Abstract
Saccharomyces cerevisiae strains used for winemaking show a wide range of fermentation phenotypes, and the genetic background of individual strains contributes significantly to the organoleptic properties of wine. This strain-dependent impact extends to the organic acid composition of the wine, an important quality parameter. However, little is known about the genes which may impact on organic acids during grape must fermentation. To generate novel insights into the genetic regulation of this metabolic network, a subset of genes was identified based on a comparative analysis of the transcriptomes and organic acid profiles of different yeast strains showing different production levels of organic acids. These genes showed significant inter-strain differences in their transcription levels at one or more stages of fermentation and were also considered likely to influence organic acid metabolism based on existing functional annotations. Genes selected in this manner were ADH3, AAD6, SER33, ICL1, GLY1, SFC1, SER1, KGD1, AGX1, OSM1 and GPD2. Yeast strains carrying deletions for these genes were used to conduct fermentations and determine organic acid levels at various stages of alcoholic fermentation in synthetic grape must. The impact of these deletions on organic acid profiles was quantified, leading to novel insights and hypothesis generation regarding the role/s of these genes in wine yeast acid metabolism under fermentative conditions. Overall, the data contribute to our understanding of the roles of selected genes in yeast metabolism in general and of organic acid metabolism in particular.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wine acidity is influenced by the level of organic acids in grape berries at harvest, as well as the organic acids produced or utilised during alcoholic fermentation. Grape-derived organic acids include primarily tartaric, malic and citric acid. They contribute the highest proportion of titratable acidity in wines (Defilippi et al. 2009). Yeast-derived organic acids, primarily succinic, acetic and pyruvic acid, also contribute significantly to the sensorial properties and organoleptic characteristics of wines (Lambrechts and Pretorius 2000). Importantly, individual acids not only contribute to general or total acidity but also have their own sensory character when present above individual perception thresholds. While most organic acids are considered as positive contributors to wine flavour and aroma when present at levels above their sensory thresholds, high concentrations of individual acids may lead to undesirable sensory profiles. Succinic acid may for example be perceived as bitter or metallic, acetic acid as vinegary and pyruvic acid as slightly sour (Mato et al. 2005).
The metabolic pathways responsible for the production of these acids are primarily linked to central carbon metabolism but are also impacted by nitrogen and phosphate metabolic pathways in particular. While these metabolic pathways have been well studied and mapped, little information exists on which genes might be responsible for the significant differences in acid production observed among different yeast strains (Magyar et al. 2014; Erasmus et al. 2004; Pigeau et al. 2007; Rossouw et al. 2009). Understanding the roles of these genes will lead to a better understanding of the genetic regulation of acid metabolising pathways and could aid the selection of yeast strains for the production of wines with well-balanced organic acids levels to achieve desirable sensory outcomes in a given scenario.
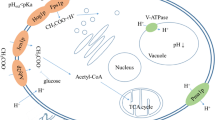
In S. cerevisiae, central carbon metabolism has been studied in depth; however, organic acid metabolism and the production and release of these acids under fermentative conditions (as well as the regulation of the metabolic networks involved) are not fully understood. Metabolic pathways such as glycolysis, the glyoxylate cycle and Krebs cycle (TCA) all have organic acids as intermediates or by-products and therefore have the potential to impact on the concentration of organic acids in wine. Besides carbon metabolism, nitrogen metabolism-related pathways may also act as a major source of acids such as pyruvic and succinic acid (Camarasa et al. 2003; Magyar et al. 2014; Torrea and Henschke 2004; Vilanova et al. 2007). Figure 1 presents an overview of the major pathways involved in organic acid metabolism.
Diagrammatic representation of pathways associated with organic acid production under anaerobic conditions. (Gene names encoding the relevant enzymes are indicated in bold italics, and only those genes that were targeted in the deletion study are indicated on the pathway maps). The diagram also shows specific points that are required for organic nitrogen fixation as well as metabolic pathways that are discussed in this study
Transcriptomic studies provide useful information regarding the specific function of genes or groups of genes and their characteristics (Hirasawa et al. 2010). Transcriptomic studies of wine yeast have been employed successfully to identify genes that influence the production of volatile aroma compounds during fermentation (Rossouw et al. 2008). The aim of our study was to identify genes which may have an impact on organic acid production during alcoholic fermentation using a similar approach. For this purpose, previously generated transcriptomic data of five industrial yeast strains at three different time points were analysed in order to identify differentially expressed genes that may be linked to changes in organic acid profiles. From the list of candidate genes identified in this unbiased manner, target genes for further experimentation were subsequently selected based on their known roles in or association with carbon and/or nitrogen metabolism in order to narrow down the list of candidate genes for experimental validation.
A final set of 10 genes identified in this manner were evaluated in our study using the corresponding knockout strains from the EUROSCARF deletion library. Fermentations were carried out using these strains to determine the metabolic impact of gene deletion on the organic acid profiles produced by these strains.
