Abstract
Jasmonates enhance the expression of various genes involved in terpenoid indole alkaloid (TIA) biosynthesis in Catharanthus roseus. We applied precursor feeding to our C. roseus suspensions to determine how methyl jasmonate (MJ) alters the precursor availability for TIA biosynthesis. C. roseus suspensions were induced with MJ (100 μM) on day 6 and fed loganin (0.30 mM), tryptamine (0.15 mM), loganin plus tryptamine, or geraniol (0.1–1.0 mM) on day 7. While MJ increased ajmalicine production by 3-fold, induced cultures were still limited by terpenoid precursors. However, both induced and non-induced cultures became tryptamine-limited with excess loganin. Geraniol feeding also increased ajmalicine production in non-induced cultures. But MJ appeared to increase geraniol availability in induced cultures, due presumably to the increased expression of Dxs with MJ addition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Precursor feeding studies are performed in plant culture systems to evaluate and identify the metabolic flux limitations in the biosynthesis of secondary metabolites (Moreno et al. 1993; Morgan and Shanks 2000; Whitmer et al. 1998, 2002a, b). By adding specific precursors to the cultures, limitations in secondary metabolism for a given metabolic state can be assessed. As gene expression and hence enzyme profiles are altered with environmental conditions, precursor limitations can change with culture conditions including growth stage (Morgan and Shanks 2000), hormone composition (El-Sayed and Verpoorte 2002), and elicitation (Moreno et al. 1993). In this paper, we investigated the precursor limitations of Catharanthus roseus suspension cultures optimally induced with methyl jasmonate (MJ).
Jasmonates are signal transduction molecules that trigger increases in secondary metabolism in a variety of plant species (Gundlach et al. 1992), including the production of terpenoid indole alkaloids (TIAs) in C. roseus cultures (Aerts et al. 1994; Gantet et al. 1998; Lee-Parsons et al. 2004; Rijhwani and Shanks 1998). Jasmonates increase TIA biosynthesis by activating the production of transcriptional regulators known as ORCAs (Memelink et al. 2001; Menke et al. 1999b; Van der Fits and Memelink 2000; Van der Fits and Memelink 2001). ORCAs then bind to the promoter region of various genes associated with TIA biosynthesis and enhance their expression. The genes that are over expressed with MJ are those involved in the biosynthesis of TIA precursors as well as downstream TIAs (Van der Fits and Memelink 2000). Figure 1 shows the biosynthetic pathway of TIAs.
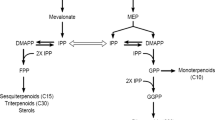
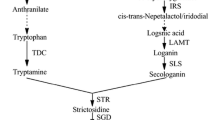
The biosynthesis of TIAs in C. roseus. Thin and dashed arrows represent single and multiple enzymatic steps, respectively: anthranilate synthase (AS), tryptophan decarboxylase (TDC), 1-deoxy-d-xylulose-5-phosphate synthase (DXS), DXP reductoisomerase (DXR), geraniol 10-hydroxylase (G10H), cytochrome P450 reductase (CPR), strictosidine synthase (STR). IPP and DMAPP represent isopentenyl diphosphate and dimethylallyldiphosphate, respectively.
TIAs are formed from the backbone compound, strictosidine. Strictosidine production is catalyzed by strictosidine synthase (STR) through the condensation of the precursors, tryptamine and secologanin, generated from the indole and terpenoid precursor branch, respectively. The first committed steps in the production of the indole and terpenoid precursors are 1) the conversion of tryptophan to tryptamine by tryptophan decarboxylase (TDC) and 2) the conversion of geraniol to 10-hydroxygeraniol by geraniol 10-hydroxylase (G10H) and cytochrome P450 reductase (CPR). The genes coding for TDC, G10H, CPR, and STR increased in the presence of MJ (Collu et al. 2001; Menke et al. 1999a; Van der Fits and Memelink 2000). In addition, genes coding for the alpha subunit of anthranilate synthase (ASα) and 1-deoxy-d-xylulose-5-phosphate synthase (DXS), upstream enzymes leading to the synthesis of tryptophan and geraniol, respectively, were also increased in the presence of MJ (Van der Fits and Memelink 2000).
