Abstract
Telomere length homeostasis is essential for cell survival. In humans, telomeres shorten as a function of age. Short telomeres are known determinants of cell senescence and longevity. The yeast Saccharomyces cerevisiae expresses telomerase and maintains a strict telomere length homeostasis during vegetative growth. We have previously reported that different environmental signals promote changes in telomere length in S. cerevisiae. In particular, exposure to ethanol induces an extensive telomere elongation response due to a reduction in RAP1 mRNA and protein levels. Here we show that the reduction in Rap1 protein levels disrupts the physical interaction between Rap1 and Rif1, which in turn reduces the recruitment of these two proteins to telomeres during G2-phase. Although elongation of the shortest telomeres has been shown to depend on the Rif2 telomeric protein and on the Tel1(ATM) protein kinase, we show here that the extensive telomere elongation in response to ethanol exposure is Rif1 and Mec1 (ATR)-dependent. Our results fit a model in which Rif1 and Rap1 form a complex that is loaded onto telomeres at the end of S-phase. Reduced levels of the Rap1–Rif1 complex in ethanol lead to continuous telomere elongation in a Mec1-dependent process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Telomeres are nucleoprotein structures located at the ends of the eukaryotic chromosomes, essential for chromosome function, stability and replication. Telomeres protect chromosome ends and prevent them from being recognized as double strand breaks and repaired, an event that could lead to undesired chromosomal rearrangements (Chan and Blackburn 2002; de Lange 2009; Levy et al. 1992). In most eukaryotes, the telomeric DNA consists of tandem repeat tracts whose overall length is highly regulated (Blackburn 2000). This telomeric DNA sequence is synthesized by a ribonucleoprotein enzyme named telomerase (Smogorzewska and de Lange 2004).
Despite the striking variation in telomere length between organisms, telomere length is tightly regulated, as it affects telomere and cell function (Harrington 2003). An inverse correlation between age and telomere length has been observed in humans, and short telomeres are also associated with age-related disorders and cancer (Blasco 2005). In higher eukaryotes, telomerase is highly expressed mainly at the early stages of development (Blackburn 2001; Collins and Mitchell 2002). In somatic cells, however, telomerase expression is low and telomeres shorten with each cell division (Harley et al. 1994; Hayflick 1965). This progressive telomere shortening represents a ‘molecular clock’ that underlies cellular aging (Blasco 2005; Holt et al. 1996). Reactivation of telomerase in cultured cells results in extended life span leading to their apparent immortalization (Bodnar et al. 1998). In cultures of cells that lack telomerase activity, there is a progressive decline in the fraction of growing cells (Shay and Wright 2011). It has been shown that replenishing telomeres by an activated telomerase or by recombination (ALT) is one of the few essential steps that a normal human fibroblast cell must take on its way to become malignant (Hahn et al. 1999). Thus, understanding how telomere length is monitored has significant medical implications especially in the fields of aging and cancer.
The relative uniformity in telomere size is achieved by a mechanism able to “count” telomere-binding proteins (e.g., Rap1 in yeast and TRF1 in humans) that presumably affects chromatin structure and accessibility of the telomerase and nucleases to the chromosomal ends (Marcand et al. 1999). Rap1 is the major component of telomeric chromatin in the budding yeast. The RAP1 gene is essential, mainly due to its roles as a transcription regulator of ribosomal genes (Chymkowitch et al. 2015; Garbett et al. 2007; Schawalder et al. 2004; Wade et al. 2004). However, a deletion of Rap1 C-terminal region leads to telomere lengthening and to telomeric ssDNA accumulation due to its inability to recruit the Rap1-binding proteins Rif1 and Rif2 (Wotton and Shore 1997). This elongation depends on the conserved MRX complex, consisting of the Mre11, Rad50 and Xrs2 (Tsukamoto et al. 2001); its mammalian counterpart, MRN, has the Nbs1 protein replacing Xrs2. Deletion of either RIF1 or RIF2 leads to substantial elongation of telomeres, demonstrating their function as negative regulators of telomere length. The two proteins seem to act by different mechanisms, as a Δrif1 Δrif2 double mutant exhibits longer telomeres than each of the double mutants (Wotton and Shore 1997). In the absence of either one of the Rif proteins, the frequency of telomerase recruitment events (but not the extent of telomerase activity of each event) is increased (Shore and Bianchi 2009; Teixeira et al. 2004). Thus, the presence of the Rif proteins precludes recruitment of telomerase and limits telomerase action (which only takes place at late S-phase), rather than controlling its activity once it is recruited (Gallardo et al. 2011). In yeast cells, telomerase is constitutively active, and is more likely to be associated with elongation of the shortest telomeres in the cell (Teixeira et al. 2004). This preferential elongation of short telomeres is induced by depletion of Rif2, which leads to preferential recruitment of MRX and Tel1, thereby promoting elongation of the shortest telomeres (McGee et al. 2010; Sabourin et al. 2007).