Materials and methods
Strains, media and culture conditions
Five commercial wine yeast strains were selected based on their divergent fermentation properties (Rossouw et al. 2008). The deletion mutants used in this study were obtained from the EUROSCARF deletion library. The genes and their current annotation (obtained from Saccharomyces Genome Database) describing likely or established roles in cellular metabolism are listed in Table 1. Cells were maintained on solid medium YPD which was supplemented with 2 % agar (Biolab, South Africa). Pre-cultures were carried out in 50 ml shake-flasks at 30 °C, 250 rpm in YPD synthetic media containing 1 % yeast extract (Biolab, South Africa), 2 % peptone (Fluka, Germany) and 2 % dextrose (Sigma, Germany). Fermentations were inoculated at an OD600 of 0.1 (i.e. a final cell density of approximately 106 cfu.ml-1) after harvesting and washing pre-cultured cells with sterile distilled water.
Fermentation medium
Fermentation experiments using wine yeast strains BM45, EC1118, 285, DV10 and VIN13 were conducted in the synthetic must MS300 which resembles a natural must as previously described (Bely et al. 1990). The medium contained equivalent amounts of 100 g/L each of glucose and fructose (total sugars of 200 g/L), and the pH was set to 3.5 using sodium hydroxide. Fermentations were carried out under anaerobic conditions in 250 ml Erlenmeyer flasks (containing 100 ml of the fermentation medium) at a temperature of 25 °C. Flasks were sealed with rubber stoppers with a CO2 opening, and no stirring was done during the course of the fermentation. These batch fermentations were carried out in triplicate. The fermentation progress was monitored by daily weight measurements to determine CO2 loss, and samples from these fermentations were taken at days 2 (exponential phase), 5 (early stationary) and 14 (late stationary phase). Likewise, fermentations inoculated with deletion strains were conducted in synthetic must MS300 at 200 g/L, pH 3.5 and 25 °C. Fermentations were monitored by weight loss and samples were taken at days 3, 6, 12, 16, 22 and 30 for chemical analysis. These sampling days were selected to cover the range of growth phases of the yeast (exponential, early and late stationary), which in the case of the deletion strains followed a more extended cycle across a longer period of time compared to the wine yeast strains. For most of the deletion strains, fermentations were complete by day 30 as opposed to day 14 as in the case for the industrial yeasts. This is due to the lower fermentative capacity of the haploid laboratory strain BY4742 which serves as the genetic background for all the gene knockouts in our study. Cell growth was monitored at 2 day intervals by measuring the optical density (at 600 nm) using a spectrophotometer (PowerwaveX, Bio-Tek Instruments).
Chemical analyses
High-performance liquid chromatography
Culture supernatants were obtained and analysed for glucose, fructose, glycerol and ethanol by high-performance liquid chromatography (HPLC) on an AMINEX HPX-87H ion exchange column at 55 °C using 5 mM H2SO4 as the mobile phase at a flow rate of 0.5 ml min−1. Agilent RID and UV detectors were used for detection and quantification. Analyses were carried out using the HP Chemstation software package (Eyeghe-Bickong et al. 2012).
Enzymatic assays
Culture supernatants were filtered through 0.45 uM nylon membranes and analysed by an enzyme robot (Arena 20XT; Thermo Electron, Finland) using the Enzytec™ Fluid Acetic acid test kit (Thermo Fisher Scientific, Finland) for acetic acid quantification, Boehringer Mannheim/R-Biopharm Succinic acid kit (R-Biopharm AG, Darmstadt) for succinic acid determination as well as the Megazyme Pyruvic acid kit (Megazyme International Ireland) for pyruvic acid quantification. NADH consumption in these assays was measured by the decrease in absorbance at 340 nm.
Microarray analysis and data processing
Microarray data generated for the five yeast strains under similar fermentation conditions at identical sampling points (days 2, 5 and 14) were generated by Rossouw et al. (2008). The transcript data can be obtained from the Gene Expression Omnibus (GEO) repository under Accession Number GSE11651.
Principal component analysis
Principal component analysis (PCA; Latentix 2.0, BRANDON GRAY INTERNET SERVICES, INC. DBA) was used in order to evaluate the impact of gene deletions on glycerol, succinic, acetic and pyruvic acid production at different physiological stages of yeast during fermentation (days 3, 6, 12, 16, 22 and 30). The transformation of the data was carried out using the autoscale function with no validation.
Statistical analysis
SAM (Significance Analysis of Microarrays) version 2 was used to determine the differential gene expression between experimental parameters as described by Rossouw et al. (2008). Genes with a Q value less than 0.5 were regarded as differentially expressed using the two-class, unpaired setting. Consideration was given to those genes with a fold change higher than two (positive or negative) for inter- and intra-strain comparisons. Statistical analysis of differences in organic acid concentrations was performed by means of T tests (Excel), tail 1 (directional test/one tailed distribution) and type 1 (repeated measure design/paired test).