Previously, we optimized the production of ajmalicine in our C. roseus suspension cultures with respect to MJ dosage and timing using 100 μM MJ in the rapid growth phase (i.e. day 6, Lee-Parsons et al. 2004). As the expression of several genes involved in the production of TIA precursors is affected by MJ, we applied precursor feeding to our C. roseus suspensions to determine how MJ alters the precursor availability for TIA biosynthesis. In particular, we monitored the production of ajmalicine and serpentine, representative TIAs produced in this cell suspension line. The precursor branch limiting TIA biosynthesis was initially assessed by feeding the precursors, loganin and tryptamine, to non-induced and MJ-induced C. roseus suspensions. As the cultures were terpenoid-limited, the extent to which geraniol availability contributed to terpenoid precursor limitations was also assessed.
Materials and methods
Maintenance of cell suspension cultures
Cell suspension cultures of C. roseus (cell line A11) were obtained from Dr. Robert Verpoorte (University of Leiden, The Netherlands) and maintained as described previously (Contin et al. 1998). Every 2 weeks, fresh growth medium (100 ml) was added to 14-day-old suspensions (50 ml) in a 250-ml Erlenmeyer flask, mixed, and divided equally between three 250-ml flasks. Fresh medium consisted of Gamborg's B5 basal salts with minimal organics medium (Gamborg et al. 1968; Caisson Laboratories, Inc., www.caissonlabs.com), 2% w/v sucrose (Caisson), and 1.86 mg/l naphthalene acetic acid (Sigma-Aldrich Corp., http://www.sigmaaldrich.com), adjusted to a pH of 5.8. The cultures were capped with Bellco foam closures (http://www.bellcoglass.com), shaken at 80 rpm, and maintained at 25°C under light in an incubator shaker (Forma Scientific, Model 4585, http://www.thermo.com).
For experiments, the mixture of fresh medium (100 ml) and 14-day-old suspensions (50 ml) was suction-filtered; 125-ml Erlenmeyer flasks were each inoculated with 25 ml of suction-filtered medium (consisting of conditioned plus fresh growth medium) and 4 g fresh weight of cells (0.17 g dry weight).
Preparation of methyl jasmonate solution
In the precursor feeding experiments, methyl jasmonate (MJ) was added to suspension cultures on day 6 at a final concentration of 100 μM. Previous findings with this C. roseus cell line showed that ajmalicine production was optimized when MJ was added on day 6, during the rapid growth phase, at a concentration of 100 μM MJ (Lee-Parsons et al. 2004).
The MJ solution (50 mM) was prepared by diluting the MJ stock (95+% purity; Sigma-Aldrich Corp.) in ethanol. In induced cultures, 50 μl of the MJ solution (50 mM) was added to suspensions (29 ml) in 125-ml flasks to yield a desired MJ concentration of 100 μM. Ethanol (50 μl) was added to non-induced cultures.
Precursor feeding experiment with loganin and tryptamine
The precursor branch that limits TIA biosynthesis in both non-induced and MJ-induced suspensions was assessed using precursor feeding. The precursors (loganin and tryptamine) were added to cell suspensions on day 7. Loganin (L) and tryptamine (T) were added either alone or in combination (LT) to a final concentration of 0.3 mM loganin and 0.15 mM tryptamine; the level of precursor addition was similar to that reported in the literature (Moreno et al. 1993; Morgan and Shanks 2000; Whitmer et al. 1998). Loganin was fed instead of secologanin as loganin was more efficiently utilized by C. roseus cells (Naudascher et al. 1989a, b).
Loganin (Extrasynthese, www.extrasynthese.com) was dissolved in water and 100 μl of the loganin solution (87.1 mM) was added to suspensions (29 ml) in 125-ml flasks to yield a desired concentration of 0.3 mM. Tryptamine (Acros Organics, www1.fishersci.com/acros/index.jsp) was dissolved in ethanol and 50 μl of the tryptamine solution (87.5 mM) was added to suspensions to yield a desired concentration of 0.15 mM. No precursors were added to the controls (C); instead, ethanol (50 μl) was added. Triplicate flasks were sampled on day 9 and analyzed for growth and alkaloid content. Maximum ajmalicine and serpentine production was achieved by day 9 in the cells induced with MJ on day 6 (data not shown). No significant difference was observed in the fresh weight of cultures with precursor feeding (data not shown).
