary metabolites, commonly named “phytochemicals”, are present only in some taxonomic groups and are not essential for plant growth and development. Nevertheless, they exert a crucial function in the defense of plants from biotic stress caused by phytophagous organisms (herbivores and insects), microbial pathogens, the competition with other plants (allelopathy) and, in a few cases, the defense of plants from some types of abiotic stresses such as UV stress, sunlight excess, water stress and oxidative stress. They are usually present at low concentrations, chiefly in vacuoles. Phytochemicals often display pharmacological or nutraceutical effects and, depending on the dosage, they impact man’s health. Their pharmaceutical or nutraceutical value is “fortuitous”, due to their capability of interfering with target proteins in herbivores or phytopathogenic organisms as well with homologous targets in man (see next chapter).
There are three main classes of plant secondary metabolites: terpenes and terpenoids, nitrogen-containing compounds, phenolic compounds. The first category includes molecules that allow plants to fight the attacks of phytophagous organisms. They are often endowed with pungent smells or aromas (essential oils), but some also with various pharmacological activities (for instance cardenolides and the antimalarial drug artemisinin), or nutraceutical action (for example lutein and lycopene). The second consists of different classes of nitrogen-containing molecules, the most abundant of which are the alkaloids that deter herbivores from predatory activity onto plant organs. Some alkaloids act on the human nervous system as stimulant (nicotine, caffeine), or as drugs (scopolamine, morphine, codeine, etc.). The third category, phenolic compounds, encompasses many substances exerting both ecological and defensive roles and mostly possessing a wide range of nutraceutical actions often supported by their relevant antioxidant activity. The examples of phenolic nutraceuticals are numerous, especially among flavonoids, ranging from many flavones to anthocyanins and proanthocyanidins (Caleja et al. 2017).
Properties and pharmaceutical or nutraceutical properties of phytochemicals
Since ancient times, medicinal herbs and spices have been used as natural remedies against morbid conditions in the form of teas, infusions and decoctions. With the development of highly sensitive analytical methods, it has been possible to identify and characterize a great variety of these substances. Meanwhile, an increasing number of epidemiological data have been demonstrating the beneficial action on human health of many phytochemicals.
Based on their effect, phytochemicals may belong to two distinct categories: drugs and nutraceuticals.
The first have pharmacological activity and, when assumed in appropriate amount, cure specific diseases; indeed, even today many commonly used drugs derive, directly or indirectly, from phytochemicals. In the early twentieth century, progress in the chemical synthesis of drugs and antibiotics led to a decline in the use of plant extracts and, consequently, a reduction in the interest of scientists. In the past 20 years, some side effects of synthetic active chemicals on human health made the interest in phytochemistry, phytopharmacology, phytomedicine and phytotherapy resurface.
Biochemical, physiological and epidemiological studies showed that nutraceutical molecules, even if do not cure diseases, are able to prevent some of them. In fact, it has been reported that many enzymes, receptors, transcription factors, ion channels, transporters, or cytoskeletal proteins involved in various cell functions may be directly or indirectly influenced, altered, inactivated, inhibited or stimulated through the interaction with nutraceuticals countering specific diseases or health disorders (Wink 2015).
Even if there is not yet clarity about the meaning of the word nutraceutical (Aronson 2017), a “nutraceutical substance” might be defined as a bioactive natural substance present in the tissues of many plant species, including seeds, fruit, leaves and other edible plant organs, which is useful to prevent (more rarely to treat) human diseases. Therefore, in the past two decades it became clear that food not only provides nutrients but is also a source of molecules that positively influence human health. Some phytochemicals of medical interest are present in fruits and vegetables at high concentrations (e.g. polyphenols), whereas others are present only in trace amount.
Both drugs and nutraceutical molecules can be extracted and used as pharmaceutical preparations of various kind or, in the case of nutraceuticals, in the form of concentrate, such as “integrators”, i.e. pills, capsules or tablets, providing doses higher than those introduced with the diet. In recent years, the use of extracts containing phytochemicals is constantly increasing also in the cosmetic field. These so-called “cosmeceuticals” are directed to the care of skin and hair or in the formulation of makeup products (https://www.medicinenet.com/script/main/art.asp?articlekey=25353).
Table 1 shows a few examples of phytochemicals of pharmaceutical, nutraceutical or cosmetic interest.
About 30 out of 252 drugs that WHO declares essential for human health derives from plants (Rates 2001) and most of them cannot be chemically synthesized or replaced by synthetic analogues. With regard to nutraceuticals, there is a growing demand for bioactive ingredients for integrators and functional foods driven by various health concerns (Ochoa-Villarreal et al. 2016). However, their mode of action is less specific, since most promote health due to antioxidant activity and/or anti-inflammatory properties. This explains their preventing role since it is now widely recognized that oxidative stress and inflammation create the conditions for the development of the majority of chronic-degenerative diseases such as diabetes, atherosclerosis and cancer (Costa et al. 2017).
Some phytochemicals of medical interest cited above are present in fruits and vegetables at high concentrations (e.g. polyphenols), whereas others are present only in trace amount; in the latter case, processing of a large amount of plant material is necessary to obtain a significant yield. In other cases, the effects are more “direct”, being attributable to mild anti-microbial or immunostimulant activity, hypocolesterolemic or anti-hypertensive action (Chen et al. 2008), hypolipidemic action (Chen et al. 2014a, b) or for demonstrated capacity of preventing cancer and Alzheimer’s disease (Sadhukhan et al. 2018).
An alternative source of high added value phytochemicals is represented by agro-industrial wastes, which are abundant, renewable, inexpensive and not in competition with the food chain, is been considered: currently, research is focused on lowering processing costs and increasing recovery yields while preserving the quality of phytochemicals (Baiano 2014).
Finally, it is worth mentioning that many plant secondary metabolites, besides being used as drugs and nutraceuticals, can be profitably employed as agrochemicals (biopesticides: insecticides and fungicides), essences, fragrances and natural pigments.
Cell, tissue and organ culture as tools to produce high amount of bioactive phytochemicals
Why to use biotechnological tools for producing phytochemicals?
Several goals are being pursued in both public and private research laboratories concerning the identification and characterization of novel plant molecules of pharmaceutical or nutraceutical interest aiming at increasing their amount. The rapid increase in the world population exerts a strong pressure on the demand for agricultural land used for producing food and other plant raw materials. It is therefore easy to foresee land shortage for growing medicinal plants. There is also the need to protect wild medicinal plants that may risk extinction because of the reduction of natural environment due to the agriculture expansion.
As mentioned above, some of the most desirable phytochemicals are found only in small amounts in specific plant tissues. For example, saffron spice compounds are present only in the stigmas of crocus flowers (Sharma and Piqueras 2010), thus outstandingly increasing the cost of spice harvest. Additional problems may arise when phytochemical production depends on wild plants growing in underdeveloped countries unable to warrant a constant provision of good quality plant material.
For these reasons, if a chemical synthesis strategy is not possible or economically sustainable, it is necessary to develop biotechnological solutions allowing large-scale production of those secondary metabolites of industrial interest that are present in low amounts in producer plants. The same is true in the case of phytochemicals produced by species difficult to cultivate.
Moreover, plant cells can make stereo- and regio-specific biotransformation reactions to obtain new valuable compounds from low-cost precursors (Hidalgo et al. 2018).
A biotechnological production system must meet two conditions: (1) constant and economically sustainable production of substances with uniform quality; (2) fast production and efficient recovery of the product.
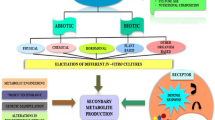
In synthesis, two main approaches can be used to increase or ameliorate in producing species the biosynthesis of phytochemicals of interest: in vitro tissue or organ culture and genetic engineering manipulations (Fig. 1). The choice between these two approaches depends primarily on the capital investments required for research and development and on the final yield.
Here we shall highlight the general features and the current limitations of both technologies.
Use of plants cell, tissue and organ cultures for phytochemicals production
Although genetic engineering can be considered a more direct and straightforward strategy, the present social opposition to the GMOs decreases its applicability in favor of a more traditional approach based on plant cell or tissue or organ cultures. In the literature, it is possible to find many articles reporting examples of phytochemicals that, following effective and often complex approaches of either cellular or molecular plant biotechnology, have been produced on a laboratory scale. However, few examples of successful exploitation at the industrial level have been reported (Knockaert et al. 2015). For obvious secrecy reasons, little information is available on the technologies used at an industrial scale for the production, extraction and purification of pharmaceuticals obtained by plant cell cultures among which Shikonine, anti-inflammatory and antitumor agent of Lithospermum erythrorhizon (Hu 2004), Berberine, antimicrobial agent of Coptis japonica and Coscinium fenestratum (Nair et al. 1992), Rosmarinic acid, antioxidant, anti-inflammatory agent, antimicrobial agent of Coleus blumeii (Bauer et al. 2015), Sanguinarine, anti-inflammatory agent of Eschscholzia californica (Balažová et al. 2018), Paclitaxel, a taxoid compound found in Taxus brevifolia partially produced in suspension cultured cells (Wickremesinhe and Arteca 1994), Vincristine, antitumoral of Catharanthus roseus (Lee-Parsons and Royce 2006).
By definition, plant cells grown in vitro are in the form of calli or of suspension cells, in both cases in an undifferentiated or semi-differentiated state. Conversely, secondary metabolite pathways are usually activated in tissues of plant organs. Nonetheless, in some cases even undifferentiated calli or suspension cells produce phytochemicals of interest. For example, Papaver somniferum (Verma et al. 2018) and Lithospermum erythrorhizon (Hu 2004) cell suspensions produce, respectively, Sanguinarine and Shikonin at an amount higher than that of the entire plant. Consequently, since many years these phytochemicals are produced industrially in this way.