Results
Fermentation kinetics and organic acid profiles of wine yeast
The selected commercial wine yeast strains were inoculated to ferment synthetic must under representative winemaking conditions. The strains show some variation in their fermentation kinetics (Fig. 2), which is in line with previous observations (Rossouw et al. 2008). For the purpose of this study, fermentation conditions similar to those of Rossouw et al. (2008) were selected for the acid analysis and subsequent identification and modelling of key genes. The only difference between the fermentation conditions used in our study and those of Rossouw et al. (2008) was that the initial sugar concentration was 200 g/L as opposed to 250 g/L. However, the fermentation kinetics (and total duration of fermentation) were similar in our fermentations suggesting that the alignment of transcriptome and organic acid production was feasible. Sampling for organic acids was conducted at the same time points and physiological stages (days 2, 5 and 14) of fermentation in our study to replicate the experiments of Rossouw et al. (2008).
Carbon dioxide release (indicative of the rate of fermentation) was similar in fermentations with BM45, 285, VIN13 and EC1118, while DV10 released less carbon dioxide (Fig. 2a). Higher biomass formation (measured as optical density) was observed for strain 285, while less biomass formation was observed for strain DV10 (Fig. 2b). Growth rates and biomass formation of the other three strains were similar in the conditions used.
Differences in organic acid concentrations produced by the five strains at different time points were investigated (Fig. 3). The concentrations of fermentation-derived organic acids (succinic, pyruvic and acetic acid) were evaluated at day 2 (exponential phase), day 5 (early stationary phase) and day 14 (late stationary phase). All strains behaved similarly with regard to acetic, succinic and pyruvic acid production trends though the absolute concentrations of acids produced varied between strains. A continuous increase in succinic acid throughout fermentation was noted in all strains. The levels of pyruvic and acetic acid remained more or less constant across time points for all strains indicating that acetic and pyruvic acid production by these strains occurred mostly during the early stages of fermentation.
Strain identity significantly affected the level of individual organic acid production, with some strains consistently producing higher or lower levels of specific acids. For example, VIN13 produced significantly higher succinic acid concentrations throughout fermentation, while DV10 produced significantly lower succinic acid concentrations across all time points compared to the other strains. VIN13 showed significantly higher pyruvic acid levels throughout fermentation, while concentrations in DV10 fermentations were the lowest compared to other strains. Strain 285 also produced significantly higher pyruvic acid levels compared to DV10, EC1118 and BM45 at day 2 (Fig. 4a) and day 5 (Fig. 4b). The levels of acetic acid at days 2 and 5 were higher for fermentations conducted by strain 285 compared to the other strains tested. DV10 showed slightly lower acetic acid levels at all three time points considered.
It is evident from these data that organic acid profiles of the fermenting wines are both strain and time point dependent. These variations present the opportunity to establish genetic and metabolic relationships between relevant genes and organic acids for the yeast at different time points.
Transcriptional analysis
Considering the central metabolic pathways that are primarily producing organic acids as intermediates or sometimes end-products, it is obvious that a very large number of metabolic enzymes could potentially impact the production and release of these compounds during fermentation. In order to limit the number of target genes and to identify likely role players in the context of wine fermentation conditions, we analysed previously generated gene expression data for differential gene expression between strains showing different production levels of wine-relevant acids. Genes were first identified based on the intra- and inter-strain comparisons of expression levels obtained from the transcriptome data of BM45, VIN13, 285, DV10 and EC1118 (Rossouw et al. 2008). A large number of metabolic genes were identified which showed differential expression (between strains) at one or more stages of fermentation, or between time points. To narrow down these outputs, a subset of genes was selected from the differentially expressed gene list based on their known link to glycerol or organic acid metabolism, organic acid transportation, amino acid metabolism and redox balance. Nine organic acid-related transcripts/genes that were significantly up/down regulated based on the microarray analysis at days 2, 5 and 14 (Tables 2, 3; Supplementary Tables S1, S2) were selected in this manner for further investigation and experimentation. An additional three genes (OSM1, SER1 and SER33) were selected based solely on a priori knowledge of gene function. This was done to ensure that the selected gene set was representative of a number of major acid contributing pathways. The inter-strain fold changes of the total set of 12 selected genes (Table 2 and Supplementary Table S1) highlight the differences in expression of these genes between strains at the different time points, while Table 3 (and Supplementary Table S2) shows the fold changes for the selected genes across time points for each of the five strains.