Yeast cells are an excellent model system to study telomere biology (Adamczyk et al. 2016; Millet and Makovets 2016; Simonicova et al. 2015). Yeast cells adapt rapidly to different stressful conditions (Chidi et al. 2016, Eleutherio et al. 2015; Ho and Gasch 2015; Nishida-Aoki et al. 2015; Nishida et al. 2014). In previous work we have shown that upon exposure to ethanol yeast telomeres become extensively elongated (Romano et al. 2013). This telomere elongation response is due to a reduction in RAP1 transcription levels, which in turn result in reduced Rap1 protein levels (Romano et al. 2013). Here we show that upon ethanol exposure, the reduced physical interaction between Rap1 and Rif1 proteins causes reduced recruitment, particularly at the G2/M-stage of the cell cycle. Moreover, we have found an unexpected role for the Mec1 kinase (the yeast ortholog of ATR), a central protein of the response to DNA damage, in the extensive telomere elongation response upon ethanol exposure. Altogether, these results shed new light to our understanding of telomere length regulation.
Materials and methods
Yeast strains
Name | Phenotype | References |
|---|---|---|
BY4741 | MATA Δura3 Δmet15 Δleu2 Δhis3 | (Brachmann et al. 1998) |
Δsml1 | MATalpha ade2::hisG his3-200 Δleu2 Δlys2 Δmet15 trp1-63 Δura3 Δsml1::HIS3 | (Chakhparonian et al. 2005) |
Δsml1Δtel1 | MATalpha ade2::hisG his3-200 Δleu2 Δlys2 Δmet15 trp1-63 Δura3 Δsml1::HIS3 tel1::LEU2 | (Chakhparonian et al. 2005) |
Δsml1Δmec1 | MATalpha ade2::hisG his3-200 Δleu2 Δlys2 Δmet15 trp1-63 Δura3 Δsml1::HIS3 mec1::TRP1 | (Chakhparonian et al. 2005) |
Δsml1Δtel1Δmec1 | MATalpha ade2::hisG his3-200 Δleu2 Δlys2 Δmet15 trp1-63 Δura3 Δsml1::HIS3 mec1::TRP1 tel1::LEU2. | (Chakhparonian et al. 2005) |
YhRif1-HA | MATa Δura3 Δmet15 Δleu2 Δhis3 Δbar1 RIF1-HA | This study |
YhRif2-Myc | MATa Δura3 Δmet15 Δleu2 Δhis3 Δbar1 RIF2-13Myc | This study |
YhDoubleRif-Tag | MATa Δura3 Δmet15 Δleu2 Δhis3 Δbar1 RIF1-HA RIF2-13Myc | This study |
Mec1-100 | MATa, sml1::kanMX mec1-100::LEU2 mec1::HIS3 DDC2-GFP::TRP1 | (Tercero et al. 2003) |
Growth media
YPD (yeast rich medium) 1% Bacto yeast extract (DIFCO), 2% Bacto peptone (DIFCO), 2% Glucose. Any other stressing agent is being added according to its final concentration into the medium. For selection, 200 mg/l of G418 Geneticin (CalBioChem) or 200 mg/l of Hygromycin B (Invitrogen) is added.
YPD + ethanol Normal YPD medium containing also 5% ethanol.
SD Complete (yeast defined medium) 0.67% Bacto yeast nitrogen base w/o amino acids (DIFCO), 2% glucose. All amino acids and nucleobases are added according to requirements.
SD for G418/Hygromycin −0.17% YNB w/o amino acids and ammonium sulfate (DIFCO), 0.1% MSG (l-glutamic acid sodium salt hydrate) (SIGMA), 2% glucose containing 200 mg/l of G418 Geneticin (CalBioChem) or 200 mg/l of Hygromycin B (Invitrogen).
Flow cytometry
200 µl of a logarithmic cell culture (0.6 OD600) were harvested, resuspended in 60 µl of 50 mM Tris pH 7.5 and 140 µl of ethanol was added; cells were then kept overnight at 4 °C. Fixed cells were centrifuged and washed once in 200 µl of 50 mM Tris pH 7.5 buffer and resuspended in 100 µl RNAse (0.2 mg/ml in 50 mM Tris pH 7.5) for 2 h at 37 °C. In addition, proteinase-K (0.2 mg/ml in 50 mM Tris pH 7.5) was added to each tube and cells were incubated for 60 additional minutes at 50 °C. 20 µl of the sample was taken into a new tube and a 180 µl of 18 µg/ml propidium iodide 50 mM Tris pH 7.5 was added. The samples were kept in the dark at 4 °C overnight, sonicated twice at low setting (20% power) for 3–5 s and stored in the dark at 4 °C. The flow cytometry MACSQuant system was used for reading. Results were analyzed using either the Flowing Software or the FlowJo program.
Telomere southern blot (Telo-blot)
2.5 µg of genomic DNA was digested with XhoI and incubated for 16 h at 37 °C. The DNA is separated on a 1% agarose gel and blotted onto NYTRAN nylon membrane. The membranes are hybridized to an S. cerevisiae-specific telomeric probe and size-control fragments (Romano et al. 2013). Hybridizations were carried out overnight in Church buffer (BSA 1%, buffer phosphate 0.5 M, EDTA 1 mM and SDS 7%) in 30 ml, and washed three times (each one for 20 min in 40 ml) with the following dilutions of SCC × 20 (0.5 M NaCl, 0.05 m C6H5Na3O7): SSC × 2 + 0.1% SDS, SSC × 0.2 + 0.1% SDS and finally with SCC × 2. Hybridizations and washes were performed at 65 °C. A Fujicom film was exposed (at −70 °C) for 3 days.
Telomere length analysis
Telomere length was measured using the Tel-Quant program (Rubinstein et al. 2014).
