Abstract
Novel diamine containing thiazole ring and double sulfide bond was synthesized, and the related polyamides were prepared by a direct polycondensation reaction of this diamine and various aromatic diacids. The polyamides were obtained in good yields and were characterized by differential scanning calorimetry, thermal gravimetric analysis, Fourier transform infrared, viscosity, solubility, and elemental analysis. The glass transition temperatures of synthesized polyamides were in the range of 120–192 °C. The inherent viscosity of these polyamides was in the range of 0.4–0.62 dL/g. The solubility of these polyamides was investigated in some solvents such as dimethyl sulfoxide, N-methyl-2-pyrrolidinone, and N,N-dimethylformamide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyamides (aramids), duo to high thermal stability, chemical resistance, low flammability, and excellent mechanical were classified as high-performance polymers [1, 2]. Kevlar [poly(p-phenyleneterephthalamide)] and Nomex [poly(m-phenyleneisophthalamide)] are most famous polyamides that are commercially marketed as high-performance polymers [3]. Moreover, they have recently been developed in advanced and interesting applications such as thin-film composite membranes [4, 5], high refractive materials [6, 7], optically active materials [8], and biomaterials [9].
However, the poor solubility, high softening temperatures or melting temperatures (Tm’s) caused by their high crystallinity, and the high stiffness of the polymer backbone lead to a difficult processability for these aromatic amides [10]. Many attempts have been made to increase the solubility of aromatic polyamide with minimal effect on thermal stability including the introduction of flexible (ether) linkages into the polymer backbone [11, 12], bulky pendant groups [13], long flexible side-chains [14], aliphatic parts [15], noncoplanar or unsymmetrical structures [16], and heterocycles rings [17].
It is known that the thioether bond is flexible. It can be incorporated into the polymer backbone to improve the processability of such resins [18] as poly(phenylene sulfide) [19], poly-(phenylene sulfide sulfone) [20], and poly(p-phenylene sulfide sulfone/ketone) [21]. The resulting materials have excellent processability as well as amazing mechanical, thermal, and antioxidant properties [18]. Moreover, recently, considerable attention has been devoted to the high sulfur containing polymer. It was found that the incorporation of sulfur atom into polymer backbones resulted in great benefits for high refractive index as well as low birefringence and good optical transparency of polymer which might be attributed to the its high polarizability. Such materials are strongly demanded in advanced optoelectronic applications including optical encapsulates or adhesives for antireflective coatings, substrates for display devices, microlens for complementary metal oxide semiconductor (CMOS) image sensors, or charge coupled device (CCD) [6, 7, 22, 23]. Regarding these parameters, we designed and synthesized a novel double sulfide-bridged diamine monomer (DA) containing thiazole units and bulky pendant phenyl rings to improve their processability.
Experimental
Materials
Acetophenone (Sigma-Aldrich), thiourea (Sigma-Aldrich), iodine (Sigma-Aldrich), and triphenyl phosphite (TPP, Merck) were used without further purification. N-Methyl-2-pyrrolidone (NMP, Merck), as polymerization solvent and pyridine (Py, Fluka), was purified by distillation under reduced pressure over calcium hydride (CaH2) and was stored over 4 A molecular sieves prior to use. Lithium chloride (LiCl, MERCK) was dried at 200 C for 24 h under vacuum before use. The aromatic dicarboxylic acids such as terephthalic acid (Merck), isophthalic acid (Merck), pyridine 2,5-dicarboxylic acid (3; Merck), and pyridine 2,5-dicarboxylic acid (4; Merck) were used as received.
Characterization
The melting points (mp) were measured by an Electrothermal engineering Ltd 9200 digital (UK) melting point apparatus. The elemental analyses of the synthesized monomer and polymers were obtained using a PerkinElmer 2004 (CHN) analyzer (USA). The Fourier transform infrared (FT-IR) spectra were measured in potassium bromide (KBr) pellets by means of a PerkinElmer FT spectrum RX1 (USA). The inherent viscosities (ηinh) were measured at a concentration of 0.5 g/dL by an Ubbelohde suspended-level viscometer using N,N-dimethylacetamide (DMAc) at 30 °C. The solution-state 1HNMR (300 MHz) and 13CNMR (75 MHz) spectra of the resulting monomer and polymers were obtained on a Bruker DRX 300 AVANCE spectrometer (Germany) using deuterated dimethyl sulfoxide (DMSO-d6) as a solvent and trimethylsilane (TMS) as the reference (0 ppm). Thermogravimetric analyses (TGA) were performed with a Du Pont 2000 thermal analysis system (Mettler Toledo, Switzerland) under nitrogen and air atmospheres at a heating rate of 10 °C/min. Glass transition temperatures (Tg) were recorded on a 2010 differential scanning calorimetry (DSC) thermal analysis (Mettler Toledo, Switzerland) instrument with a heating rate of 10 °C/min.