In other cases, to induce secondary metabolites synthesis, it is necessary to repeatedly select clones that maintain a suitable pattern of partial differentiation even in in vitro conditions (Hussain et al. 2012). This latter case often occurs mostly when the phytochemical of interest is produced in specialized plant tissues or glands.
A strategy to bypass the limits imposed by the undifferentiated state of cell suspensions and calli relies on growing in vitro organs such as roots or buds. In particular, isolated roots of several medicinal plants have been cultured in vitro in the past 30–40 years to produce a few drugs, for instance tropane alkaloids and Hyoscyamine. (Veerport et al. 2002; Ochoa-Villarreal et al. 2016). Morever, there is a lot of interest on trichomes, many of which perform a glandular function (Huchelmann et al. 2017).
Since roots are characterized by slow growth when cultured in vitro, an alternative approach based on “hairy-roots” is often used. This system has been made available following the discovery of the ability of the two species of the genus Agrobacterium to infect plants and transfer specific genes forcing them to produce phytormones. In the case of Agrobacterium rhizogenes containing Ri plasmids, the transferred genes tms1 and tms2 induce the synthesis of auxin that in turn stimulates the production of adventitious roots (hairy roots) in infected plants (White et al. 1985). Excised hairy roots grow rapidly and are genetically more stable than cultured cells. So, it is not surprising that the number of papers reporting the use of hairy roots to produce various phytochemicals has been remarkably increasing in recent years (Tian 2015). However, this approach cannot be applied to phytochemicals that are produced in the aerial part of the organism.
Independently of the be differentiation state of the cultured tissue, it is not always easy to obtain a phytochemical yield high enough to be remunerative and it is frequently necessary to enhance phytochemical production by means of a number of strategies employing experimental tricks and genetic manipulations. Furthermore, in particular cases, production of phytochemicals in undifferentiated plant cultures is problematic or even impossible because of their intrinsic toxicity, a problem that in planta is avoided by sequestrating the molecules into toxicity-resistant differentiated cells such as trichomes. This is the case of cannabinoids produced by Cannabis sativa (Schachtsiek et al. 2018).
Already about 30 years ago, Zenk (1991) proposed a number of general rules for improving by plant cell cultures the production of phytochemicals of interest. These “guidelines” have been followed by several researchers and basically are still valid. In the following paragraphs the different steps leading to achievement of the objective (production yield increase) will be updated and illustrated in more detail with the help of examples and comments about merits and limits of each operational step.
-
1.
Identification of the starting material (individual plant, organ or plant tissue) containing the largest amount of the secondary metabolite to be extracted and thus theoretically susceptible to give origin to more productive in vitro cultures. Once the best starting experimental material has been identified and/or selected, various explants (leaf-disks, stem segments, roots, seeds, etc.) can be sterilized and grown on semi-solid culture medium.
-
2.
Identification of culture medium and of experimental conditions favoring optimal growth and secondary metabolite synthesis. Most of the components of a culture medium (micro- and macronutrients, source of carbon nitrogen, vitamins and growth regulators) and growing conditions (light, temperature, oxygen) influence biomass growth rate and level of accumulation of secondary metabolites. Nitrogen concentration obviously influences the amount of amino acid and protein in cultured calli or suspension cells. Plant tissue culture media contain both ammonium and nitrate as sources of nitrogen and their ratio can be modified to enhance phytochemical production (Efferth 2018). The phosphate concentration in the medium has a major effect on the production of secondary metabolites in plant cell cultures. In the case of Solanum laciniatum, high phosphate levels enhanced cell growth, but negatively affected the production of secondary metabolites (Chandler and Dodds 1983). However, there are exceptions to this rule: for instance, an increased phosphate level was shown to stimulate the synthesis of Digitoxin in Digitalis purpurea (Corchete et al. 1990) and of Betacyanin in Chenopodium rubrum (Berlin et al. 1986). Conversely, reduced phosphate levels induced production of Ajmalicine and phenolics in Catharantus roseus (Balathandayutham et al. 2008), of Caffeoyl Putrescines in Nicotiana tabacum (Schiel et al. 1984) and of Harman alkaloids in Peganum harmala (Sasse et al. 1982).
In addition, the concentrations of auxins (IAA, 2,4-D, NAA, etc.) and citokynines (6-BAP, Kinetin, Zeatin), the hormones which stimulate cell expansion and division, are obviously crucial factors for the success of in vitro culture of plant cells and, therefore, they should be established for each plant since different species may behave differently (Efferth 2018).
Similarly, the ratio between these two hormones is very important in determining whether cells will remain in a completely undifferentiated state or will tend to differentiate leaf primordia or roots. Therefore, preliminary experiments are required to define the hormonal conditions favoring the highest yield of phytochemical accumulation. For example, the in vitro production of phenylpropanoids by blueberries (Vaccinum corymbosum) cells in temporary immersion bioreactors (TIBs) was optimized by adjusting hormonal conditions and reducing the amount of sugar to obtain photomixotrophic growth and partial differentiation. (Arencibia et al. 2018). Likewise, photomixotrophic growth of sunflower (Heliantus annuus) suspension cultures was obtained modulating light intensity, sugar concentration and age of culture. Under these conditions, the accumulation of α-tocopherol increased up to 230% that of heterotrophic cell cultures (Geipel et al. 2014).
The production of secondary metabolites was also found to be influenced by light, temperature, pH and oxygen concentration. However, it is difficult to predict how variations of the the first parameter affect phytochemical synthesis in different plant species. For instance, it was found that light influenced positively the accumulation of anthocyanins and sesquiterpenes in, respectively, carrot suspension cultures (Dougall et al. 1980) and Matricaria chamomilla (Tatli 2012). On the contrary, dark conditions stimulate the production of the sesquiterpenoid Zerumbone in Zingiber sp. cell cultures Rajkumpari and Sanatombi 2018).
Temperatures ranging from 17 °C to 25 °C were also found to stimulate or inhibit the production of secondary metabolites in cultures of different species. Low temperatures favored the production of alkaloids in C. roseus cell cultures (Courtois and Guern 1980), while high temperatures favored the formation of Ubiquinone in tobacco cell cultures (Ikeda et al. 1977).
Finally, oxygen concentration and stirring, as well as gaseous composition, are particularly crucial for large-scale production. For example, a dissolved oxygen level of 50% allowed a production of alkaloids of about 3 g/l of culture (Courtois and Guern 1980), while carbon dioxide stimulated the synthesis of monoterpenes in grape cell cultures (Ambid and Fallot 1981), prevented the browning of cells and supported the production of Berberine in Thalictrum minus (Kobayashi et al. 1991).
-
3.
Addition to in vitro cultures of precursors, intermediates, inhibitors or of other means that can drive and speed up the metabolic flow leading to the phytochemical of interest. A well-known case of this notion is the addition of Phenylalanine, a precursor of phenolic compounds, to suspension cultures of Coleus blumei and Salvia officinalis in order to increase the production of rosmarinic acid (Koca and Karaman 2015), and to suspension cultures of Taxus cuspidata to enhance the synthesis of N-benzoilphenylisoserine, an intermediate in the synthesis of paclitaxel (Fett-Neto et al. 1994).
Another example of “precursor feeding” regards the production of Vanillin, an aroma molecule of relevant economic interest: it has indeed been reported that the addition of Ferulic acid to cultures of Vanilla planifolia boosts Vanillin accumulation (Romagnoli and Knorr 2009).
An “indirect” way to increase the synthesis of phytochemicals was reported in the case of Artemisinin, a sesquiterpene endowed with effective antimalarial activity. Artemisia annua L. cell suspension cultures were added with Miconazole (200 mM), an inhibitor of sterol biosynthesis, a pathway competing with that of sesquiterpenes. HPLC analysis showed that after a 24 h treatment, the level of Artemisinin was 2.5 times higher than that of untreated cultures. Moreover, the addition of Miconazole was found to induce (after 30 min) the expression of CPR gene encoding cytochrome P450 reductase and (after 4 h) that of DBR2 gene encoding artemisinic aldehyde reductase (Caretto et al. 2011).
Failure to accumulate the metabolite of interest in cultured cells may be due either to feedback-inhibition of key biosynthetic enzymes or to non-enzymatic degradation of the product in the culture medium. In this regard, it has been observed that the addition to the culture medium of a solid or liquid phase that adsorbs and/or sequestrates the product of interest increases its production by creating an artificial “accumulation compartment”. For example, the addition of activated carbon to Matricaria chamomilla cultures allowed a 20- to 60-fold increase in the amount of Coniferic aldehyde (Beiderbeck and Knoop 1984). The use of XAD-7 amberlite resin also allowed a 15-fold increase in Anthraquinone production by Cinchona ledyeriana cell cultures (Robins and Rhodes 1986).
-
4.
Addition of elicitors of secondary metabolite synthesis. Elicitors are molecules of various origin and structure that are formed following the interactions between plant cells and biotic or abiotic stresses. These molecular signals are recognized by plant cells leading them to react with the synthesis of secondary metabolites that alleviate the stress and, in some cases, possesse pharmaceutical or nutraceutical properties (Ramirez-Estrada et al. 2016).
The addition to the culture medium of elicitors of fungal and bacterial origin or of heavy metal salts stimulates the production of various plant secondary metabolites. For example, the addition of Botrytis homogenate to cell suspensions of Papaver somniferum increases the content of Sanguinarine up to 3% of the dry cell weight (Bobak et al. 1995). In the case of transformed root cultures of Datura stramonium, it was observed that heavy metals (copper and cadmium) caused an increase of defense sesquiterpenoids (Furze et al. 1991).