Fitness and competition experiments ~106 cells were grown for 24 h in a Tecan Horizon robot, and OD was automatically measured every 30 min. For competition experiments cells marked with either the KanMX or HygMX markers were mixed in a 1:1 ratio, and grown as described.
ChIP
Typically, 50 ml of a log culture (5 × 107 cells/ml) were cross-linked for 30 min in 1% formaldehyde. The cross-linker was quenched by the addition of glycine to 125 mM and the cells were incubated for 5 min at room temperature. Cells were washed twice with TBS + 10% glycerol. Cells were vortexed for 45 min in 600 µl of lysis buffer (50 mM HEPE pH 7.5, 1% triton, 0.1% SDS, 0.1% deoxycholate, 2.5 mM EDTA, 0.5 M NaCl) supplemented with protease inhibitors (Roche) and glass beads. The crude lysate was sonicated to an average fragment size of 500 bp (8 × 10 s pulses at 80% power levels using a Sonic Vibra cell sonicator) after which the supernatant was clarified (14,000 rpm, 20 min). Protein concentrations were used to normalize all samples. 450 µl of the clarified lysates were used for immunoprecipitations. The immune complexes were retrieved using protein G beads (Adar Biotech) and washed using lysis buffer, wash buffer (250 mM LiCl, 0.5% NP40, 0.5% deoxycholate, 5 mM EDTA) and TE. DNA was eluted, cross-linking was reversed and the DNA was ethanol precipitated and resuspended in 100 µl of TE. 5 µl were used for RT-PCR (ABI StepOnePlus™ Real-Time PCR System); primer concentration and cycles number were calibrated individually for each reaction. Every RT-PCR was carried out simultaneously on input DNA and on the relevant IP to control for changes in PCR conditions.
The association of each relevant protein the Y′-element telomeres was detected using Santa Cruz Mouse anti-Myc or anti-HA monoclonal IgG antibodies or the Rap1 polyclonal antibody. Real-time PCRs (RT-PCRs) were carried out using the following primers:
Y′-element 5′-GGCTTGATTTGGCAAACGTT-3′, and 5′-GTGAACCGCTACCATCAGCAT-3′.
ARO1 (control) 5′-GTCGTTACAAGGTGATGCC-3′, and 5′- CGAAATAGCGGCAACAAC-3′.
The relative fold enrichment\depletion of the telomere-associated protein was calculated as follows: [tel-IP/ARO1-IP]/[tel-input/ARO1-input].
Co-IP
Yeast cells were grown to a concentration of ~2 × 107 cells/ml. Cells (100 ml) were harvested, washed twice with DDW and resuspended in 4 ml of PBS buffer (0.5% tween, 10% glycerol, 1 mM PMSF [Sigma], protease inhibitors). An equal volume of glass beads (diameter 0.5 mm) was added. Breakage was achieved by vortexing for 60 min at 4 °C. The supernatant was used for input and for immunoprecipitation. One microgram of antibody was added and incubated overnight at 4 °C. 20 µl of protein A-Sepharose and G-Sepharose beads (Invitrogen) were added, and the incubation continued for 2 h. The immune precipitates were washed 5 times for 5 min with PBS buffer and subsequently resuspended in 40 µl of SDS-PAGE sample buffer. 30–40 µl of the eluted proteins were analyzed by SDS-PAGE and Western blotting with anti Rap1 anti-HA and anti-Myc antibodies.
Western blot
Yeast cells were collected by centrifugation, resuspended in 600 μl of phosphate-buffered saline with 1% Triton X-100 (PBST), supplemented with a protease inhibitor cocktail (ROCHE), and subjected to mechanical rupture using glass beads. The cell debris was removed by centrifugation, and the supernatants were applied onto 0.1 M dithiothreitol, and incubated at 80 °C for 10 min before SDS-PAGE (Resolving gel: 29:1 acrylamide, 1.5 M Tris–HCl pH 8.8, 10% SDS (pH 7.2), 9.7 ml H2O, 100 µl 10% APS and 10 µl TEMED. Stacking gel: 30% bis/acrylamide, 1 M Tris–HCl pH 6.8, 10% SDS (pH 7.2), 5.5 ml H2O, 800 µl 10% APS and 8 µl TEMED). The samples were run with SDS-PAGE buffer (Tris–Glycine) at 100 V until the samples have passed the stacking gel and then at 160 V till the samples have been fully separated. Transfer to nitrocellulose was done in transfer buffer (200 ml of methanol, 3.03 gr Tris base, 14.4 gr glycine) at 500 m AMP and verified by staining with Ponceau-S dye. The blot was blocked with milk (5%) for at least 60 min at room temperature. Primary antibody was added for 12 h at 4 °C. The blot was washed 3 × 5 min with TBST (Tris-buffered saline Tween-20) and secondary antibody was added for 1 h. The blot was washed 3 × 5 min with TBST and subjected to ECL (Amersham). Protein quantitation was carried out with the ImageJ software (Girish and Vijayalakshmi 2004).
Results
As previously reported, exposure to ethanol induces an extensive telomere elongation response in S. cerevisiae (Fig. 1a), due to a reduction in Rap1 protein levels [Fig. 1b, (Romano et al. 2013)], but not Rif1 or Rif2 levels (Fig. 1c, d). This telomere elongation response is accompanied by an increase in telomere length variability (Fig. 1a), indicating that cells have lost their homeostasis-specific ability to particularly elongate the short telomeres. Indeed, the fact that mutants in the Tel1-MRX pathway [which preferentially elongates the shortest telomeres (Arneric and Lingner 2007); (Chang et al. 2007)] do not impair the ethanol telomere elongation response supports these observations (Romano et al. 2013).