Some of the genes with the greatest up/down regulation between the five different industrial yeast were ARO10 (−19.7 fold for 285 vs BM45) and AAD6 (24.7 fold for BM45 vs VIN13) at day 2 of fermentation (Table 2a). Genes such as GPD2 (7.99-fold increase for DV10 compared to 285) and AAD6 (6.89-fold increase for BM45 vs VIN13) also showed high fold change differences in the inter-strain comparisons at day 5 (Table 2b). Of interest to the current study, ARO10 exhibited the highest fold change between days 5 and 2 for VIN13 (−9.42), 285 (14.27), BM45 (−12.15), DV10 (−3.94) and EC1118 (−9.86-fold change; Table 3). At day 14, some of the genes with the highest differential expression between strains were AAD6 (−19.97-fold change for DV10 vs BM45), ARO10 (−5.04-fold change for DV10 vs VIN13) and GLY1 (3.17 for DV10 vs 285 and EC1118; Supplementary Table S1).
Deletion studies
In order to further investigate the metabolic roles of these potentially important acid-related genes, the corresponding deletion strains were selected to conduct fermentations in synthetic must. The aim was to evaluate the impact of gene deletion on pyruvic, succinic and acetic acid production during fermentation. While complete deletion of a gene does present an extreme scenario and may lead to metabolic impacts that are not directly related to the genetic perturbation in some cases, gene deletion still presents the standard approach to provide preliminary insights into the role of a gene or genes in a particular context.
BY4742 is the haploid laboratory strain which serves as the genetic background for all the deletions investigated in this study. Due to its ease of use and genetic manipulation, the availability of databases and tools for this strain, and the large amount of published research related to this yeast, it is a favourite model yeast strain in wine research. Numerous studies in the field of wine and fermentation research have been conducted using this laboratory strain as a model for wine yeast primary and secondary metabolism (Styger et al. 2011; Rossouw et al. 2013). BY4742 is a slow growing and fermenting strain under wine-like anaerobic fermentation conditions. As previously stated, the fermentation timescales for this strain are different to those of commercial wine yeast strains. Based on growth curves, day 6 of BY4742 fermentation is similar to day 2 (exponential phase) of most commercial yeast fermentations, and 16 fall comparable to day 5 in commercial yeast fermentations (early stationary phase). Day 30 corresponds to the end of fermentation for the deletion strains, comparable to day 14 in the case of the commercial wine yeast fermentations. While organic acid concentrations were determined at various time points (days 2, 6, 12, 16, 22 and 30), most of our discussion is focussed on data obtained at days 6, 16 and 30 for this reason.
Fermentation kinetics and organic acid profiles produced by deletion mutants
Kinetics of growth and carbon dioxide release (indicative of the rate of fermentation) of all deletion mutants showed similar trends (Fig. 5). Compared to the control strain (BY4742), some deletion mutants exhibited significant changes to their glycerol and organic acid profiles, while others did not show any notable differences by the end of fermentation (Figs. 6, 7; Supplementary Figs. S1–S3).
Acetic acid (frame A), succinic acid (frame B), pyruvic acid (frame C) and glycerol (frame D) production (g/L) at the end of fermentation. BY4742 (control) bars are indicated in black. Values are the average of three independent repeats ± standard deviation. The asterix indicates those values that are statistically significantly different from the control (p < 0.05)
As a product of fermentation, pyruvic acid can be found in small quantities in wine. Compared to other acids, pyruvic acid production was significantly influenced by the deletion of most genes (Fig. 7c). The deletion of redox-related genes such as KGD1, AAD6, ARO10 and SER33 resulted in less pyruvic acid production compared to the control at the end of fermentation. Other deletion strains which showed a significant decrease in pyruvic acid production during fermentation were ICL1 (isocitrate lyase) and AGX1 (alanine: glyoxylate aminotransferase). These genes are directly involved in the glyoxylate pathway during yeast growth. GLY1 (l-threonine aldolase) and OSM1 (fumarate reductase) deletion significantly increased pyruvic acid production at the end of fermentation, as well as at the earlier time points of fermentation (Supplementary Fig. S3).
Compared to the reference strain, osm1∆, kgd1∆, aad6∆, icl1∆, agx1∆ and aro10∆ mutants resulted in fermentations with slightly higher acetic acid levels at the end of fermentation (Fig. 7a). Similar trends were also observed at other time points (i.e. days 12, 16 and 22; Supplementary Fig. S2).
The most significant increase in succinic acid production was observed in the case of SER33 and KGD1 deletion (Fig. 7b). Strains icl1∆ and ser1∆ (Supplementary Fig. S1C) resulted in fermentations with increased succinic acid production at day 12 of fermentation while gly1∆ mutants led to increased concentrations of this acid at day 6 (Supplementary Fig. S1B).
The impact of gene deletion on glycerol levels in wine is shown in Fig. 7d. Although several deletion strains (i.e. adh3∆, sfc1∆ and ser1∆) appeared to produce slightly higher concentrations of glycerol compared to the control, these differences were not statistically significant at the end of fermentation. Only the deletion of GLY1 led to significantly increased glycerol levels, while GPD2 deletion led to the expected decrease in glycerol concentrations.