Jasmonic acid or its derivative methyl-jasmonate represent another kind of elicitors. These regulatory molecules stimulate secondary metabolism, thus adapting plants to environmental changes by the action of newly synthesized phytochemicals that increase the fitness of the species. This approach was used with cell cultures of Artemisia annua L that were challenged with 22 µM Methyl Jasmonate eliciting a threefold increase of the Artemisinin content after 30 min and a simulatneous sevenfold increase in the expression of CYP71AV1 gene (cytochrome P 450) (Caretto et al. 2011).
-
5.
Selection of the most productive lines. Mutation strategies have been adopted to obtain highly productive cell lines. The use of selective agents is an alternative approach to select lines characterized by a high content of a given secondary metabolite by exposing a cell population to an inhibitor or to an environmental stress the cytotoxic effects of which are lessened by the presence of the metabolite. In these conditions, only cells that resist the selection procedure by producing the desired metabolites at a higher rate will survive and grow. For instance, mutations that lead to phenylalanine overproduction avoids the incorporation of the analog P-fluorophenylalanine (PFP) into cell protein thus averting cell death. Such overproduction depends on increased amount and activity of Phenylalanine ammonia lyase, the key enzyme of phenolic metabolism that stimulates the metabolic flux towards phenolic compounds among which many flavonoids of pharmaceutical or nutraceutical interest (Howles et al. 1994). Therefore, PFP has been widely used to select mutant cell lines showing a high-production rate of the above cited classes of phenolics. For example, it has been reported that the selection of Capsicum annuum cell lines resistant to PFP allowed to remarkably increase the production of Capsaicin (Salgado-Garciglia and Ochoa-Alcjo 1990).
-
6.
Cell immobilization. In some cases, it is convenient to resort to cell immobilization in gels (mostly sodium or calcium alginate), entrapping the cells at high density and thus favoring an increased synthesis of phytochemicals. This approach avoids cell damage due to mechanical agitation of the culture and allows regular withdrawal of an aliquot of the culture medium permitting the removal of products as well as inhibitors. The above described strategy has been employed, although not at industrial level, for producing Vanillin, Ajmalicine and Capsaicin (Rao and Ravishankar 2002).
-
7.
Cell permeabilization. In most cases the secondary metabolites are stored in the vacuole and, therefore, a permeabilization step may be applied to facilitate their release and subsequent recovery from the culture medium without cell disruption. This step consists in the transient formation of pores in the membranes (tonoplast and plasmalemma), thus facilitating the release of phyochemicals in the culture medium. A series of agents (Chitosan and surfactants such as Tween, Triton, SDS) or physical methods (ultrasonication, electroporation and iontophoresis) can be used to this end (Zhao et al. 2014).
-
8.
Scale-up through the use of appropriate bioreactors. On a large scale, the growth of biomass and the production of secondary metabolites depend also on additional factors such as the dimension of the inoculum and most of all aeration and agitation rate that may influence the scale up success. In the case of suspension cultured plant cells, special bioreactors are used, most of which are stirred tank reactors and gas sparged reactors (Weathers et al. 2010; Steingroewer et al. 2013). Since purchase and operating costs of bioreactors are central in the large-scale production of phytochemicals, the choice of the bioreactor is crucial for economic profitability. Therefore, in attempts to maximize production at a reduced cost, different types of bioreactors have been designed including mechanical bioreactors, air/bubble bridge, fixed bed, mixed devices and single-use bioreactors (Eibl and Eibl 2008). The choice of the production process is further influenced by the type of culture (cell suspension vs. hairy roots), by the possible requirement of lighting devices and also by the mode of product accumulation. In fact, as already mentioned, many secondary metabolites accumulate in the vacuole, making it necessary to extract the molecules contained in the vacuole with various solvents. For these phytochemicals, a “batch” culture process is advisable, i.e. the cells are grown in suitable bioreactors in the environmental conditions previously set up at a laboratory scale to maximize the synthesis and accumulation of the product of interest, then are collected and mechanically broken in order to extract with various solvents the molecules contained in the vacuole. In the case of compounds that are secreted in the culture medium, a continuous growth process can be used by which a suitable amount of medium is withdrawn and filtered at intervals and replaced with an equal quantity of “fresh” culture medium. Consequently, no extraction is required: the metabolite of interest can be directly purified from the spent medium while the culture is maintained in stable and productive growth phase (Chattopadhyay et al. 2004).
As concerns hairy root cultures, in order to be grown in large amount, they necessitate of peculiar bioreactions designed to alleviate the mechanical stress produced by stirring and aerating the massive cultures. Indeed, while suspension cultured cells generally tolerate this mechanical stress, roots are more susceptible, and growth is impaired. Moreover, because of their branched mass, some measures are necessary to avoid the blockage of the rotating parts. So, mist reactor (in which hairy roots are hung on a support and grow immersed not in the liquid, but in a mist generated from the liquid medium) and roller drum reactor have been employed successfully, as reported and illustrated in the review by Georgiev et al. (2013).
Emerging topics
Cambial meristemoid cells
The isolation of cambial meristemoid cells (CMCs) from the cells of the procambium has recently been achieved with various plants such as Taxus cuspidata, Ginseng panax, Ginkgo biloba and Solanum lycopersicum (Lee et al. 2010). These cells, which are naturally undifferentiated, grow indefinitely and function as “plant stem cells” (Ochoa-Villarreal et al. 2015; Lee and Kim 2014). Therefore, they have the potential to elude many of the current drawbacks of dedifferentiated cultured cells and hairy roots. Moreover, CMCs can stably carry out the synthesis of secondary metabolites. Further useful traits of CMCs are the reduced size of cell aggregates (2–3 cells/cluster) that facilitates the response to various elicitors, high tolerance to the mechanical stress endured in bioreactor and, above all, the ability to release phytochemicals in the culture medium. This facilitates product recovery and avoids feedback inhibition of the metabolic pathway involved. CMCs of Catharanthus roseus have been recently used to produce vindoline, catharanthine and ajmalicine (Moon et al. 2015; Zhou et al. 2015) while CMCs of P. ginseng were successfully used to produce ginsenosides at an industrial level (Ochoa-Villarreal et al. 2015; Lee et al. 2010).
Mutants selected by expressing in plant cell genes encoding human target proteins
This emerging topic has been recently explored by Brown et al. (2016), who described a novel experimental system aiming at manipulating plant cells to re-direct their metabolism towards producing metabolites that interact with human target proteins. This can be achieved by producing in plant cells human target proteins whose inhibition may be of “therapeutic” interest, followed by the selection of mutant cells whose altered metabolome allows to overcome the inhibiting action of the inhibitory target protein. Applying this strategy to cultured hairy roots of a Lobelia species, Brown and colleagues selected mutants capable of increased biosynthesis of a metabolite that inhibits the human dopamine transporter. Many of the selected mutants were shown to produce an excess of the active metabolite found in the wild plant, but others overproduced novel active metabolites not readily detectable in control cells. This technology can probe the entire genomic capacity of a plant species and exploit its potential to synthesize secondary metabolites interfering with a specific target of therapeutic interest. Therefore, it has a potential for the identification of plant pharmaceuticals, or to optimize the therapeutic value of medicinal plant extracts.
Eco-friendly bioextraction of phytochemicals from plants and in vitro tissue cultures
A major challenge is the identification of the best extraction conditions, i.e. the conditions that can overcome the problems relative to the release of phytochemicals from a vegetable matrix. Conventional methods commonly described in the literature are based on the use of liquid–liquid or solid–liquid extraction processes primarily using organic solvents the manipulation of which might be economically and environmentally unsustainable (Prado et al. 2015). A recent trend is indeed focused on finding solutions that minimize the use of organic solvents. The efficiency of extraction is a key issue since it can influence the yield and consequently, the economic sustainability of the process (Smith 2003). Much work has been dedicated to the improvement of extraction processes based on technologically advanced but costly and energy-demanding equipments. These methods include subcritical water extraction (Luque-Rodriguez et al. 2006), supercritical fluid extraction (SFE) (Wang et al. 2008), ultrasound-assisted extraction (Chukwumah et al. 2009), microwave-assisted extraction (Moreira et al. 2012), pulsed electric field extraction (Segovia et al. 2015), pressurized fluid extraction (Herrero et al. 2015), counter-current extraction (Solana et al. 2016). In addition, combinations of different extraction technologies are under investigation (Loyola-Vargas and Ochoa-Alejo 2018). These ongoing studies will surely contribute to extract phytochemicals from fruit and vegetable wastes, thereby attaining fundamental goals of the circular economy.
Compared to the above mentioned techniques, “enzyme assisted extraction” is an emerging eco-friendly alternative to conventional solvent-based and complex physical methods, since it offers several benefits: rapidity, maximal yield, minimal solvent and energy consumption (Wijesingh and Jeon 2012; Chandini et al. 2011; Khandare et al. 2011).
The principle of this methods is based on the fact that plant cells are surrounded by a resistant cell wall made of complex biopolymers such as cellulose, hemicellulose, pectin and lignin that are rather recalcitrant to the attack of solvents of any type, thus hindering the leakage of phytochemicals. In fact, the treatment of plant materials with enzymatic mixtures, containing cellulases, hemicellulases and pectinases produced industrially by growing ligno-cellulosytic microorganisms promotes the hydrolytic degradation of cell wall polymers (Khandare et al. 2011; Puri et al. 2012). The use of cell wall degrading enzymes does not pose any environmental issue or require sophisticated equipment; nevertheless, it loads the extraction process with additional costs. Therefore, research is underway to discover less expensive methods, for instance the usage of engineered bacterial strains over-producing plant-degradative enzymes directly inside the plant biomass used as a bacterial feedstock, or from which it is possible to extract the overproduced enzymes at low cost.