Precursor feeding experiment with geraniol
The suspensions were fed with geraniol to determine if the limitation in terpenoid precursors could be attributed to the availability of geraniol. Geraniol was added on day 7 to non-induced cells to a final concentration of 0.5 mM and to MJ-induced cells to final concentrations of 0.1, 0.5, or 1.0 mM, levels similar to that added by Morgan and Shanks (2000).
Geraniol (TCI America, http://www.tciamerica.com) was diluted in ethanol and 50 μl of the geraniol solution (58.3, 291.7, or 583.5 mM) was added to suspensions (29 ml) in 125-ml flasks to yield a desired concentration of 0.1, 0.5, or 1.0 mM, respectively. Triplicate flasks were sampled on day 9 and analyzed for growth and alkaloid content. No significant difference was observed in the fresh weight of the cultures with precursor feeding (data not shown).
Isolation and concentration of alkaloids from cell samples
The cells were freeze-dried using a Flexi-Dry MP freeze dryer (Kinetics Thermal Systems, http://www.ftssystems.com) and their dry weights were recorded. The freeze-dried cells (250 mg) were extracted with 25 ml methanol twice while being agitated at 75–90 rpm, as previously described by Wong et al. (2004). The first extraction was for 3 h and the second for 1 h. Methanol extracts from both extraction steps were combined and evaporated in a Savant Speedvac Plus concentrator (www.thermo.com).
Crude cell extracts were redissolved in 10 ml methanol/hydrochloric acid (1 mM) (10:90 v/v) and subsequently fractionated using Sep-Pak Plus C18 liquid chromatography cartridges (Waters Associates, http://www.waters.com), as previously described by Wong et al. (2004). Cartridges were pre-conditioned with methanol followed by methanol/hydrochloric acid (1 mM) (10:90 v/v) and then by the solubilized crude extracts. Alkaloids of interest were eluted with 13 ml methanol/hydrochloric acid (1 mM) (75:25 v/v), evaporated, redissolved in 1 ml methanol, and syringe-filtered for HPLC analysis.
Separation and quantification of alkaloids by HPLC
Alkaloids in the extracts were separated by HPLC (Waters Corp.) and quantified by UV absorbance at 254 nm, as previously described by Wong et al. (2004). Separation was achieved using a Phenomenex Luna C18(2) column (150×4.6 mm, 5 μm particles) with a pre-column (Phenomenex, http://www.phenomenex.com). Samples (10 μl) were injected and a flow rate of 1.0 ml/min was maintained. Elution was performed isocratically with acetonitrile (ACN)/water/formic acid (FA) (11.9:88:0.1 v/v/v) for 18 min, increased to ACN/water/FA (20:79.9:0.1 v/v/v) in 2 min, and eluted in the same condition for another 30 min. The column was washed with ACN/FA (99.9:0.1 v/v) for 10 min and equilibrated with ACN/water/FA (11.9:88:0.1 v/v/v) for 18 min before the next injection.
Tryptamine eluted at 5 min, ajmalicine at 34 min, and serpentine at 37 min. The identity of tryptamine, ajmalicine, and serpentine in the chromatograms were verified in two ways: (1) spiking extracts with pure standards (Acros Organics; TCI America; Sigma, respectively) and (2) comparing MS and NMR spectra of collected peaks to those of standards.
Results and discussion
Identifying precursor limitations in TIA biosynthesis under MJ-induced conditions
Previously, we determined that ajmalicine production was optimized when MJ (100 μM) was added to our C. roseus cell cultures on day 6, during the rapid growth phase (Lee-Parsons et al. 2004). Next, precursor feeding was applied to these cultures to assess the precursor limitations in TIA biosynthesis under MJ induction. Suspensions were induced with MJ (100 μM) on day 6 and both non-induced and MJ-induced cultures were fed loganin (L), tryptamine (T), or loganin combined with tryptamine (LT) on day 7. The production of ajmalicine and serpentine on day 9 in these cultures is shown in Fig. 2.
Ajmalicine and serpentine production in non-induced and MJ-induced C. roseus cultures fed with loganin (L), tryptamine (T), or loganin+tryptamine (LT). In induced cultures, MJ was added on day 6 to a final concentration of 100 μM. Precursors were added on day 7: loganin (0.3 mM), tryptamine (0.15 mM), or loganin (0.3 mM)+tryptamine (0.15 mM). No precursors were added to the controls (C). Alkaloid concentrations represent the average production on day 9 from triplicate flasks; the error bars represent standard deviations. In non-induced cultures, the addition of loganin and loganin+tryptamine significantly increased ajmalicine production compared to non-fed cultures (asterisk (*) denotes 0.05<P< 0.02). MJ also significantly increased ajmalicine production compared to non-induced cultures (asterisks (**) denote 0.01<P<0.001). In MJ-induced cultures, the addition of loganin and loganin+tryptamine significantly increased ajmalicine production compared to non-fed cultures.