Principal component analysis
Principal component analysis was carried out using organic acid data for the five strains at different time points (days 2, 6, 12, 16, 22 and 30). The results show a clear separation of samples into time point clusters (data not shown). To highlight strain-dependent groupings, the PCA plot below (Fig. 8) shows sample groupings based on organic acid concentrations at two time points only (days 6 and 16) for all 12 deletion strains. Groupings were observed based on the two different stages of fermentation as well as acid-gene relationships.
Principal component analysis of succinic, acetic, pyruvic acid and glycerol data at different time points (day 6; purple and day 16; green). Samples are labelled based on the gene name and day of sampling (e.g. GLY1-6 represents the GLY1 deletion strain at day 6 of fermentation). Biological repeats (in triplicate) are shown
The PCA in Fig. 8 accounts for 91.55 % of the total explained variance in the dataset. Separation along the first principal component axis was dominated by differences in glycerol, succinic and acetic acid concentrations. As expected the time point, stage of fermentation was the main factor driving the separation of clusters in the first principal component, which accounts for the majority (73 %) of explained variance. Differences in pyruvic acid between treatments were clearly a strong contributor to variance explained by both the first and second principal components.
In terms of strain impacts, gly1∆, icl1∆, gpd2∆ and osm1∆ mutants formed clearly distinct groupings at the exponential phase (day 6), while adh3∆, aro10∆ and ser33∆ mutants were separated from the rest of the strains based on organic acid profiles at early stationery phase (day 16).
Discussion
In the current study, efforts have been applied to understand organic acid metabolism in yeast through exploration of yeast strains carrying deletions for genes which may contribute towards acidity in wine. These genes were identified through a combination of biased and un-biased methods. Firstly, we analysed transcriptional differences between strains displaying differences in acid production but focused on genes with known or expected impacts on carbon and nitrogen metabolism. Genes selected in this manner were ARO10, SER1, SER33, GPD2, OSM1, AGX1, KGD1, GLY1, ADH3, SFC1, ICL1 and AAD6. The impact of the deletion of these 12 differentially expressed metabolic genes on organic acid metabolism was investigated in fermentation studies using the corresponding deletion strains. The deletion of several genes affected organic acid metabolism, while a few did not appear to impact acid production during fermentation in a significant manner. A summary of the organic acid outcomes for the deletions throughout fermentation are shown in Fig. 9 below. Only those genes which showed a significant impact on glycerol and/or organic acid concentrations at either the exponential (day 6), early stationary (day 16) and late stationary (day 30) growth phases are indicated in the figure.
A pathway representation showing the involvement in organic acid metabolism of the genes which were absent in the deletion strains used to conduct fermentations. The data boxes for each gene highlight statistically significant changes (up arrow for an increase and down arrow for a decrease) in the levels of the organic acids at the exponential phase (day 6), early stationary phase (day 16) and end of fermentation (day 30) for the deletion strain compared to the control (BY4742). The width of the arrows represents the magnitude of the increase/decrease relative to the control
Impact of deletion of redox-related genes on growth, organic acids and glycerol production
The fermentation kinetics of the deletion strains that were selected in this study showed similar trends in the fermentation conditions used. However, the GPD2 mutant showed reduced glucose/fructose consumption and ethanol and glycerol production (Fig. 6). This can be explained by the growth-inhibiting accumulation of glycerol-3-phosphate in the cells in the absence of GPD2, as proposed by Pahlman et al. (2001). Relative to the reference (BY4742) strain, the gpd2∆ deletion mutants showed lower glycerol production at the end of fermentation (Fig. 7d) confirming the importance of GPD2 for glycerol production. Overexpression of this gene is indeed well characterised in terms of increased glycerol production in S. cerevisiae (Michnick et al. 1997; Remize et al. 1999; De Barros Lopes et al. 2000). These authors also reported significant increases in acetic acid when GPD2 is over-expressed. In our study, however, GPD2 deletion only led to a small, though statistically insignificant, decrease in acetic acid levels at days 12 (Supplementary Fig. S2C) and 16 (Supplementary Fig. S2D), suggesting that the production of acetic acid in this mutant may not be stringently linked to redox balancing. Otherwise, the GPD2 mutation did not significantly affect any of the other organic acids considered.
The role of SER33 and SER1 on organic acid metabolism in yeast
SER33 and SER1 deletion led to an increase in succinic acid levels at the end of fermentation (Fig. 7b). This is in line with the results of a previous study attempting to increase succinic acid concentrations. Otero et al. (2013) noted higher yields of succinic acid in a chemically defined minimal medium in shake flask cultures when ser33∆, ser3∆ and sdh1∆ deletion mutants were tested. Ser33p plays an important role in the biosynthesis of amino acids and is one of the key enzymes in the glyoxylate pathway. A possible explanation for this increase in succinic acid in our study is based on the disruption of serine and glycine biosynthesis in ser33∆ mutants. Under these conditions, cells will use the alternative pathway from isocitrate to produce glycine and serine. In this pathway, isocitrate is converted to glyoxylate and succinate by Icl1p. Succinate is thus indirectly produced as a by-product of the reaction, which accounts for the increased succinate concentrations when serine and glycine biosynthesis are forced to proceed via isocitrate (Fig. 1). Ser1p is likewise responsible for catalysing one of the final reactions in serine biosynthesis. Disruption of this gene would be expected to have the same metabolic impact as SER33 deletion in terms of increasing succinic acid production as a by-product of glyoxylate and ultimate serine biosynthesis from isocitrate.