Genetic engineering aimed at enhancing or improving the synthesis of phytochemicals
The genetic manipulation strategy is also rather multifaceted. Progresses in the field of in vitro culture of plant cells and organs and of the ability to introduce and modify genes in plants has opened the possibility to use successfully this approach to produce metabolites of interest thus further widening the possibility of increasing and improving the production of nutraceutical and therapeutic molecules both in planta and in vitro. This strategy is particularly important whenever there are difficulties in obtaining the plant material containing the compounds of interest or in obtaining them by chemical synthesis.
The demonstration of the feasibility of plant transformation occurred over three decades and half ago (Herrera-Estrella et al. 1983), opening the way to plant genetic engineering techniques allowing the modification of agronomic and qualitative traits leading to the cultivation in many Countries of transgenic crops (https://www.isaaa.org/inbrief/pdf/isaaa-brochure.pdf). In parallel, the increasing knowledge of metabolic pathways and their regulation along with genomic, transcriptomic and metabolomics approaches have paved the way to the planning of biotechnological modifications aimed at both quantitative and qualitative improvements of the synthesis of the phytochemicals of interest and of their production at the industrial level.
Figure 2 schematizes the main strategies adopted to alter metabolic pathways and improve phytochemical production. (Oksman-Caldentey and Inzé 2004):
Strategies developed to alter metabolic pathways and improve phytochemical production. PM primary metabolite, P phytochemical of interest, A, B, C, intermediate pathway metabolites, Y new metabolite, Z unwanted product of a pathway branch, nE foreign recombinant enzyme. The production of the desired phytochemicals, P or Y can be obtained using the following strategies: (1) gene insertion (GI) by nuclear transformation of genes encoding either novel or modified enzymes, or of a transcription factor; (2) gene insertion by genome editing (GEi); (3) introduction of a gene-silencing construct (GS); (4) gene disruption by genome editing (GEd); (5) increased accumulation of a pathway enzyme due to overexpression of the relevant gene (SGOE); (6) increased accumulation of a transcription factor as a result of ectopic overexpression of the relevant gene (TFOE) an event that can contribute to augment to content of most or all pathway enzymes; (7) production of a new phytochemical Y due to the accumulation of a recombinant enzyme that uses the pathway intermediate B as substrate (nE). This can be obtained either by GI or GEi; (8) reduced content of an enzyme that diverts a pathway intermediate to phytochemical Z, thus reducing the accumulation of the final product P. This result can be obtained by gene silencing (GS) or gene disruption by genome editing (GEd). The red arrow indicates the increase in final product concentration
-
gain-of-function of a gene of the pathway;
-
loss-of-function of a gene of the pathway;
-
ectopic expression of transcription factors (TFs) responsible for expressing all or most pathway genes;
-
over-expression of a single gene encoding a rate-limiting enzyme.
The latter strategy, however, has not been always successful since in a complex pathway it is difficult to single out an enzyme responsible of the metabolic flux, which rather is the consequence of the interaction of different components and for this reason the use of TFs has been studied (Grotewold 2008).
There are several early reports of successful examples of these approaches that have been extensively reviewed over the years (Dixon and Steele 1999; Forkmann and Stefan Martens 2001; Verpoorte and Memeling 2002; Hashimoto and Yamada 2003; Ncube and Van Staden 2015; Tatsis and O’Connor 2016). Consequently, only selected examples will be reported in this review. For instance, the introduction in Atropa belladonna of a gene encoding hyoscyamine 6β-hydroxylase from Hyoscyamus niger under the control of a constitutive promoter (pCaMV-35S) caused the almost complete conversion of hyoscyamine into scopolamine, which is a more valuable drug (Yun et al. 1992). Likewise, when the same gene was expressed constitutively in H. muticus (Egyptian henbane) a remarkable increase of tropane alkaloids was observed in transformed clones with a scopolamine content up to 100 times higher than that of controls. Interestingly, in the hairy root clones the extent of conversion of hyoscyamine to scopolamine was proportional to the expression of the transgene (Jouhikainen et al. 1999).
A biolistic approach was used for the constitutive expression in Nicotiana tabacum of two H. niger cDNA sequences encoding tropinone reductase and hyoscyamine-6b-hydroxylase. Besides the expected products, the detached leaves of regenerated plants produced acetylated tropine. An increased content of nicotine was also observed along with nicotine-related compounds (anatabine, nornicotine, bipyridine, anabasine and myosmine) which are undetectable in untransformed control plants (Rocha et al. 2002). The alteration of the nicotine biosynthetic pathway following the introduction of foreign genes producing metabolic alteration is reminiscent of the combinatorial biochemistry effect described by Laurila et al. (1996). These authors observed that somatic hybrids between Solanum tuberosum and S. brevidens produced a novel glycoalkaloid, Demissidine, which was not present in the parental species, possibly due to the activity of the hydrogenase contributed by S. brevidens on Solanidine molecules synthesized by S. tuberosum. In the case of N. tabacum (Rocha et al. 2002), the notable difference is that the two enzymes were produced by genetic engineering rather than by complementation of pre-existing ones.
Once more, a H. niger gene encoding hyoscyamine-6 β-hydroxylase was expressed in hairy roots of N. tabacum and H. muticus. These species differ with respect to the synthesis of hyoscyamine, which is produced in H. muticus, but not in tobacco. Interestingly, it was observed that tobacco hairy roots were far more efficient in bio-transforming and releasing scopolamine and other alkaloids in the culture medium when hyoscyamine was exogenously supplied (Häkkinen et al. 2005).
Although biofortification is out of the scope of this review, it is of interest to analyze some of the results obtained by genetic engineering of metabolic pathways that can be considered paradigmatic. A milestone in the development of biofortified food by metabolic engineering was established by Potrykus’ and Beyer’s groups with the development of Golden Rice that is characterized by the production of β-carotene in the endosperm through the ectopic expression of foreign genes encoding enzymes of the pathway (phytoene synthase, phytoene desaturase and lycopene cyclase) under the control of endosperm-specific and constitutive promoters (Ye et al. 2000). A follow-up of this approach was the development of Golden Rice 2 where the gene of Narcissus pseudonarcissus encoding phytoene synthase, which was found to be responsible for the poor accumulation of β-carotene, was substituted with a maize orthologue gene that allowed to increase up to 23-fold the content of pro-vitamin A (Paine et al. 2005).
Similarly, in order to obtain biofortified potato tubers, three bacterial genes encoding phytoene synthase, phytoene desaturase and lycopene β-cyclase were expressed under the control of tuber-specific promoters. The resulting Golden tubers, which were deep yellow, featured a dramatic increase of β-carotene (Diretto et al. 2007). The analysis of the complex relationship between levels of expression of foreign and endogenous genes of the carotenoid pathway and carotenoid accumulations in transgenic leaves and tubers was analyzed in a subsequent paper using a hierarchical clustering and pairwise correlation analysis along with a novel network correlation tool (Diretto et al. 2010). Results of this study point out the complex regulation of metabolic pathways and the uncertain outcome of attempts aiming at increasing the content of phytochemicals of interest by altering the expression of only one or few genes of the pathway, an issue that will be addressed below when analyzing the overexpression of transcription factor genes.
Downregulation of the activity of the gene encoding putrescine N-methyltransferase (PMT) was used to alter the content of pyridine alkaloid of N. tabacum (Chintapakorn and Hamill 2011). In untransformed lines, nicotine is prevalent while anatabine, which is used as an anti-inflammatory and against nicotine addiction, is present at low levels. The transformed root lines showing a drastic reduced PMT activity were similarly characterized by a reduced nicotine content and a considerable increase of anatabine. According to authors, the reduced PMT activity favors the accumulation of nicotinic acid which is then directly transformed in anatabine.
The role played by miRNAs in the regulation of eukaryotic gene expression has been proven in recent years and this recognition has stimulated the analysis of their role also in the biosynthesis of phytochemicals. In the case of Papaver somniferum, the role of miRNAs in the synthesis of benzylisoquinoline alkaloids (BIA) (Boke et al. 2015) has been analyzed in depth using molecular tools (second-generation sequencing and direct cloning). Out of 327 miRNAs present in opium poppy, among which 11 novel ones, this study has identified three miRNAs possibly involved in BIA synthesis and three additional putative ones. The issue concerning the identification of miRNAs and of the role that they play in the biosynthesis of phytochemicals has been recently addressed (Gupta et al. 2017). In this review, authors discuss the possible use of this approach for improving the production of plant secondary metabolites.
Virus-induced gene silencing (VIG) is a molecular tool that uses engineered viral genomes to target specific plant host genes (Lu et al. 2003). In the past 15 years this mechanism has been widely used as a functional genomics tool to understand the role of host genes in abiotic and biotic stresses (Ramegowda et al. 2014; Zhang et al. 2016). VIG was used to silence the expression of a gene for ornithine decarboxylase (ODC) of N. tabacum using both Agrobacterium rhyzogenes and A. tumefaciens transformation systems. ODC-silenced hairy roots showed an increased anatabine content and a reduced level of nicotine (DeBoer et al. 2011). A similar alteration of the pyridine alkaloid profile was observed in regenerated tobacco plants. Notably, treatments that stimulate nicotine synthesis in untransformed plants such as the addition of methyl jasmonate to hairy roots or decapitation of transgenic plants resulted in a significant increase of anabatine content.
A virus-induced gene silencing approach was used to analyze the actual role of six genes of Papaver somniferum whose relevant products were supposedly involved in the biosynthesis of papaverine. Only the genes encoding coclaurine N-methyltransferase, (S)-3′hydroxy-N-methylcoclaurine 4-O-methyltransferase and norreticuline 7-O-methyltransferase were shown to be involved in papaverine biosynthesis. This can be regarded as a significant achievement since biochemical studies had failed to provide conclusive evidence concerning the pathway. Remarkably, the papaverine content was found to increase following the silencing of the first gene while decreased following the silencing of the other two. Interestingly, this silencing approach enabled to establish that papaverine biosynthesis does not involve (S)-reticuline but implicates N-desmethylated compounds (Desagagné-Penix and Facchini 2012). The importance of a combined use of biochemical, molecular genetics and functional genomic tools to better understand the biosynthesis of benzylisoquinoline alkaloid in opium poppy was comprehensively discussed by Beaudoin and Facchini (2014).