In non-induced cultures, feeding tryptamine had little effect on ajmalicine or serpentine levels compared to the non-fed cultures (C; Fig. 2). Hence, non-induced cells did not appear to be limited by endogenous tryptamine levels. However, feeding loganin more than doubled the ajmalicine levels compared to the controls, but no effect on the serpentine levels was observed. The increase in ajmalicine production with loganin addition suggests that these cultures were limited by the availability of terpenoid precursors. When both loganin and tryptamine were fed, ajmalicine levels were further enhanced, i.e. 6-fold of that in non-fed cultures and 2.5-fold of that in loganin-fed cultures; again the serpentine levels remained unaffected. Thus, in the presence of excess loganin, ajmalicine production became limited by tryptamine availability. Whitmer et al. (2002a, b) also observed that the terpenoid-limited C. roseus cultures (over-expressing either Tdc or Str) became tryptamine-limited when fed excess loganin (0.8–1.6 mM). When TIA production leveled off with increasing loganin addition, Whitmer et al. noted further enhancements in production with the combined addition of loganin and tryptamine.
In the presence of MJ, ajmalicine production was tripled compared to non-induced controls (Fig. 2). Feeding tryptamine to MJ-induced cells did not increase ajmalicine or serpentine levels compared to non-fed induced cells. Feeding loganin or loganin plus tryptamine to MJ-induced cultures enhanced the ajmalicine levels, i.e. 2-fold and 3-fold of that in non-fed induced cells, respectively. Serpentine levels were not significantly affected by MJ addition or by precursor addition, despite the increases in ajmalicine levels with MJ addition. Previously, we observed increases in both ajmalicine and serpentine with MJ addition (Lee-Parsons et al., 2004).
Thus, even with the presumed increase in G10H, CPR, and TDC enzyme levels with MJ addition (Collu et al. 2001; Van der Fits and Memelink 2000), ajmalicine production in our induced cultures appeared to be terpenoid-limited, exhibiting greater availability of indole precursors to terpenoid precursors. The increase in ajmalicine production in our cultures with the addition of loganin or loganin plus tryptamine also suggests that the enzymes downstream of loganin and tryptamine, such as strictosidine synthase, were not currently limiting.
Morgan and Shanks (2000) also investigated the limitations in TIA biosynthesis of C. roseus cultures under jasmonic acid-induced conditions. Morgan and Shanks (2000) initially determined that their C. roseus hairy root cultures were limited by the terpenoid precursors during the early stationary phase. For example, the addition of the terpenoid precursors such as geraniol, 10-hydroxygeraniol, or loganin improved the specific production of tabersonine by 50–70% but the addition of tryptamine or tryptophan had no effect on tabersonine production. The addition of jasmonic acid to these hairy root cultures further increased the specific production of alkaloids (serpentine, ajmalicine, tabersonine, horhammericine, and lochnericine) by 2.5-fold. However, the addition of loganin or tryptamine, either a day before or after jasmonic acid addition, did not enhance the specific yields of these five alkaloids. Hence, under jasmonic acid-induced conditions, the terpenoid branch was no longer limiting but potentially a step downstream in the TIA pathway (after loganin and tryptamine).
El-Sayed and Verpoorte (2002) observed that alkaloid production in MJ-induced C. roseus suspensions (A12A2 cell line) was limited by the availability of the precursors rather than a downstream step in the TIA pathway. The individual precursor branch limiting TIA biosynthesis was not determined; rather an excess of both precursors was added to overcome the limitation of either precursor alone. In non-induced cultures, the addition of excess loganin and tryptamine (20 mM each) increased the ajmalicine and strictosidine production by 6-fold and 20-fold, respectively, compared to non-fed controls. The addition of MJ (100 μM) to these precursor-fed cultures resulted in a 2-fold increase in ajmalicine and a 30% increase in strictosidine over precursor feeding alone. This cell line exhibited a high capacity to produce ajmalicine and strictosidine and thus was limited by the availability of precursors; under MJ-induced conditions, flux to ajmalicine production was further enhanced.