Rap1 depletion induces telomere elongation. a Kinetics of telomere length change upon exposure to ethanol. Wild-type yeast strain BY4741 was grown in the presence of 5% ethanol for 300 generations. b The level of Rap1 protein is reduced upon exposure to ethanol. c Western blot analysis shows that unlike Rap1, the protein levels of Rif1 and Rif2 are not reduced upon exposure to ethanol. d Quantitation of the Western blots shown in b and c above
Since telomere regulation is dynamic, it is possible that Rif1 or Rif2 protein levels are slightly affected by ethanol at a very specific stage of the cell cycle, and thereby we are unable to detect these changes in unsynchronized cultures. To deal with this problem, we followed the levels of Rif1 and Rif2 proteins throughout the cell cycle. Cells were grown overnight in YPD, diluted and allowed to reach the mid-logarithmic stage either in YPD or YPD + 5% ethanol. At this stage, alpha-factor was added to synchronize the cells in G1. Then cells were released back into YPD or YPD + 5% ethanol medium (the same in which the cells were grown prior to cell cycle arrest). Cells were collected every 10 min for Western blot (WB) analysis, and DNA content was used to determine cell cycle progression by flow cytometry (FC) analysis (Fig. 2a). When comparing the initial protein levels in G1-arrested cells between the YPD and ethanol-containing media, we can detect a moderate reduction in Rap1 protein levels (as previously described in Fig. 1b). When cells progress through the cell cycle in YPD, Rap1 protein levels remain unchanged, the level of Rif1 decreases and that of Rif2 increases towards the G2-phase (Fig. 2b). In ethanol-containing medium, however, we can see a dramatic upregulation of Rif1 when cells enter S-phase, which continues to increase and peaks at the G2/M-stage. In contrast, and only a moderate elevation in Rap1 and Rif2 levels can be observed towards the end of S-phase. These results by themselves cannot explain the dramatic telomere elongation seen upon exposure to ethanol, as Rif1 is considered a negative regulator of telomerase, and an increase in its activity should thus lead to decreased telomerase activity.
Levels of Rap1, Rif1 and Rif2 and their interactions during the cell cycle in YPD and YPD + 5% ethanol. a Western blot analysis for the total protein levels of Rap1, Rif1 and Rif2, in YPD and in YPD containing 5% ethanol, in a cell cycle dependent manner (The invariable Cdc28 protein serves as loading control). In the lower figure section, representative flow cytometry results. b Quantitation of the level of the different proteins compared to their level in G1-arrested cells. Left cells grown in YPD; right cell grown in YPD + ethanol. The green line shows the absolute level of the proteins in YPD in the G1 phase. c Co-Immunoprecipitation assay of Rap1 (using anti-Rap1 polyclonal antibody) with Rif1 and Rif2 proteins (HA and Myc-tagged, respectively), in an unsynchronized cell culture, and in G1- and M-phase arrested cultures (treated with either alpha-factor or Benomyl). d Quantitation of the Rap1–Rif1 interaction (upper panel) and Rap1–Rif2 interaction (lower panel) results
To further explore the effect of ethanol exposure on the nucleoprotein structure of telomeres during the different cell cycle stages, we performed Co-Immunoprecipitation (Co-IP), using an anti-Rap1 polyclonal antibody, in an unsynchronized cell culture, and in G1- and G2/M- phase arrested cultures. This allowed us to map the timing of interaction between Rap1 and Rif proteins, and whether this interaction is affected by the exposure to ethanol. In an unsynchronized YPD culture, we can detect protein interactions between Rap1 and both Rif1 and Rif2 proteins (Fig. 2c, d). However, upon ethanol exposure, the interaction between Rap1 and Rif1 becomes almost undetectable, whereas the interaction between Rap1 and Rif2 seems to be intact (Fig. 2c, d). When cells are arrested in G1 (using alpha-factor) the interaction between Rap1 and Rif1 cannot be detected, either in YPD or ethanol-containing media, whereas the interaction between Rap1 and Rif2 is still unaffected. This result suggests that during G1-phase, Rif1 plays no role in telomere regulation, and that telomere homeostasis is mainly directed through the Rap1–Rif2 interaction. In contrast, at the end of S/G2/M-phase, when the association of Rif1 to telomeres is suggested to be at its peak (Sabourin et al. 2007), we were able to detect a strong interaction between Rap1 and Rif1 in YPD medium, but this interaction is reduced by a factor of 2 in ethanol (Fig. 2c, d). These results suggest that ethanol exposure induces telomere elongation by dysregulating the interaction between Rap1 and Rif1. The fact that ethanol exposure seems to have no effect on Rap1–Rif2 interaction fits with the results that telomere elongation in response to ethanol is Tel1-MRX independent (Romano et al. 2013), as this is the regulatory branch negatively regulated by Rif2.