A breakthrough in the development of metabolic engineering aimed at the alteration of the biosynthesis of phytochemicals has been the ectopic overexpression of genes encoding transcription factors, which can activate all at once several genes of the metabolic pathway instead of varying the expression of one only. Indeed, transcription factors have shown to be new molecular tools for plant metabolic engineering, in particular when aiming at increasing the production of valuable compounds (Gantet and Memelink 2002).
One of the first successful attempts was the overexpression of ORCA3 gene from Catharanthus roseus, an APETALA2-domain transcription factor responsive to jasmonate, which was identified by activation tagging as a master regulator of terpenoid indole alkaloids. The overexpression of this gene in the tagged cell line resulted in an augmented level of these alkaloids thanks to the enhanced expression of several genes of the pathway (Van der Fits and Memelink 2000).
An increased content of flavanols in tomato fruits was achieved by engineering tomato plants with transcription factor (LC and C1) genes from maize. The ectopic expression of these two genes allowed the upregulation of flavonoid pathway genes and the accumulation in berry flesh of these antioxidant compounds (Bovy et al. 2002).
Another notable example was the engineering of the “purple” tomato that is characterized by the accumulation of anthocyanins in berry cells (Butelli et al. 2008). This was achieved by expressing two genes of Antirrhinum majus encoding the transcription factors Delila and Rosea1. The purple color of the berries resulted from an increased content of hydrophilic anthocyanins that caused a threefold increase of their antioxidant capacity. Remarkably, when knockout mice with a reduce content of p53, which develop tumors precociously, were subjected to a diet supplemented with purple tomatoes powder, their life expectancy increased significantly.
These encouraging results have emphasized the need for the identification of transcription factors genes controlling secondary metabolic pathways. In the case of Chenopodium quinoa, a genome sequencing approach contributed to the identification of master genes allegedly controlling the production of triterpenoid saponins that accumulate in seeds and act as anti-nutritional compounds, a piece of information useful to improve the nutritional value of this crop (Jarvis et al. 2017). In addition to widening the general knowledge of plant genetic complexity and species phylogenesis, genomics can contribute to improve the production of phytochemicals. For instance, genome sequencing and transcriptomic analysis of Capsicum hot pepper indicates how the neofunctionalization of capsaicin synthase has influenced the biosynthesis of capsaicinoids (Kim et al. 2014). Additional examples of the effectiveness of this approach for understanding the evolution of phytochemical biosynthetic pathways have been reported for other species such as Coffea canephora and Olea europea (Denoeud et al. 2014; Unver et al. 2017). In the case of C. canephora, it was observed that several gene families—among which those encoding N-methytransferases and alkaloid and flavomoid enzymes, underwent gene expansion to produce the present secondary metabolism. Similarly, genome annotation of wild olive revealed that events of duplication, expansion and neofunctionalization of gene and gene families involved in oil biosynthesis accounts for the increased content of unsaturated fatty acids (oleic and linoleic acids) compared to sesame. Although these reports are not strictly related to the production of the recognized phytochemical classes, they emphasize the need of a broader genomic knowledge to modify the secondary metabolic pathways of interest.
Recently, genome editing technologies based on zinc finger nucleases (ZFNs), transcription activator-like endonucleases (TALENs) and clustered regularly interspaced short palindromic repeats along with protein 9 endonuclease (CRISPR/Cas9) have been extended to plants (Petolino 2015; Chen et al. 2014a, b; Doudna and Charpentier 2014; Bortesi and Fischer 2014; Norman et al. 2016; Belhaj et al. 2016) and used successfully for modifying agronomic traits of some crop species (Pennisi 2016). Besides the remarkable site-specificity of DNA modification, an important advantage of this approach derives from the fact that, when editing is limited only to gene disruption, edited plants may not be subjected to the strict and costly procedure required by current regulatory standards for transgenic plants.
As foreseeable, genome editing has been used for engineering secondary metabolic pathways. The knocking out of 3′-hydroxyl-N-methylcoclaurine 4′-O-methyltransferase (4′ OMT2) of Papaver somniferum was obtained by non-homologous end-joining by using a CRISPR/SpCas9 type II approach. Edited plants were characterized by a reduced content of morphine and thebaine and the appearance of a novel benzylisoquinoline alkaloid (Alagoz et al. 2016).
A multiplex pYLCRISPR/Cas9 system, which allows to generate multiple DNA breaks thus targeting several genes at once, was used to increase the tomato content of γ-aminobutyric acid (GABA), molecule that has received most attention due to its properties as a nutraceutical and as a neurotransmitter inhibitor (Li et al. 2018). When five genes of GABA metabolism were targeted, genome-edited plants were found to have up to four mutated genes. Remarkably, the GABA level in leaves of quadruple mutants was 19-fold higher than that of control plants. These results indicate that multiplex CRISPR/Cas9 genome editing represents a promising method to modify plant secondary metabolite pathways.
It has been anticipated that the use of synthetic biology for engineering microbial metabolic pathways might meet the increasing request of phytochemicals independently of environmental and seasonal conditions that could limit the growth of crop species. With the respect to the production of polyphenols, this approach has been envisaged as an alternative to phytochemical extraction from native plants. Yet, it was recently reported that this strategy has not fully met the expectations for phytochemical production (Chouhan et al. 2017).
However, not all scientists are convinced that microbial engineering might be an alternative for producing phytochemicals and that new approaches to engineer plant metabolic pathways could provide better results. In a recent review Fu et al. (2018) consider an ancient, ingenious and self-regulating Chinese irrigation system built over 22 centuries ago on the Minjang river as a paradigm for developing new metabolic engineering strategies. The three components of the irrigation system that influence flow and distribution of waters are considered similar to those acting in a metabolic pathway. Based on these considerations, it is foreseen that an improved production of the desired metabolites will be obtained developing new strategies by carefully playing with the various components of the system: introgression of biosynthetic genes, appropriate choice of promoters and transcription factors and elimination of branching pathways along with the modification of biochemical conditions with respect to energy and reducing power.
Conclusions
Plants can be regarded as natural chemical factories due to their extraordinary ability to react to environmental cues by synthesizing a large variety of secondary metabolites, many of which are endowed with biological activities of pharmaceutical, nutraceutical or cosmeceutical interest and can be successfully used to protect human health and well-being. For several reasons, among which the need to spare agricultural soil for edible crops and avoid the risk of extinction of rare medicinal plants, in the past years attempts have been made to use in vitro culture as an alternative to plants for phytochemical production. This effort has stimulated the development of new cell and tissue culture methods required for the industrial production of the phytochemicals of interest. Nevertheless, a more advanced knowledge of physiological, biochemical and environmental factors controlling the synthesis and accumulation of the desired phytochemicals in plants and in in vitro cultures is needed. For example, plant stem cells can be very useful to bypass many of the limitations associated with the use of dedifferentiated cell cultures and hairy roots for secondary metabolites’ production. It is expected that these undifferentiated cells following the treatment with suitable elicitors, the application of metabolic precursors or of appropriate stressful environmental conditions, might improve significantly the production of the phytochemicals of interest.
In addition, the progress made with regards to phytochemical extraction methods such as permeabilization, cellular immobilization and improved bioreactor designs will contribute to increase the phytochemical production yield.
Moreover, the remarkable advancements made in the past years in functional genomics and metabolomics along with the availability of valid transformation protocols as well as silencing and genome-editing methods, suggest that significant advances will allow the modification of metabolic pathways of cultured plant cell and organs resulting in an enhanced production of drugs and nutraceuticals. This will contribute to an increasingly standardized production of specific, valuable and new phytochemicals meanwhile avoiding the risk of extinction of rare medicinal plants that represent an invaluable biological resource.
Author contribution statement
The three authors contributed similarly to the ideation, planning, articles research and drawing up of the text.