Assessing geraniol availability
Under MJ-induced conditions, our C. roseus suspensions appeared to be terpenoid-limited, as determined by loganin feeding. Therefore, the production of terpenoid precursors in these cultures may be limited by the availability of an upstream precursor such as geraniol or a likely enzymatic bottleneck such as G10H. Evidence for G10H as a bottleneck is based on precursor feeding studies and a positive association between TIA production and G10H levels (Collu et al. 2002; Schiel et al. 1987). Next, the availability of geraniol was assessed by feeding varying concentrations of geraniol to non-induced and MJ-induced suspensions on day 7. The production of ajmalicine and serpentine on day 9 in these suspensions is shown in Fig. 3.
Ajmalicine and serpentine production in non-induced and MJ-induced C. roseus cultures fed with geraniol. In induced cultures, MJ was added on day 6 to a final concentration of 100 μM. Geraniol was added on day 7. Alkaloid concentrations represent the average production on day 9 from triplicate flasks; the error bars represent standard deviations. In non-induced cultures, the addition of geraniol significantly increased ajmalicine production compared to non-fed cultures (asterisk (*) denotes 0.1<P<0.05). MJ also significantly increased ajmalicine production compared to non-induced cultures (asterisks (**) denotes 0.01<P<0.001).
Interestingly, feeding geraniol to non-induced suspensions more than doubled the ajmalicine levels while the serpentine levels remained unaffected (Fig. 3). These results suggest that geraniol availability may be inadequate in our non-induced cultures. Similarly, Morgan and Shanks (2000) and Hong et al. (2003) also observed inadequate geraniol availability in their C. roseus hairy root cultures, which exhibited terpenoid-limitation based on feeding of 10-hydroxygeraniol or loganin. For example, Morgan and Shanks (2000) detected an increase in tabersonine production in non-induced cultures fed with geraniol (0.05, 0.1, 0.5 mM) during the early stationary phase. The feeding of 1-deoxy-d-xylulose, a precursor to geraniol, also favorably increased the production of tabersonine and lochnericine (Hong et al. 2003).
However, feeding geraniol to our MJ-induced cells, even at increasing concentrations (0.1, 0.5, 1.0 mM), did not significantly affect ajmalicine or serpentine production (Fig. 3). Thus, geraniol did not appear to be the limiting factor in our MJ-induced cells, even with the presumed increase in G10H and CPR expression with MJ induction (Collu et al. 2001; Van der Fits and Memelink 2000). Both the mevalonate pathway and the DXP pathway provide precursors leading to geraniol. The increased availability of geraniol in our MJ-induced cells may be due to the increased expression of Dxs with MJ (Van der Fits and Memelink 2000); Dxs codes for 1-deoxy-d-xylulose-5-phosphate synthase, an enzyme involved in the DXP pathway leading to the production of geraniol precursors. Hong et al. (2003) demonstrated that the DXP pathway was a significant contributor of precursors leading to the presence of geraniol in C. roseus hairy root cultures. For instance, the addition of fosmidomycin, an inhibitor of an enzyme in the DXP pathway (i.e. DXP reductoisomerase, DXR), significantly reduced the production of ajmalicine, tabersonine, and lochnericine in their hairy root cultures during the exponential phase.
Conclusions
In our study, we monitored the production of ajmalicine and serpentine, two alkaloids from the Corynanthe family, and illustrated that the production of ajmalicine appeared to be terpenoid-limited. Monitoring the production of alkaloids from multiple alkaloid families may yield different conclusions with regard to precursor limitations. For example, Morgan and Shanks (2000) observed that the addition of several monoterpenoid precursors (i.e. geraniol, 10-hydroxygeraniol, and loganin) to C. roseus hairy root cultures (in late-exponential growth phase) had varying effects on the production of alkaloids either from the same family (i.e. tabersonine, lochnericine, horhammericine from the Aspidosperma family) or from different families (i.e. alkaloids from Aspidosperma versus Corynanthe families). Hence, cultures may exhibit differences in the precursor limitations for different alkaloid families.