To directly test the cell cycle dependent effect of the Rap1–Rif1 protein interaction dysregulation on telomere structure, we performed Telomeric Chromatin Immunoprecipitation (Telo-ChIP) assays for Rap1, Rif1 and Rif2 at specific cell cycle stages. We started by monitoring the association of Rap1 to telomeres in synchronized cell cultures grown in YPD or in YPD + ethanol media throughout the different cell cycle stages (Fig. 3a, b). In YPD medium, the association levels of Rap1 remain fairly constant during the transition from G1 to S, increasing dramatically at late S-phase. The pattern of Rap1 recruitment to telomeres very much resembles previously reported pattern of recruitment to telomeres of Rif1 (Sabourin et al. 2007). When cells are exposed to ethanol the pattern of Rap1 recruitment to telomeres is altered and the peak of Rap1 association to telomeres is now shifted toward early S-phase, followed by a reduction in protein association levels to telomeres in late S/G2. An additional set of Telo-ChIP data for arrested cells in G1- or G2/M-phase (using either alpha-factor or Benomyl) supports these findings (Fig. 3c). As we can see, in G1-arrested cells Rap1 is recruited to telomeres at higher levels in the presence of ethanol than in its absence. The level of Rif1 is slightly elevated too, whereas the recruitment of Rif2 is unaffected (Fig. 3c). In G2/M arrested cells, the situation seems to be different: a dramatic reduction in Rap1 association levels to telomeres upon ethanol exposure is detected. Concomitantly, Rif1 association to telomeres is also significantly decreased. In contrast, the association levels to telomeres of Rif2 seem to be intact. As an additional control, we also measured the recruitment of Sir4, an additional protein known to bind Rap1 (Luo et al. 2002); as with Rif2, we found no effect of ethanol (Fig. 3d). We conclude that ethanol particularly affects the recruitment of Rap1 and Rif1 to telomeres during G2/M.
Telomere Chromatin Immunoprecipitation throughout the cell cycle in YPD or YPD + ethanol. a Anti-Rap1 Telo-ChIP at different stages of the cell cycle in YPD medium. Cells were synchronized using alpha-factor, and then released back to fresh medium. Synchronization and cell cycle progression were monitored by flow cytometry, as presented below the graph. b Same, in YPD + 5% ethanol medium. c Relative enrichment of Telo-ChIP results of Rap1, Rif1-HA and Rif2-Myc on YPD + ethanol in G1-phase (alpha-factor arrested cells). d Same in M-phase (Benomyl-arrested cells)
As stated above, Tel1 regulates the preferential elongation of short telomeres (Arneric and Lingner 2007) by a pathway that also includes the MRX complex [Mre11, Rad50, Xrs2; (Tsukamoto et al. 2001)]. The fact that telomeres can be elongated by ethanol in the absence of Tel1 or of components of the MRX complex is surprising; notably, the wide size distribution of telomeres observed upon exposure to ethanol is consistent with a mechanism independent of the one that preferentially elongates the shortest telomeres, which depends on the Tel1 pathway (Chang et al. 2007).
This observation led us to question whether the ATR-related protein Mec1 could be the kinase necessary for the telomerase-dependent telomere elongation in the presence of ethanol. We tested the telomeric response to ethanol in single Δtel1 and Δmec1 mutants, and in freshly created double mutant strains (as MEC1 is essential, these strains are deleted for the SML1 gene to suppress the essential function of MEC1). Our results show that, as expected, the Δtel1 mutant has very short telomeres; however, it experiences a significant telomere elongation upon exposure to ethanol (Fig. 4a, b). In contrast, the Δmec1 mutant, which has telomeres of wild-type size, shows no change in telomere length when ethanol is added; a similar result is seen for the double mutant Δtel1 Δmec1. Thus, Mec1 (ATR ortholog), rather than Tel1 (ATM ortholog) is responsible for the response to ethanol. As a confirmation, we tested the telomeric response to ethanol of a strain that carries the mec1-100 allele, which is defective in both the G1/S and intra-S DNA damage checkpoints (Paciotti et al. 2001). Cells of this strain were also unresponsive to ethanol (Fig. 4b), confirming that Mec1 is indeed the main kinase involved in the extensive telomere elongation in response to exposure to ethanol.