References
Alagoz Y, Gurkok T, Zhang B, Unver T (2016) Manipulating the biosynthesis of bioactive compound alkaloids for next-generation metabolic engineering in opium poppy using CRISPR–Cas 9 genome editing technology. Sci Rep 6:30910. https://doi.org/10.1038/srep30910
Alavizadeh SH, Hosseinzadeh H (2014) Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol 64:65–80. https://doi.org/10.1016/j.fct.2013.11.016
Ambid C, Fallot J (1981) Role of the gaseous environment on volatile compounds production by fruit cell suspension cultures in vitro. In: Scherir (ed) Proceedings of the international conference on flavour, 3rd Weurman symposium De Gruyter, Berlin, New York, pp 529–538
Arencibia AD, Aleydis Gómez A, Mora P, Orellana F, Alarcón JE (2018) Photomixotrophic cultures of blueberries (Vaccinium corymbosum) accumulate or release phenylpropanoids via inductive treatments. Acta Physiol Plant 40:36. https://doi.org/10.1007/s11738-018-2615-x
Aronson JK (2017) Defining nutraceuticals: neither nutritious nor pharmaceuticals. Br J Clin Pharmacol 83:8–19. https://doi.org/10.1111/bcp.12935
Baiano A (2014) Recovery of biomolecules from food wastes—a review. Molecules 19(9):14821–14842. https://doi.org/10.3390/molecules190914821
Baikenova GG, Abdulina GA, Gazaliev AM, Fazylov SD, Kudaibergenova SZ (2004) Synthesis and antimicrobial activity of anabasine, piperidine, and morpholine dithiocarbamates. Pharm Chem J 38(1):19–20. https://doi.org/10.1023/b:phac.0000027638.24327.bd
Balathandayutham K, Cheruth AJ, Changxing Z, Melvin JM, Jothi S, Muthukumar D (2008) The effect of AM fungi and phosphorous level on the biomass yield and ajmalicine production in Catharanthus roseus. Eur J Biosci 2:26–33
Balažová A, Urdová J, Bilka F, Holková I, Horváth B, Forman V, Mučaji P (2018) Evaluation of manganese chloride’s effect on biosynthetic properties of in vitro cultures of Eschscholzia californica Cham. Molecules 23:971. https://doi.org/10.3390/molecules23040971
Bauer N, Vuković R, Likić S, Jelaska S (2015) Potential of different Coleus blumei tissues for rosmarinic acid production. Food Technol Biotechnol 53:3–10. https://doi.org/10.17113/ftb.53.01.15.3661
Beaudoin GA, Facchini PJ (2014) Benzylisoquinoline alkaloid biosynthesis in opium poppy. Planta 240:19–32. https://doi.org/10.1007/s00425-014-2056-8
Beiderbeck R, Knoop B (1984) Ein adsorbens als Speicher fur phytohormone in einer pflantzlichen suspensionkulture. Z Naturforsch. 39c:45–49. https://doi.org/10.1515/znc-1984-1-208
Belhaj K, Chaparro-Garcia A, Kamoun S, Patron NJ, Nekrasov V (2016) Editing plant genomes with CRISPR/Cas9. Curr Opin Biotechnol 32:76–84. https://doi.org/10.1016/j.copbio.2014.11.007
Berlin J, Sieg S, Strack D, Bokern M, Harms H (1986) Production of betalains by suspension cultures of Chenopodium rubrum L. Plant Cell Tissue Org Cult 5:163–174. https://doi.org/10.1007/bf00040126
Bobak M, Nádaska M, Samaj J, Blehova A, Erdelsky K, Ovecka M, Kristín J (1995) The influence of elicitation on the subcellular localization and content of sanguinarine in callus cells of Papaver somniferum L. Biol Plant 37(4):501–506. https://doi.org/10.1007/bf02908827
Boke H, Ozhuner E, Turktas M, Parmaksiz I, Ozcan S, Turgay Unver T (2015) Regulation of the alkaloid biosynthesis by miRNA in opium poppy. Plant Biotechnol J 13:409–420
Bortesi L, Fischer R (2014) The CRISPR–Cas9 system for plant genome editing and beyond. Biotechnol Adv 33:41–52. https://doi.org/10.1016/j.biotechadv.2014.12.006
Bovy A, De Vos R, Kemper M, Schijlen E, Almenar Pertejo M, Muir S, Collins G, Robinson S, Verhoeyen M, Hughes S, Santos-Buelga C, Van Tunen A (2002) High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell 14:2509–2526. https://doi.org/10.1105/tpc.004218
Brown DP, Rogers DT, Gunjan SK, Gerhardt GA, Littleton JM (2016) Target-directed discovery and production of pharmaceuticals in transgenic mutant plant cells. J Biotechnol 238:9–14. https://doi.org/10.1016/j.jbiotec.2016.09.007
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EGWM, Hall RD, Bovy AG, Jie Luo J, Martin C (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26:1301–1308. https://doi.org/10.1038/nbt.1506
Caleja C, Barreiro MF, Ferreira I (2017) Phenolic compounds as nutraceuticals or functional food ingredients. Curr Pharm Des 23:2787–2806. https://doi.org/10.2174/1381612822666161227153906
Caretto S, Quarta A, Durante M, Nisi R, De Paolis A, Blando F, Mita G (2011) Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol 13:51–58. https://doi.org/10.1111/j.1438-8677.2009.00306.x
Chandini SK, Rao LJ, Gowthaman MK, Haware DJ, Subramanian R (2011) Enzymatic treatment to improve the quality of black tea extracts. Food Chem 127:1039–1045. https://doi.org/10.1016/j.foodchem.2011.01.078
Chandler SF, Dodds JH (1983) The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep 2(4):205–208. https://doi.org/10.1007/bf00270105
Chattopadhyay S, Srivastava AK, Bisaria VS (2004) Production of phytochemicals in plant cell bioreactors. Plant Biotechnol Mol Mark. https://doi.org/10.1007/1-4020-3213-7_7
Chen ZY, Jiao R, Ma KY (2008) Cholesterol-lowering nutraceuticals and functional foods. J Agric Food Chem 56:8761–8773. https://doi.org/10.1021/jf801566r
Chen K, Shan Q, Gao C (2014a) An efficient TALEN mutagenesis system in rice. Methods 69:2–8. https://doi.org/10.1016/j.ymeth.2014.02.013
Chen G, Wang H, Zhang X, Yang ST (2014b) Nutraceuticals and functional foods in the management of hyperlipidemia, critical reviews. Food Sci Nutr 54:1180–1201. https://doi.org/10.1080/10408398.2011.629354
Chintapakorn Y, Hamill JD (2011) Antisense-mediated down-regulation of putrescine N-methyltransferase activity in transgenic Nicotiana tabacum L. can lead to elevated levels of anatabine at the expense of nicotine. Plant Mol Biol 53:87–105. https://doi.org/10.1023/b:plan.0000009268.45851.95
Chouhan S, Sharma K, Zha J, Guleria S, Koffas MAG (2017) Recent advances in the recombinant biosynthesis of polyphenols. Front Microbiol 8:22–59. https://doi.org/10.3389/fmicb.2017.02259 (eCollection 2017)
Chukwumah YC, Walker LT, Verghese M, Ogutu S (2009) Effect of frequency and duration of ultrasonication on the extraction efficiency of selected isoflavones andtrans-resveratrol from peanuts (Arachis hypogaea). Ultrason Sonochem 16:293–299. https://doi.org/10.1016/j.ultsonch.2008.07.007
Corchete MP, Sanchez JM, Cacho M, Moran M, Fernandez-Tarrago J (1990) Cardenolide content in suspension cell cultures derived from root and leaf callus of Digitalis thapsi L. J Plant Physiol 137:196–200. https://doi.org/10.1016/s0176-1617(11)80081-7
Costa C, Tsatsakis A, Mamoulakis C, Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis E, Kouretas D, Fenga C (2017) Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem Toxicol 110:286–299. https://doi.org/10.1016/j.fct.2017.10.023
Courtois D, Guern J (1980) Temperature response of Catharanthus roseus cells cultivated in liquid medium. Plant Sci Lett 17:473–482. https://doi.org/10.1016/0304-4211(80)90135-2
DeBoer KD, Dalton HL, Edward FJ, Hamill JD (2011) RNAi-mediated down-regulation of ornithine decarboxylase (ODC) leads to reduced nicotine and increased anatabine levels in transgenic Nicotiana tabacum L. Phytochemistry 72:344–355. https://doi.org/10.1016/j.phytochem.2010.12.012
Denoeud F, Carretero-Paulet L, Dereeper A, Droc G, Guiot R, Pietrella M et al (2014) The coffee genome provides insight into the convergent evolution of caggeine biosynthesis. Science 345:1181–1184
Desagagné-Penix I, Facchini PJ (2012) Systematic silencing of benzylisoquinoline alkaloid biosynthetic genes reveals the major route to papaverine in opium poppy. Plant J 72:331–334. https://doi.org/10.1111/j.1365-313x.2012.05084.x
Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, Giuliano G (2007) Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS One 2:e350. https://doi.org/10.1371/journal.pone.0000350
Diretto G, Al-Babili S, Tavazza R, Scossa F, Papacchioli V, Migliore M, Beyer P, Giuliano G (2010) Transcriptional-metabolic networks in β-carotene-enriched potato tubers: the long and winding road to the golden phenotype. Plant Physiol 154:899–912. https://doi.org/10.1104/pp.110.159368
Dixon RA, Steele CL (1999) Flavonoids and isoflavonoids—a gold mine for metabolic engineering. Trends Plant Sci 4(10):394–400. https://doi.org/10.1016/S1360-1385(99)01471-5
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR–Cas9. Science 346:1258096. https://doi.org/10.1126/science.1258096
Dougall DK, Johnson JM, Whitten GH (1980) A clonal analysis of anthocyanin accumulation by cell cultures of wild carrot. Planta 149:292–297. https://doi.org/10.1007/bf00384569
Efferth T (2018) Biotechnology applications of plant callus cultures. Engineering. https://doi.org/10.1016/j.eng.2018.11.006
Eibl R, Eibl D (2008) Design of bioreactors suitable for plant cell and tissue cultures. Phytoc Rev 7:593–598. https://doi.org/10.1007/s11101-007-9083-z
Fett-Neto AG, Melanson SJ, Nicholson SA, Jason J, Pennington JJ, Di Cosmo F (1994) Improved taxol yield by aromatic carboxylic acid and amino acid feeding to cell cultures of Taxus cuspidate. Biotechnol Bioeng 44:967–971. https://doi.org/10.1002/bit.260440813