In our cultures, MJ-induction alone increased ajmalicine production by 3-fold, presumably through the increased expression of genes involved in TIA biosynthesis. However, these MJ-induced cultures still exhibited limitations in the terpenoid precursors based on feeding studies with loganin and tryptamine. Geraniol feeding to non-induced cultures also increased ajmalicine production. However, geraniol availability appeared to be increased with MJ-induction as ajmalicine production was not affected by geraniol feeding in induced cultures. Hence, our results suggest that an enzymatic bottleneck exists between geraniol and loganin in MJ-induced cultures. In the literature, the conversion of geraniol to 10-hydroxygeraniol by G10H and CPR is considered to be a likely bottleneck in the biosynthesis of terpenoid precursors.
References
Aerts RJ, Gisi D, De Carolis E, De Luca V, Bauman TW (1994) Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J 5:635–643
Collu G, Unver N, Peltenburg-Looman AMG, van der Heijden R, Verpoorte R, Memelink J (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508:215–220
Collu G, Alonso Garcia A, van der Heijden R, Verpoorte R (2002) Activity of the cytochrome P450 enzyme geraniol 10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci 162(1):165–172
Contin A, van der Heijden R, ten Hoopen HJG, Verpoorte R (1998) The inoculum size triggers tryptamine or secologanin biosynthesis in a Catharanthus roseus cell culture. Plant Sci 139:205–211
El-Sayed M, Verpoorte R (2002) Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tissue Org Cult 68(3):265–270
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:155–158
Gantet P, Imbault N, Thiersault M, Doireau P (1998) Necessity of a functional octadecanoid pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol 39:220–225
Gundlach H, Muller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89:2389–2393
Hong S-B, Hughes EH, Shanks JV, San K-Y, Gibson SI (2003) Role of the non-mevalonate pathway in indole alkaloid production by Catharanthus roseus hairy roots. Biotechnol Prog 19(3):1105–1108
Lee-Parsons CWT, Ertürk S, Tengtrakool J (2004) Enhancement of ajmalicine production in Catharanthus roseus cell cultures with methyl jasmonate is dependent on timing and dosage of elicitation. Biotechnol Lett 26(20):1595–1599
Memelink J, Verpoorte R, Kijne, W (2001) ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci 6:212–219
Menke FLH, Parchmann S, Mueller MJ, Kijne JW, Memelink J (1999a) Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol 119:1289–129
Menke FLH, Champion A, Kijne JW, Memelink J (1999b) A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J 18:4455–4463
Moreno PRH, van der Heijden R, Verpoorte, R (1993) Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep 12(12):702–705
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145
Naudascher F, Doireau P, Guillot A, Viel C, Thiersault M (1989a) Time-course studies on the use of secologanin by Catharanthus roseus cells cultured in vitro. J Plant Physiol 134:608–612
Naudascher F, Doireau P, Guillot A, Thiersault M (1989b) Time-course studies on the use of loganin by Catharanthus roseus cells cultured in vitro. J Plant Physiol 135:366–368
Rijhwani SK, Shanks JV (1998) Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol Progr 14:442–449
Schiel O, Witte L, Berlin J (1987) Geraniol-10-hydroxylase activity and its relation to monoterpene indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus. Z. Naturforsch C 42(9–10):1075–1081
Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Van der Fits L, Memelink J (2001) The jasmonate-inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J 25:43–53
Whitmer S, Canel C, Hallard D, Goncalves C, Verpoorte R (1998) Influence of precursor availability on alkaloid accumulation by transgenic cell line of Catharanthus roseus. Plant Physiol 116(2):853–857
Whitmer S, van der Heijden R, Verpoorte, R (2002a) Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tiss Org Cult 69(1):85–93
Whitmer S, van der Heijden R, Verpoorte R (2002b) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96(2):193–203
Wong PL, Royce AJ, Lee-Parsons CWT (2004) Improved ajmalicine production and recovery from Catharanthus roseus suspensions with increased product removal rates. Biochem Eng J 21:253–258
Acknowledgements
This work was supported in part by the National Science Foundation (NSF-CAREER, Grant No. BES-0134511). A. Royce was supported in part by Giner, Inc. We would like to thank Dr. Robert Verpoorte (University of Leiden, The Netherlands) for the C. roseus cultures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. K. Kamo
Rights and permissions
About this article
Cite this article
Lee-Parsons, C.W.T., Royce, A.J. Precursor limitations in methyl jasmonate-induced Catharanthus roseus cell cultures. Plant Cell Rep 25, 607–612 (2006). https://doi.org/10.1007/s00299-005-0109-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0109-y