Synthetic procedures
Monoamine synthesis
Monoamine was synthesized by a modified procedure [24]. A mixture of 10 mmol of acetophenone, 20 mmol of thiourea, and 10 mmol of diiodine was heated and stirred at 110 °C in a solvent free condition (Scheme 1). After the completion of the reaction, the mixture was dissolved in 50 mL of boiling water and the filtered clear solution was neutralized with aqueous solution of sodium hydroxide. The crude product was filtered, washed with water, and recrystallized from H2O/EtOH (1:1) to afford monoamine (MA) (mp = 144–147).
Diamine synthesis
Sulfur monochloride (5 mmol) was added cautiously to a mixture of MA (10 mmol) and acetic acid (15 mL) followed by stirring of the content at room temperature for 12 h. Then aqueous ammonium hydroxide (25%) was added dropwise till the pH of solution became 10. The precipitated disulfides were collected by filtration, washed with cold water, and crystallized from hot ethanol (mp = 191–194).
Polymer synthesis
Polymerization under conventional heating of a mixture of 1 mol of diacid, 1 mmol of diamine (DA), 0.3 g of lithium chloride, 0.5 mL of TPP, 0.5 mL of Py, and 3 mL of NMP was refluxed for 6 h. After cooling, the reaction mixture was poured into 100 mL of methanol with constant stirring. Afterward, the precipitate was washed thoroughly with methanol and hot water, collected on a filter, and dried under vacuum.
Results and discussion
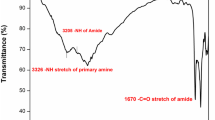
Dimer synthesis
Dimer containing double sulfide and thiazole ring was synthesized according to a two-step procedure as shown in Scheme 1. The chemical structure of this diamine was fully characterized by FT-IR and NMR spectroscopy. FT-IR spectrum of DA monomer showed the absorption bands due to the primary amino groups at 3436 and 3275 cm−1 Fig. 2. Also it revealed distinct stretching peak features of –C=N– thiazole at 1617 cm−1, N–H bending at 1603 cm−1, and aromatic –C=C stretching vibrations at 1509 cm−1. As shown in Fig. 1, the 1H NMR and 13C NMR spectra of the diamine, all the protons are in total agreement with its structure. 13C NMR (75 MHz, DMSO-d6) δ 101.44, 125.48, 127.13, 128.40, 134.84, 149.73, 168.14. 1H NMR (300 MHz, DMSO-d6) δ 6.99 (d, 4H), 7.25 (s, 6H), 7.68 (4H, d).
Polymer synthesis
The new double sulfide bond containing polyamides (PAs) were synthesized by phosphorylation polycondensation of diamine DA with various commercially available aromatic diacid using triphenyl phosphite (TPP) and pyridine as condensing agents (Scheme 2). The reactions were carried out in NMP solution of the diacid and diamine in the presence of LiCl in the nitrogen atmosphere and at a temperature of 110 °C. All the polycondensations progressed readily in a homogeneous solution. Tough and fibrous precipitates formed when the viscous polymer solutions were trickled into the stirring methanol. As shown in Table 1, the inherent viscosities of polymers were in the range of 0.40–0.62 dL/g.
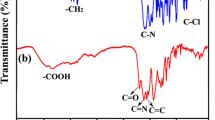
The elemental analysis values generally agreed with the calculated values for the proposed structures of polymers (Table 1). Structural features of these polyamides were verified by IR and proton NMR spectroscopy. They exhibited characteristic FT-IR absorption bands of the amide group around 3337 cm−1 (N–H stretch) 1526 cm−1 (N–H bending), and the strong absorption bands around 1667 is assigned to symmetrical stretching vibrations of carbonyl groups of amide group (Fig. 2). 1H NMR spectra of the polyamide (PA 2) showed amide groups (NH) protons at the most downfield region about (12.5) ppm and aromatic protons at the region of about (7–8.5) ppm (Fig. 3).