Telomere length response of tel1 and mec1 mutants to ethanol stress. a Southern blot analysis of ∆sml1, ∆tel1 ∆sml1, ∆mec1 ∆sml1 and ∆tel1 ∆mec1 ∆sml1 cultures grown in YPD + 5% ethanol for 100 generations. Each passage (P) represents ~25 generations. b Same, mec1-100 mutant. Each lane represents the telomere length of at 10 generations intervals
Discussion
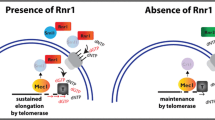
Telomeres are dynamic and their elongation is cell cycle regulated [reviewed in (Harari and Kupiec 2014; Kupiec 2014)]. Telo-ChIP results of different telomeric components have determined the specific time points in which these proteins are recruited to telomeres during the cell cycle. Previous work by the Zakian lab has established that Rif1 is present at telomeres throughout the cell cycle. However, during late S and G2 stages, Rif1 levels at telomeres are particularly elevated (Sabourin et al. 2007), suggesting that this is the step were Rif1 function in telomere regulation takes place. Our Telo-ChIP results show that the Rap1 pattern of recruitment to telomeres resembles the telomeric recruitment pattern of Rif1 (Fig. 3d). In addition, we have shown by Co-IP that the physical interaction between Rap1 and Rif1 is dramatically enriched during G2/M-phase (compared to that seen during G1-phase), and that upon exposure to ethanol this interaction is reduced (Fig. 3), in contrast to the physical contact between Rap1 and Rif2, which seems to be stable throughout the cell cycle, and appears also to be unaffected by ethanol exposure (Fig. 2b, d). One possible model to explain these results (Fig. 5) is that the Rap1–Rif1 complex is created in early/mid S-phase, but only recruited to telomeres at late S/G2-phase to fulfill Rif1’s role as a negative telomerase regulator. Together with the previously discussed data, these results favors a model in which Rap1 and Rif1 are recruited to telomeres during late S and G2/M stages as a complex, which negatively regulates telomerase activity. In the presence of ethanol, we have detected a reduction in Rap1–Rif1 protein interaction during G2/M-phase (Fig. 2d). In addition, our Telo-ChIP data detects a reduction in Rap1 and Rif1 protein levels at telomeres during G2/M-phase, but not in G1-phase (Fig. 3c, d). This supports a model in which a reduction in protein–protein interaction between Rap1 and Rif1 due to lower Rap1 levels leads to a reduction in the recruitment of these proteins to telomeres (as a complex) during late S and G2 stages, and thereby leads to non-regulated extensive telomerase activity at telomeres. The fact that the reduced interaction between Rap1 and Rif1 upon ethanol exposure is specific and cannot be detected in the case of Rif2, suggest that this reduction in protein–protein interaction is not the consequence of a global Rap1 dysfunction at telomeres due to the reduced Rap1 protein levels (Fig. 5).
A model for the Mec1(ATR) dependent mechanism of telomere length regulation. In the G1 phase of the cell cycle Rif1 and Rif2 are present at telomeres, bound to Rap1. Rif2 activity prevents Tel1-dependent telomerase activity. At the end of S-phase, the negative regulation on Tel1 is abolished, and short telomeres are elongated in a Tel1-dependent fashion. The level of Rif1 keeps increasing, Rif1 and Rap1 form a complex that is loaded to telomeres, eventually stopping telomerase activity at the end of S/G2. In the presence of ethanol, the Rap1–Rif1 complex is not formed, and telomerase continues to elongate telomeres irrespectively of their length. This activity is Tel1(ATR)-independent, but depends instead on the Mec1(ATR) kinase
Only a few telomeres are elongated in a given cell cycle. In a single telomere extension assay (STEX), it was found that ~10% of wild-type length telomeres (~300 bp long) are elongated by telomerase, while very short telomeres (~100 bp long) are lengthened ~50% of the time (Teixeira et al. 2004). Rif2 was found to be quite evenly associated with telomeres throughout the cell cycle. However, at short telomeres the levels of Rif2 were shown to be reduced (Sabourin et al. 2007). This reduction in Rif2 levels, which leads to recruitment of the MRX complex and the Tel1 kinase, is the signal which marks short telomeres for elongation by telomerase. In the absence of Rif2 (Δrif2 cells), Tel1 no longer binds to short telomeres better than to those of normal size (McGee et al. 2010). Our results indicate that Δtel1 strains exhibit no defect in telomere elongation in response to ethanol and suggest that Tel1 is not the kinase that regulates this extensive telomere elongation. In other words, telomere elongation upon exposure to ethanol does not depend on the same mechanism that leads to the preferential elongation of short telomeres.
Mec1, the main checkpoint kinase in yeast, was thought to have a minor role in telomere length regulation since mutations in MEC1 exhibit only a mild telomeric phenotype compared to tel1 mutants (Ritchie et al. 1999). Consistent with this observation, Mec1 recruitment to telomeres is only detected at ultra-short telomeres which are already considered to be nonfunctional (Abdallah et al. 2009; Gadaleta et al. 2016; Hector et al. 2012; McGee et al. 2010). We show that two different mec1 mutants (Δmec1 and mec1-100) exhibit telomeres of wild-type length (and thus are capable of coping with the “end-replication problem”) but show no telomere elongation upon exposure to ethanol, indicating that Mec1 activity is necessary for this extensive telomere elongation response (Fig. 4). Thus, whereas Tel1 activity is necessary mainly for the maintenance of short telomeres and for normal telomerase activity during late S-phase, Mec1 may have additional functions in extensive telomerase activity during G2/M. Mec1 has been proposed to play a role in promoting telomere recombination in senescent cells with short telomeres (Simon et al. 2016). We propose that Mec1 may facilitate telomerase activity in cells with stalled replication forks as a way to solve replication problems at the end of the S-phase. This is consistent with models of telomere length maintenance in which the replication fork plays a central role in determining the activity of telomerase (Greider 2016). Mec1 is a central regulator of late origin firing and the resolution of stalled replication forks. Ethanol could cause its effect by affecting fork progression; future work should concentrate on testing possible fork stalling at telomeres in the presence of alcohols. It will also be interesting to analyze industrial yeast strains, used in the ethanol and biotechnology industries (Chidi et al. 2016; Cubillos 2016; Piccirillo et al. 2016; Yadav et al. 2016).
Although these new observations regarding telomere regulation were mostly concluded from experiments involving exposure to ethanol, they provide us also with new insights into normal telomere regulation and emphasize the complexity of telomere length regulation.