Forkmann G, Stefan Martens S (2001) Metabolic engineering and applications of flavonoids. Curr Opin Biotechnol 12:155–160. https://doi.org/10.1016/s0958-1669(00)00192-0
Fu R, Martin C, Zhang Y (2018) Next-generation plant metabolic engineering inspired by ancient Chinese irrigation system. Mol Pant 11:47–57. https://doi.org/10.1016/j.molp.2017.09.002
Furze JM, Rhodes MJ, Parr AJ, Robins RJ, Withehead IM, Threlfall DR (1991) Abiotic factors elicit sesquiterpenoid phytoalexin production but not alkaloid production in transformed root cultures of Datura stramonium. Plant Cell Rep 10(3):111–114. https://doi.org/10.1007/bf00232039
Gajowik A, Dobrzyńska MM (2014) Lycopene-antioxidant with radioprotective and anticancer properties. A review. Rocz Panstw Zakl Hig 65:263–271
Gantet P, Memelink J (2002) Transcription factors: tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol Sci 23:563–569. https://doi.org/10.1016/S0165-6147(02)02098
Geipel K, Song X, Socher ML, Kümmritz S, Püschel J, Bley T, Ludwig-Müller J, Steingroewer J (2014) Induction of a photomixotrophic plant cell culture of Helianthus annuus and optimization of culture conditions for improved α-tocopherol production. Appl Microbiol Biotechnol 98:2029–2040. https://doi.org/10.1007/s00253-013-5431-7
Georgiev MI, Eibl R, Zhong JJ (2013) Hosting the plant cells in vitro: recent trends in bioreactors. Appl Microbiol Biotech 97:3787–3800. https://doi.org/10.1007/s00253-013-4817-x
Grotewold E (2008) Transcription factors for predictive plant metabolic engineering: are we there yet? Curr Opin Biotechnol 19:138–144. https://doi.org/10.1016/j.copbio.2008.02.002
Gupta OP, Karkute SG, Banerjee S, Meena NL, Dahuja A (2017) Contemporary understanding of miRNA-based regulation of secondary metabolites biosynthesis in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00374
Häkkinen ST, Moyano E, Cusidó RM, Palazón J, Piñol MT, Oksman-Caldentey KM (2005) Enhanced secretion of tropane alkaloids in Nicotiana tabacum hairy roots expressing heterologous hyoscyamine-6b-hydroxylase. J Exp Bot 56:2611–2618. https://doi.org/10.1093/jxb/eri253
Hashimoto T, Yamada Y (2003) New genes in alkaloid metabolism and transport. Curr Opin Biotechnol 14:163–168. https://doi.org/10.1016/s0958-1669(03)00027-2
He J, Giusti MM (2010) Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol 1:163–187. https://doi.org/10.1146/annurev.food.080708.100754
Herrera-Estrella L, Depicker A, Van Montague M, Schell J (1983) Expression of chimaeric genes transferred into plant cells using a ti-plasmid-derived vector. Nature 303:209–213. https://doi.org/10.1038/303209a0
Herrero M, Del Pilar Sánchez-Camargo A, Cifuentes A, Ibáñez E (2015) Plants, seaweeds, microalgae and food byproducts as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trends Analyt Chem 71:26–38. https://doi.org/10.1016/j.trac.2015.01.018
Hidalgo D, Sanchez R, Lalaleo L, Bonfill M, Corchete P, Palazon J (2018) Biotechnological production of pharmaceuticals and biopharmaceuticals in plant cell and organ cultures. Curr Med Chem 25:3577–3596. https://doi.org/10.2174/0929867325666180309124317
Howles PA, Vincent JH, Sewalt V, Paiva NL, Elkind Y, Bate NJ, Lamb C, Dixon RA (1994) Overexpression of i-phenylalanine ammonia-lyase in transgenic tobacco plants reveals control points for flux into phenylpropanoid biosynthesis. Plant Physiol 112:1617–1624. https://doi.org/10.1104/pp.112.4.1617
Hu L (2004) Production of shikonin by cell cultures of Lithospermum erythrorhizon. J Chin Med Mat 27:313–314
Huchelmann A, Boutry M, Hachez C (2017) Plant glandular trichomes: natural cell factories of high biotechnological interest. Plant Physiol 175:6–22. https://doi.org/10.1104/pp.17.00727
Hussain M, Fareed S, Ansari S, Rahman A, Ahmad IZ, Saeed M (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4:10–20. https://doi.org/10.4103/0975-7406.92725
Ikeda T, Takashi Matsumoto T, Noguchi M (1977) Effects of inorganic nitrogen sources and physical factors on the formation of ubiquinone by tobacco plant cells in suspension culture. Agric Biol Chem 41:1197–1201. https://doi.org/10.1271/bbb1961.41.1197
Jarvis DE, Ho YS, Lightfoot DJ, Schmöckel SM, Li B, Borm TJA, Ohyanagi H, Mineta K, Michell CT, Saber N, Kharbatia NM, Rupper RR, Sharp AR, Dally N, Boughton BA, Woo YH, Gao G, Schijlen EGWM, Guo X, Momin AA, Negrão S, Al-Babili S, Gehring C, Roessner U, Jung C, Murphy K, Arold ST, Gojobori T, van der Linden CG, van Loo EN, Jellen EN, Maughan PJ, Tester M (2017) The genome of Chenopodium quinoa. Nature 542:307–312. https://doi.org/10.1038/nature21370
Jouhikainen K, Lindgren L, Jokelainen T, Hiltunen R, Teemu H, Teeri TH, Oksman-Caldentey KM (1999) Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 208:545–551. https://doi.org/10.1007/s004250050592
Khandare V, Walia S, Singh M, Kaur C (2011) Black carrot (Daucus carota ssp. sativus) juice: processing effects on antioxidant composition and color. FBP 89:482–486. https://doi.org/10.1016/j.fbp.2010.07.007
Kim S et al (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet 46:270–278
Knockaert M, Manigart S, Cattoird S, Verstraete W (2015) A perspective on the economic valorization of gene manipulated biotechnology: past and future. Biotechnol Rep 6:56–60. https://doi.org/10.1016/j.btre.2015.01.002
Kobayashi Y, Fukui H, Tabata M (1991) Effect of carbon dioxide and ethylene on berberine production and cell browning in Thalictrum minus cell cultures. Plant Cell Rep 9:496–499. https://doi.org/10.1007/bf00232104
Koca N, Karaman Ş (2015) The effects of plant growth regulators and l-phenylalanine on phenolic compounds of sweet basil. Food Chem 166:515–521. https://doi.org/10.1016/j.foodchem.2014.06.065
Kumar GS, Hazra S (2014) Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Adv 4:56518–56531. https://doi.org/10.1039/c4ra06456a
Laurila J, Laakso I, Valkonen JPT, Hiltunen R, Pehu E (1996) Formation of parental-type and novel glycoalkaloids in somatic hybrids between Solanum brevidens and S. tuberosum. Plant Sci 118:145–155. https://doi.org/10.1016/0168-9452(96)04435-4
Lee CH, Kim JH (2014) A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res 38:161–166. https://doi.org/10.1016/j.jgr.2014.03.001
Lee EK, Jin YW, Park JH, Yoo YM, Hong SM, Amir R, Yan Z, Kwon E, Elfick A, Tomlinson S, Halbritter F, Waibel T, Yun BW, Loake GJ (2010) Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol 28:1213–1217. https://doi.org/10.1038/nbt.1693
Lee-Parsons CWT, Royce AJ (2006) Precursor limitations in methyl jasmonate-induced Catharanthus roseus cell cultures. Plant Cell Rep 25(6):607–612
Li R, Li R, Li X, Fu D, Zhu B, Tian H, Luo Y, Zhu H (2018) Multiplexed CRISPR/Cas9-mediated metabolic engineering of ϒ-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol J 16:415–427. https://doi.org/10.1111/pbi.12781
Loyola-Vargas VM, Ochoa-Alejo N (2018) An introduction to plant tissue culture: advances and perspectives. Methods Mol Biol. https://doi.org/10.1007/978-1-4939-8594-4_1
Lu R, Martin-Hernandez AM, Peart JR, Malcuit I, Baulcombe DC (2003) Virus-induced gene silencing in plants. Methods 30:296–303. https://doi.org/10.1016/s1046-2023(03)00037-9
Luque-Rodriguez JM, Perez-Juan P, De Castro MDL (2006) Extraction of polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J Agric Food Chem 54:8775–8781. https://doi.org/10.1021/jf061855j
Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H (2007) Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf 6:609–621. https://doi.org/10.1517/14740338.6.5.609
Mitsunaga Y, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, Sawada Y (2000) Effect of bioflavonoids on vincristine transport across blood–brain barrier. Eur J Pharmacol 395:193–201. https://doi.org/10.1016/s0014-2999(00)00180-1
Moon SH, Venkatesh J, Yu JW, Park SW (2015) Differential induction of meristematic stem cells of Catharanthus roseus and their characterization. Comptes Rendus Biol 338:745–756. https://doi.org/10.1016/j.crvi.2015.05.005
Moreira MM, Morais S, Barros AA, Delerue-Matos C, Guido LF (2012) A novel application of microwave-assisted extraction of polyphenols from brewer’s spent grain with HPLC-DAD-MS analysis. Anal Bioanal Chem 403:1019–1029. https://doi.org/10.1007/s00216-011-5703-y
Nair AJ, Sudhakaran PR, Rao JM, Ramakrishna SV (1992) Berberine synthesis by callus and cell suspension cultures of Coscinium fenestratum. Plant Cell Tissue Org Cult 29:7–10
Nakase I, Lai H, Singh NP, Sasaki T (2008) Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm 354:28–33. https://doi.org/10.1016/j.ijpharm.2007.09.003
Ncube B, Van Staden J (2015) Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 20:12698–12731. https://doi.org/10.3390/molecules200712698
Norman A, Aqeel M, Shuilin He S (2016) CRISPR–Cas9: tool for qualitative and quantitative plant genome editing. Front. Plant Sci 7:1740. https://doi.org/10.3389/fpls.2016.01740
Ochoa-Villarreal M, Howat S, Jang MO, Kim IS, Jin YW, Lee EK, Loake GJ (2015) Cambial meristematic cells: a platform for the production of plant natural products. New Biotechnol 32:581–587. https://doi.org/10.1016/j.nbt.2015.02.003
Ochoa-Villarreal M, Howat S, Hong S, Jang MO, Jin YW, Lee EK, Loake GJ (2016) Plant cell culture strategies for the production of natural products. BMB Rep 49:149–158. https://doi.org/10.5483/bmbrep.2016.49.3.264