The role of KGD1 on organic acid metabolism in yeast
KGD1 (alpha-ketoglutarate dehydrogenase) encodes a key enzyme of the TCA cycle. Some work has been done to assess the potential roles of KGD1 on succinic acid production under fermentative conditions. Arikawa et al. (1999) showed 1.5-fold higher succinic acid production by kgd1∆ mutants compared to the wild-type strain K901 during sake (Japanese alcoholic beverage) fermentation. The increased succinic acid observed in our study confirms this observation (Fig. 7b).
Our findings may be linked to the fact that KGD1 catalyses the conversion of alpha-ketoglutarate to isocitrate and deletion results in the interruption of the oxidative branch of the TCA cycle. This means that carbon entering the TCA cycle would likely be channelled to succinate via the reductive branch of the TCA cycle as an alternative pathway. Alpha-ketoglutarate is required for ammonium fixation and biomass formation during fermentation. This would mean that KGD1 disruption during the exponential growth phase of the yeast would result in a build-up of alpha-ketoglutarate, which could be partially channelled to ammonium fixation and growth during the earlier stages of fermentation. This would explain why no increase in succinate (via the reductive branch of the TCA cycle) was observed during the early stages of fermentation (Supplementary Fig. S1; Fig. 9). However, when active biomass formation has ceased and no ammonium fixation takes place, carbon entering the TCA from glycolysis might be redirected to succinate via the reductive branch to avoid accumulation of alpha-ketoglutarate (Fig. 1). This again accounts for the increase in succinate concentrations towards the later stages of fermentation by the KGD1 deletion strain (Fig. 7b).
Other strains of interest
GLY1 deletion (threonine aldolase, a key enzyme involved in glycine biosynthesis) significantly increased pyruvic acid production throughout fermentation (Supplementary Fig. S3). The link between pyruvic acid production and GLY1 gene disruption has not received much consideration in literature. Glycine is primarily synthesised from threonine, which is derived from aspartate, which is derived from oxaloacetate and which is in turn derived from pyruvate. The disruption of this chain of events could have led to a build-up of pyruvic acid due to the disruption of glycine biosynthesis. This and the previous examples highlight the complexity of the metabolic interplay between reaction networks involved in central carbon metabolism and amino acid biosynthesis.
In the current study, other genes (ARO10, SER1, AGX1, SFC1 and OSM1) significantly affected pyruvic acid metabolism throughout fermentation. Although their deletion mutants showed different levels of pyruvic acid at different physiological stages, the deletion of AGX1, SFC1 and ARO10 genes resulted in significantly higher pyruvic acid levels at the early stages of fermentation but these differences diminished as fermentation progressed. These differences at the later stages of fermentation were, however, still statistically significant compared to the wild type.
There is no information available regarding how these genes impact on pyruvic acid metabolism during fermentation, however, previous reports have shown a 58.6 % decrease in pyruvic acid yield when S. cerevisiae sfc1∆ deletion mutants were evaluated under micro-aerobic conditions (Zhang et al. 2007). Interestingly, aro10∆ mutants showed significantly lower pyruvic acid levels at all stages of fermentation when compared to the wild type (Supplementary Fig. S3). ARO10 is primarily responsible for the decarboxylation of phenylpyruvate to phenyl acetaldehyde during fermentation. No plausible hypothesis for the possible indirect role of Aro10p in pyruvic acid metabolism could be formulated. The disruption of OSM1 (Osm1p is involved in the reoxidation of intracellular NADH under anaerobic conditions) resulted in a remarkable increase in pyruvic acid at the end of fermentation. This is not surprising considering that Osm1p plays a central role in TCA cycle, which accounts for the build-up of pyruvate from glycolysis as the TCA cycle is disrupted.
Apart from GPD2, the deletion of two other NADH requiring genes (KGD1 and ICL1) did not significantly affect growth in this study. Famili et al. (2003) reported increased growth on a defined complete glucose media when osm1∆, kgd1∆ and icl1∆ strains were assayed. In a different study, the deletion of OSM1 did not affect anaerobic cell growth (Camarasa et al. 2007). In contrast, our data showed a slight decrease in optical density throughout fermentation by osm1∆ mutants (Fig. 5). OSM1 is one of the genes required for the reoxidation of intracellular NADH under anaerobic conditions. The disruption of this gene, therefore, would result in an NAD+/NADH imbalance which could eventually lead to reduced cell growth as a result of the lack of ATP generation.