References
Abdallah P, Luciano P, Runge KW, Lisby M, Geli V, Gilson E, Teixeira MT (2009) A two-step model for senescence triggered by a single critically short telomere. Nat Cell Biol 11:988–993. doi:10.1038/ncb1911
Adamczyk J, Deregowska A, Panek A, Golec E, Lewinska A, Wnuk M (2016) Affected chromosome homeostasis and genomic instability of clonal yeast cultures. Curr Genet 62:405–418. doi:10.1007/s00294-015-0537-3
Arneric M, Lingner J (2007) Tel1 kinase and subtelomere-bound Tbf1 mediate preferential elongation of short telomeres by telomerase in yeast. EMBO Rep 8:1080–1085. doi:10.1038/sj.embor.7401082
Blackburn EH (2000) Telomeres and telomerase. Keio J Med 49:59–65
Blackburn EH (2001) Switching and signaling at the telomere. Cell 106:661–673
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622. doi:10.1038/nrg1656
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132. doi:10.1002/(SICI)1097-0061(19980130)14:2<115:AID-YEA204>3.0.CO;2-2
Chakhparonian M, Faucher D, Wellinger RJ (2005) A mutation in yeast Tel1p that causes differential effects on the DNA damage checkpoint and telomere maintenance. Curr Genet 48:310–322. doi:10.1007/s00294-005-0020-7
Chan SW, Blackburn EH (2002) New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 21:553–563
Chang M, Arneric M, Lingner J (2007) Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev 21:2485–2494. doi:10.1101/gad.1588807
Chidi BS, Rossouw D, Bauer FF (2016) Identifying and assessing the impact of wine acid-related genes in yeast. Curr Genet 62:149–164. doi:10.1007/s00294-015-0498-6
Chymkowitch P, Nguea AP, Aanes H, Koehler CJ, Thiede B, Lorenz S, Meza-Zepeda LA, Klungland A, Enserink JM (2015) Sumoylation of Rap1 mediates the recruitment of TFIID to promote transcription of ribosomal protein genes. Genome Res 25:897–906. doi:10.1101/gr.185793.114
Collins K, Mitchell JR (2002) Telomerase in the human organism. Oncogene 21:564–579
Cubillos FA (2016) Exploiting budding yeast natural variation for industrial processes. Curr Genet 62:745–751. doi:10.1007/s00294-016-0602-6
de Lange T (2009) How telomeres solve the end-protection problem. Science 326:948–952
Eleutherio E, Panek A, De Mesquita JF, Trevisol E, Magalhaes R (2015) Revisiting yeast trehalose metabolism. Curr Genet 61:263–274. doi:10.1007/s00294-014-0450-1
Gadaleta MC, Gonzalez-Medina A, Noguchi E (2016) Timeless protection of telomeres. Curr Genet 62:725–730. doi:10.1007/s00294-016-0599-x
Gallardo F, Laterreur N, Cusanelli E, Ouenzar F, Querido E, Wellinger RJ, Chartrand P (2011) Live cell imaging of telomerase RNA dynamics reveals cell cycle-dependent clustering of telomerase at elongating telomeres. Mol Cell 44:819–827. doi:10.1016/j.molcel.2011.09.020
Garbett KA, Tripathi MK, Cencki B, Layer JH, Weil PA (2007) Yeast TFIID serves as a coactivator for Rap1p by direct protein–protein interaction. Mol Cell Biol 27:297–311. doi:10.1128/MCB.01558-06
Girish V, Vijayalakshmi A (2004) Affordable image analysis using NIH Image/ImageJ. Indian J Cancer 41:47
Greider CW (2016) Regulating telomere length from the inside out: the replication fork model. Genes Dev 30:1483–1491. doi:10.1101/gad.280578.116
Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA (1999) Inhibition of telomerase limits the growth of human cancer cells. Nat Med 5:1164–1170. doi:10.1038/13495
Harari Y, Kupiec M (2014) Genome-wide studies of telomere biology in budding yeast. Microbial cell 1:70–80. doi:10.15698/mic2014.01.132
Harley CB, Kim NW, Prowse KR, Weinrich SL, Hirsch KS, West MD, Bacchetti S, Hirte HW, Counter CM, Greider CW et al (1994) Telomerase, cell immortality, and cancer. Cold Spring Harb Symp Quant Biol 59:307–315
Harrington L (2003) Biochemical aspects of telomerase function. Cancer Lett 194:139–154
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636
Hector RE, Ray A, Chen BR, Shtofman R, Berkner KL, Runge KW (2012) Mec1p associates with functionally compromised telomeres. Chromosoma 121:277–290. doi:10.1007/s00412-011-0359-0
Ho YH, Gasch AP (2015) Exploiting the yeast stress-activated signaling network to inform on stress biology and disease signaling. Curr Genet 61:503–511. doi:10.1007/s00294-015-0491-0
Holt SE, Shay JW, Wright WE (1996) Refining the telomere-telomerase hypothesis of aging and cancer. Nat Biotechnol 14:836–839. doi:10.1038/nbt0796-836
Kupiec M (2014) Biology of telomeres: lessons from budding yeast. FEMS Microbiol Rev 38:144–171. doi:10.1111/1574-6976.12054
Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB (1992) Telomere end-replication problem and cell aging. J Mol Biol 225:951–960
Luo K, Vega-Palas MA, Grunstein M (2002) Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev 16:1528–1539. doi:10.1101/gad.988802