Oksman-Caldentey KM, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440. https://doi.org/10.1016/j.tplants.2004.07.006
Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 23:482–487. https://doi.org/10.1038/nbt1082
Pennisi E (2016) The plant engineer. Science 353:1220–1224. https://doi.org/10.1126/science.353.6305.1220
Petolino JF (2015) Genome editing in plants via designed zinc finger nucleases. Vitro Cell Dev Biol Plant 51:1–8. https://doi.org/10.1007/s11627-015-9663-3
Prado JM, Vardanega R, Debien ICN et al (2015) Conventional Extraction. Food waste recovery processing technologies and industrial techniques. Academic Press imprint of Elsevier, Amsterdam, pp 127–148
Puri M, Sharma D, Barrow CJ (2012) Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol 30:37–44. https://doi.org/10.1016/j.tibtech.2011.06.014
Rajkumpari S, Sanatombi K (2018) In Vitro production of some important secondary metabolites from zingiber species. Biotechnol Approaches Med Aromat Plants. https://doi.org/10.1007/978-981-13-0535-1_9
Ramegowda V, Mysore KS, Senthil-Kumar M (2014) Virus-induced gene silencing is a versatile tool for unraveling the functional relevance of multiple abiotic-stress-responsive genes in crop plants. Front Plant Sci. 8(5):323. https://doi.org/10.3389/fpls.2014.00323
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó MR, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21:182. https://doi.org/10.3390/molecules21020182
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153. https://doi.org/10.1016/s0734-9750(02)00007-1
Rates SMK (2001) Plants as sources of drugs. Toxicon 39:603–613. https://doi.org/10.1016/S0041-0101(00)00154-9
Robins RJ, Rhodes MJC (1986) The stimulation of anthraquinone production by Cinchona ledyeriana cultures with polymeric adsorbents. Appl Microbiol Biotechnol 24:35–41. https://doi.org/10.1007/bf00266282
Rocha P, Stenzel O, Parr A, Walton N, Christou P, Drager B, Mark J, Leech MJ (2002) Functional expression of tropinone reductase I (trI) and hyoscyamine-6b-hydroxylase (h6h) from Hyoscyamus niger in Nicotiana tabacum. Plant Sci 162:905–913. https://doi.org/10.1016/s0168-9452(02)00033-x
Romagnoli LG, Knorr D (2009) Effects of ferulic acid treatment on growth and flavor development of cultured Vanilla planifolia cells. J Food Biotechnol 2:93–104. https://doi.org/10.1080/08905438809549678
Sadhukhan P, Saha S, Dutta S, Mahalanobish S, Sil PC (2018) Nutraceuticals: an emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol Res 129:100–114. https://doi.org/10.1016/j.phrs.2017.11.028
Salgado-Garciglia R, Ochoa-Alcjo N (1990) Increased Capsaicin content in PFP-resistant cells of chili pepper (Capsicum annuum L.). Plant Cell Rep 8:617–620. https://doi.org/10.1007/bf00270067
Sánchez-Pujante PJ, Borja-Martínez M, Pedreño MÁ, Almagro L (2017) Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 246:19–32. https://doi.org/10.1007/s00425-017-2705-9
Sasse F, Heckenberg U, Berlin J (1982) Accumulation of beta-carboline alkaloids and serotonin by cell cultures of Peganum harmala L: correlation between plants and cell cultures and influence of medium constituents. Plant Physiol 69:400–404. https://doi.org/10.1104/pp.69.2.400
Schachtsiek J, Warzecha H, Kayser O, Stehle F (2018) Current perspectives on biotechnological cannabinoid production in plants. Planta Med 84:214–220. https://doi.org/10.1055/s-0043-125087
Schiel O, Jarchow-Redecker K, Piehl GW, Lehmann J, Berlin J (1984) Increased formation of cinnamoyl putrescines by fedbatch fermentation of cell suspension cultures of Nicotiana tabacum. Plant Cell Rep 3:18–20. https://doi.org/10.1007/bf00270221
Segovia FJ, Luengo E, Corral-Pérez JJ, Raso J, Almajano MP (2015) Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields (PEF) applications. Ind Crops Prod 65:390–396. https://doi.org/10.1016/j.indcrop.2014.11.010
Sharma KD, Piqueras A (2010) Saffron (Crocus sativus L) tissue culture: micropropagation and secondary metabolite production. Funct Plant Sci Biotechnol 4:15–24
Smith RM (2003) Before the injection: modern methods of sample preparation for separation techniques. J Chromatogr A 1000:3–27. https://doi.org/10.1016/s0021-9673(03)00511-9
Solana M, Teel J, Hojilla-Evangelista M, Bertucco A, Eller F (2016) Counter-current carbon dioxide extraction of fat from soy skim. J Supercrit Fluids 113:106–111. https://doi.org/10.1016/j.supflu.2016.03.022
Steingroewer J, Bley T, Georgiev V, Ivanov I, Lenk F, Marchev A, Pavlov A (2013) Bioprocessing of differentiated plant in vitro systems. Eng Life Sci 13:26–38. https://doi.org/10.1002/elsc.201100226
Suhaj M (2006) Spice antioxidants isolation and their antiradical activity: a review. J Food Compos Anal 19(6–7):531–537. https://doi.org/10.1016/j.jfca.2004.11.005
Sun Y, Keli X, Yitao W, Xiuping C (2009) A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 20:757–769. https://doi.org/10.1097/cad.0b013e328330d95b
Tatli I (2012) Plant cell tissue and organ culture in terpenoids. In: Biotechnological production of plant secondary metabolites. Bentham Science Publishers, Chapter 07, pp 107–123. https://doi.org/10.2174/978160805114411201010107
Tatsis EC, O’Connor SE (2016) New developments in engineering plant metabolic pathways. Curr Opin Biotechnol 42:126–132. https://doi.org/10.1016/j.copbio.2016.04.012
Tian L (2015) Using hairy roots for production of valuable plant secondary metabolites. Adv Biochem Eng Biotechnol 149:275–324. https://doi.org/10.1007/10_2014_298
Unver et al (2017) Genome of wild olive and the evolution of oil biosyhtesis. PNAS 114:E9413–E9422. https://doi.org/10.1073/pnas.1708621114 (Epub 2017 Oct 9)
Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297. https://doi.org/10.1126/science.289.5477.295
Veerport R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25. https://doi.org/10.1023/a:1015871916833
Venugopala KN, Rashmi V, Odhav B (2013) Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int. https://doi.org/10.1155/2013/963248 (ID 963248, 14 pages)
Verma P, Khan SA, Mathur AK, Ghosh S, Shanker K, Kalra A (2018) Improved sanguinarine production via biotic and abiotic elicitations and precursor feeding in cell suspensions of latex-less variety of Papaver somniferum with their gene expression studies and upscaling in bioreactor. Protoplasma 251:1359–1371. https://doi.org/10.1007/s00709-014-0638-8
Verpoorte R, Memeling J (2002) Engineering secondary metabolite production in plants. Curr Opin Biotechnol 13:181–187. https://doi.org/10.1016/s0958-1669(02)00308-7
Wang L, Weller CL, Schlegel VL, Carr TP, Cuppett SL (2008) Supercritical CO2 extraction of lipids from grain sorghum dried distillers grains with solubles. Bioresour Technol 99:1373–1382. https://doi.org/10.1016/j.biortech.2007.01.055
Weathers PJ, Towler MJ, Xu J (2010) Bench to batch: advances in plant cell culture for producing useful products. Appl Microbiol Biotechnol 85:1339–1351. https://doi.org/10.1007/s00253-009-2354-4
White FF, Taylor BH, Huffman GA, Gordon MP, NesterE W (1985) Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164:3–44
Wickremesinhe ERM, Arteca RN (1994) Taxus cell suspension cultures: optimizing growth and production of Taxol. J Plant Physiol 144(2):183–188
Wijesingh WAJP, Jeon YJ (2012) Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review. Fitoterapia 283:6–12. https://doi.org/10.1016/j.fitote.2011.10.016
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. Medicines (Basel) 2:251–286. https://doi.org/10.3390/medicines2030251
Yang T, Wang L, Zhu M, Zhang L, Yan L (2015) Properties and molecular mechanisms of resveratrol: a review. Pharmazie 70:501–506. https://doi.org/10.1691/ph.2015.5571
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (β-carotene biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305. https://doi.org/10.1126/science.287.5451.303
Yun DJ, Hashimoto T, Yamada Y (1992) Metabolic engineering of medicinal plants: transgenic Atropa belladonna with an improved alkaloid composition. Proc Natl Acad Sci USA 89:11799–11803. https://doi.org/10.1073/pnas.89.24.11799
Zenk MH (1991) 6. Chasing the enzymes of secondary metabolism: plant cell cultures as a pot of gold. Phytochemistry 30(12):3861–3863
Zhang H, Hong Y, Huang L, Liu S, Tian L, Dai Y, Cao Z, Huang L, Li D, Song F (2016) Virus-induced gene silencing-based functional analyses revealed the involvement of several putative trehalose-6-phosphate synthase/phosphatase genes in disease resistance against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000 in Tomato. Front Plant Sci 7:1176. https://doi.org/10.3389/fpls.2016.01176
Zhao S, Baik OD, Choi YJ, Kim SM (2014) Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit Rev Food Sci Nutr 54:1283–1297. https://doi.org/10.1080/10408398.2011.632698
Zhou P, Yang J, Zhu J, He S, Zhang W, Yu R, Zi J, Song L, Huang X (2015) Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl Microbiol Biotechnol 99:7035–7045. https://doi.org/10.1007/s00253-015-6651-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Neal Stewart.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nielsen, E., Temporiti, M.E.E. & Cella, R. Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell Rep 38, 1199–1215 (2019). https://doi.org/10.1007/s00299-019-02415-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02415-z