Conclusions
The current study is the first to utilise comparative transcriptomic and organic acid datasets to identify genes of interest in the context of wine yeast organic acid metabolism. Deletion studies were carried out to further improve our understanding of acid evolution during fermentation. In some instances, gene deletion did not lead to the expected outcomes, and results differed from those obtained in previous studies. This is not surprising since the use of wine-like anaerobic fermentation conditions presents a different experimental framework for the analysis of gene function. Moreover, unexpected results are likely when considering the complex nature of central carbon metabolism and associated pathways. Each of the three acids considered in our study can indeed be produced through several interconnected pathways. However, it is generally assumed that during fermentation, only one or two major pathways are really responsible for the production of these acids. For succinic acid, the reductive branch of the TCA cycle has been proposed as the major production pathway under fermentative conditions, which agrees with our data. Likewise, our data showed that acetic and pyruvic acids were primarily released during the early stages of fermentation, which aligns with expectations as acetic production is mostly attributed to cellular redox balancing. Redox balancing is essential when cells are engaged in active biomass formation in the earlier stages of fermentation.
As previously stated, the regulation of carbon flux through the various interconnected pathways which together influence the levels of organic acids is highly complex and integrated. This means that expectation based on single gene considerations are likely to underestimate the integrated network response to the genetic perturbation introduced by deletion of the target gene/s. Central carbon metabolism is tightly regulated by external and internal factors. Organic acids, being mostly by-products or intermediates of these pathways, are therefore likely to be subject to the same level of regulation.
In our study, analyses were carried out at three time points during fermentation for the investigation of gene expression and acid relationships. The datasets are thus not continuous, and valuable information may be found between the discrete time points selected in our study.
Despite these limitations and potential shortfalls, the use of an approach aimed at incorporating both transcriptomic and organic acid data aided our identification of genes which play important roles in acid evolution in synthetic must under fermentative conditions. For several genes (most notably KGD1 and SER33), interesting changes in organic acid levels were observed in fermentations conducted with the deletion strains. The data presented here provide a useful platform for further investigations into the genetic factors which are responsible for differences in acid evolution between distinct wine yeast strains.
References
Albers E, Laize V, Blomberg A, Hohmann S, Gustafsson L (2003) Ser3p (Yer081wp) and Ser33p (Yil074cp) are phosphoglycerate dehydrogenases in Saccharomyces cerevisiae. J B Chem 278:10264–10272
Arikawa Y, Kuroyanagi T, Shimosaka M, Muratsubaki H, Enomoto K, Kodaira R, Okazaki M (1999) Effect of gene disruptions of the TCA cycle on production of succinic acid in Saccharomyces cerevisiae. J Biosci Bioeng 87:28–36
Bely M, Sablayrolles JM, Barre P (1990) Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J Ferment Bioeng 70:245–252
Camarasa C, Grivet JP, Dequin S (2003) Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways of succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology 149:2669–2678
Camarasa C, Faucet V, Dequin S (2007) Role in anaerobiosis of the isoenzymes for Saccharomyces cerevisiae fumarate reductase encoded by OSM1 and FRDS1. Yeast 24:391–401
De Barros Lopes M, Rehman AU, Gockowiak H, Heinrich AJ, Langridge P, Henschke PA (2000) Fermentation properties of a wine yeast overexpressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2). Aust J Grape Wine Res 6:208–215
Defilippi BG, Manríquez D, Luengwilai K, GonzálezAgüero M (2009) Aroma volatiles: biosynthesis and mechanisms of modulation during fruit ripening. Adv bot res 50:1–37
Drewke C, Thielen J, Ciriacy M (1990) Ethanol formation in Adh mutants reveals the existence of a novel acetaldehyde reducing activity in Saccharomyces cerevisiae. J Bacteriol 172:3909–3917
Erasmus DJ, Cliff M, van Vuuren HJJ (2004) Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of Icewine. Am J Enol Vitic 55:371–378
Eyeghe-Bickong HA, Alexandersson EO, Gouws LM, Young PR, Vivier MA (2012) Optimisation of an HPLC method for the simultaneous quantification of the major sugars and organic acids in grapevine berries. J Chrom 885:43–49