Marcand S, Brevet V, Gilson E (1999) Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J 18:3509–3519. doi:10.1093/emboj/18.12.3509
McGee JS, Phillips JA, Chan A, Sabourin M, Paeschke K, Zakian VA (2010) Reduced Rif2 and lack of Mec1 target short telomeres for elongation rather than double-strand break repair. Nat Struct Mol Biol 17:1438–1445. doi:10.1038/nsmb.1947
Millet C, Makovets S (2016) Aneuploidy as a mechanism of adaptation to telomerase insufficiency. Curr Genet 62:557–564. doi:10.1007/s00294-015-0559-x
Nishida N, Jing D, Kuroda K, Ueda M (2014) Activation of signaling pathways related to cell wall integrity and multidrug resistance by organic solvent in Saccharomyces cerevisiae. Curr Genet 60:149–162. doi:10.1007/s00294-013-0419-5
Nishida-Aoki N, Mori H, Kuroda K, Ueda M (2015) Activation of the mitochondrial signaling pathway in response to organic solvent stress in yeast. Curr Genet 61:153–164. doi:10.1007/s00294-014-0463-9
Paciotti V, Clerici M, Scotti M, Lucchini G, Longhese MP (2001) Characterization of mec1 kinase-deficient mutants and of new hypomorphic mec1 alleles impairing subsets of the DNA damage response pathway. Mol Cell Biol 21:3913–3925. doi:10.1128/MCB.21.12.3913-3925.2001
Piccirillo S, Kapros T, Honigberg SM (2016) Phenotypic plasticity within yeast colonies: differential partitioning of cell fates. Curr Genet 62:467–473. doi:10.1007/s00294-015-0558-y
Ritchie KB, Mallory JC, Petes TD (1999) Interactions of TLC1 (which encodes the RNA subunit of telomerase), TEL1, and MEC1 in regulating telomere length in the yeast Saccharomyces cerevisiae. Mole Cell Biol 19:6065–6075
Romano GH, Harari Y, Yehuda T, Podhorzer A, Rubinstein L, Shamir R, Gottlieb A, Silberberg Y, Pe’er D, Ruppin E, Sharan R, Kupiec M (2013) Environmental stresses disrupt telomere length homeostasis. PLoS Genet 9:e1003721. doi:10.1371/journal.pgen.1003721
Rubinstein L, Ungar L, Harari Y, Babin V, Ben-Aroya S, Merenyi G, Marjavaara L, Chabes A, Kupiec M (2014) Telomere length kinetics assay (TELKA) sorts the telomere length maintenance (tlm) mutants into functional groups. Nucleic Acids Res 42:6314–6325. doi:10.1093/nar/gku267
Sabourin M, Tuzon CT, Zakian VA (2007) Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol Cell 27:550–561. doi:10.1016/j.molcel.2007.07.016
Schawalder SB, Kabani M, Howald I, Choudhury U, Werner M, Shore D (2004) Growth-regulated recruitment of the essential yeast ribosomal protein gene activator Ifh1. Nature 432:1058–1061. doi:10.1038/nature03200
Shay JW, Wright WE (2011) Role of telomeres and telomerase in cancer. Semin Cancer Biol 21:349–353. doi:10.1016/j.semcancer.2011.10.001
Shore D, Bianchi A (2009) Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J 28:2309–2322. doi:10.1038/emboj.2009.195
Simon MN, Churikov D, Geli V (2016) Replication stress as a source of telomere recombination during replicative senescence in Saccharomyces cerevisiae. FEMS Yeast Res 16:fow85. doi:10.1093/femsyr/fow085
Simonicova L, Dudekova H, Ferenc J, Prochazkova K, Nebohacova M, Dusinsky R, Nosek J, Tomaska L (2015) Saccharomyces cerevisiae as a model for the study of extranuclear functions of mammalian telomerase. Curr Genet 61:517–527. doi:10.1007/s00294-014-0472-8
Smogorzewska A, de Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73:177–208
Teixeira MT, Arneric M, Sperisen P, Lingner J (2004) Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117:323–335
Tercero JA, Longhese MP, Diffley JF (2003) A central role for DNA replication forks in checkpoint activation and response. Mol Cell 11:1323–1336
Tsukamoto Y, Taggart AK, Zakian VA (2001) The role of the Mre11-Rad50-Xrs2 complex in telomerase-mediated lengthening of Saccharomyces cerevisiae telomeres. Curr Biol 11:1328–1335
Wade JT, Hall DB, Struhl K (2004) The transcription factor Ifh1 is a key regulator of yeast ribosomal protein genes. Nature 432:1054–1058. doi:10.1038/nature03175
Wotton D, Shore D (1997) A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev 11:748–760
Yadav KK, Singh N, Rajasekharan R (2016) Responses to phosphate deprivation in yeast cells. Curr Genet 62:301–307. doi:10.1007/s00294-015-0544-4
Acknowledgements
We thank all members of the Kupiec Group for encouragement and support. We thank Raymond Wellinger for strains and Judy Berman (Tel Aviv University) for Rap1-specific antibodies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by grants from the Israel Science Foundation, Israel Cancer Association and Israel Cancer Research Foundation to M.K.
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Harari, Y., Kupiec, M. Mec1ATR is needed for extensive telomere elongation in response to ethanol in yeast. Curr Genet 64, 223–234 (2018). https://doi.org/10.1007/s00294-017-0728-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-017-0728-1