Famili I, Forster J, Nielsen J, Palsson BO (2003) Saccharomyces cerevisiae phenotypes can be predicted by using constraint-based analysis of a genome-scale reconstructed metabolic network. PNSA 131:13134–13139
Fernandez M, Fernandez E, Rodicio R (1994) ACR1, a gene encoding a protein related to mitochondrial carriers, is essential for acetyl-CoA synthetase activity in Saccharomyces cerevisiae. Mol Gen Genet 242:727–735
Hirasawa T, Furusawa C, Shimizu H (2010) Saccharomyces cerevisiae and DNA microarray analyses: what did we learn from it for a better understanding and exploitation of yeast biotechnology. Appl Microbiol Biotechnol 87:391–400
Ivanov SL, Petrova VY, Pisareva EI, Kujumdzieva AV (2013) Ehrlich pathway study in Saccharomyces and Kluyveromyces yeasts. Biotechnol Biotec EQ 27:4103–4110
Lambrechts MG, Pretorius IS (2000) Yeast and its importance in wine aroma—a review. S Afr J Eno Vitic 21:97–129
Magyar I, Nyitrai-Sardy D, Lesko A, Pomazi A, Kallay M (2014) Anaerobic organic acid metabolism of Candida zemplinina in comparison with Saccharomyces wine yeasts. Int J Food Microbiol 16:1–6
Mato I, Suárez-Luque S, Huidobro JF (2005) A Review of the analytical methods to determine organic acids in grape juices and wines. Food Res Int 38:1175–1188
Melcher K, Rose M, Mkunzler M, Braus GH, Entian KD (1995) Molecular analysis of the yeast SER1 gene encoding 3-phosphoserine aminotransferase: regulation by general control and serine repression. Curr Genet 27:501–508
Michnick S, Roustan JL, Remize F, Barre P, Dequin S (1997) Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 13:783–793
Monschau N, Stahmann KP, Sahm H, McNeil JB, Bognar AL (1997) Identification of Saccharomyces cerevisiae GLY1 as a threonine aldolase: a key enzyme in glycine biosynthesis. FEMS Microbiol Lett 150:55–60
Otero JM, Cimini D, Patil KR, Poulsen SG, Olsson L, Nielsen J (2013) Industrial systems biology of Saccharomyces cerevisiae enables novel succinic acid cell factory. PLoS One 8:e54144. doi:10.1371/journal.pone.0054144
Pahlman AK, Granath K, Ansell R, Hohmann S, Adler L (2001) The yeast glycerol 3-phosphatases Gpp1p and Gpp2p are required for glycerol biosynthesis and differentially involved in the cellular responses to osmotic, anaerobic, and oxidative stress. J Biol Chem 276:3555–3563
Pigeau GM, Bozza E, Kaiser K, Inglis DL (2007) Concentration effect of Riesling Icewine juice on yeast performance and wine acidity. J Mol Microbiol 103:1691–1698
Porro D, Brambilla L, Ranzi BM, Martegani E, Alberghina L (1995) Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol Prog 11:294–298
Prior B, Toh T, Jolly N, Baccari C, Mortimer R (2000) Impact of yeast breeding for elevated glycerol production on fermentative activity and metabolite formation in Chardonnay wine. S Afr J Enol Vitic 21:92–99
Remize F, Roustan JL, Sablayrolles JM, Barre P, Dequin S (1999) Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol 65:143–149
Romagnoli G, Luttik MA, Kotter P, Pronk JT, Daran JM (2012) Substrate specificity of thiamine-pyrophosphate-dependent 2-oxo-acid decarboxylases in Saccharomyces cerevisiae. Appl Environ Microbiol 78:7538–7548
Rossouw D, Naes T, Bauer FF (2008) Linking gene regulation and the exo-metabolome: a comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast. BMC Genom 9:530–548
Rossouw D, Olivares-Hernandes R, Nielsen J, Bauer FF (2009) Comparative transcriptomic approach to investigate differences in wine yeast physiology and metabolism during fermentation. Appl Environ Microbiol 75:6600–6612
Rossouw D, Heyns EH, Setati ME, Bosch S, Bauer FF (2013) Adjustment of trehalose metabolism in wine Saccharomyces cerevisiae strains to modify ethanol yields. Appl Environ Microbiol 79:5197–5207
Styger G, Jacobson D, Bauer FF (2011) Identifying genes that impact on aroma profiles produced by Saccharomyces cerevisiae and the production of higher alcohols. Appl Microbiol Biotechnol 9:713–730
Torrea D, Henschke PA (2004) Ammonium supplementation of grape juice effect on the aroma profile of a Chardonnay wine. Tech Rev 150:59–63
Vilanova M, Ugliano M, Siebert T, Pretorius IJ, Henschke PA (2007) Assimilable nitrogen utilization and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine strains. App Microbiol Biotechnol 77:145–157
Zhang A, Kong Q, Cao L, Chen X (2007) Effect of FPS1 deletion on the fermentation properties of Saccharomyces cerevisiae. Lett Appl Microbiol 44:212–217
Acknowledgments
We thank Stellenbosch University for research facilities. The SARChI grant from the National Research Foundation and Winetech are thanked for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chidi, B.S., Rossouw, D. & Bauer, F.F. Identifying and assessing the impact of wine acid-related genes in yeast. Curr Genet 62, 149–164 (2016). https://doi.org/10.1007/s00294-015-0498-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-015-0498-6