Solubility of polymers
The main objective of this study was to produce polyamides with improved solubility. The solubility of polymers was investigated as 0.01 g of polymeric sample in 2 mL of solvent. Due to the presence of thiazole ring as heterocyclic group and rigid aromatic ring and double sulfide bond (–S–S–) in the structure of polyamide, the solubility of this polymer was very high in common organic solvent. All polyamides were easily soluble at room temperature in aprotic polar solvents such as N-methyl-2-pyrrolidinone (NMP), N,N-dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), soluble and partially soluble on heating at 60 °C in tetrahydrofuran (THF) and pyridine (Py), and insoluble in protic solvents such as acetone and methanol (Table 2).
It can be concluded that the presence of double sulfide linkage in the backbone and benzene ring as pendant group of PAs helps to enhance the solubility of polymers. Moreover, the existence of double sulfide linkage causes more flexible polymer chain, and bulky benzene pendant group disturbed dense chain packing. Consequently, the solvents molecules were able to penetrate easily to solubilize the polymer chain.
Thermal properties
The thermal properties of the prepared polymers were investigated by means of TGA as well as DSC. Thermogravimetric analysis was performed on the polyamides under inert atmosphere. Thermograms of these materials are described in Fig. 4, and thermal data are summarized in Table 3. The initial thermal decomposition temperatures for PA 1–4 were found between 160 and 295 °C.
Also, residual weights for PAs at 800 °C ranged from 18 and 13% in nitrogen, respectively. The temperature for 10% weight loss (T10) as one of the main criteria to determine the thermal stability of polymers is determined from the original thermograms and is tabulated in Table 3. T10 values of the PAs were in the range of 250–300 °C in nitrogen atmosphere. According to these data, it can be concluded that incorporation of –S–S– linkages in structure of polymers reduces thermal stability, despite the increase in solubility.
Differential scanning calorimetry (DSC) was used to determine the glass transition temperature values (Tg) of the samples obtained with a heating rate of 10 K/min under nitrogen. The DSC results are summarized in Table 3. The glass transition temperature (Tg) is determined in the second heating runs of DSC measurements. Tg of these polymers is about 119–192 °C which depended on the structure of the dicarboxylic acid component and decreased with increasing flexibility of the PA backbones according to the applied structure of the diacid. PA 1 exhibited the highest Tg value among these PAs, which could be attributed to its stiffness and symmetry of 1,4-phenylene segments in the polymer backbone. Moreover, PA 4 with meta-substituted Py rings had a Tg of 144 °C, whereas a higher value of 154 °C was observed for PA 3, which had para-substituted Py rings. Thus, we can speculate that incorporation of flexible groups (–S–S–) into the polymer chain serves to moderate the thermal stability and the Tg values of the PA.
Conclusions
In this work, specific synthetic methods were designed to obtain soluble polyamides, which have not precedents in the published literature. First, a novel double sulfide-bridged diamine monomer (DA) containing thiazole units and bulky pendant phenyl rings was successfully synthesized, and then a series of polyamides was prepared by phosphorylation polycondensation of diamine. The obtained polyamides had inherent viscosities in the range 0.42–0.62 dL/g. The simultaneous introduction of double sulfide bond and thiazole rings into backbone of wholly aromatic polyamides afforded soluble polymers with moderate thermal stability. Thus, these polyamides can be considered as likely processable high-performance polymeric materials.
References
Zou F, Wen H, Yan T, Cai M (2016) Synthesis and properties of novel soluble aromatic polyamides containing 4-aryl-2,6-diphenylpyridine moieties and pendant fluorinated phenoxy groups. J Polym Res 23(11):225. https://doi.org/10.1007/s10965-016-1117-z
Carter KR, Furuta PT, Gong V (1998) Soluble high-temperature polyterephthalamides. Macromolecules 31(1):208–209. https://doi.org/10.1021/ma971194e
Yu Y, Cai M, Zhang Y (2010) Study on synthesis of novel soluble aromatic polyamides with pendant cyano groups. Polym Bull 65(4):309–318. https://doi.org/10.1007/s00289-009-0198-9
Safarpour M, Khataee A, Vatanpour V (2015) Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J Membr Sci 489:43–54. https://doi.org/10.1016/j.memsci.2015.04.010
Zarrabi H, Yekavalangi ME, Vatanpour V, Shockravi A, Safarpour M (2016) Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 394:83–90. https://doi.org/10.1016/j.desal.2016.05.002
Javadi A, Shockravi A, Rafieimanesh A, Malek A, Ando S (2015) Synthesis and structure–property relationships of novel thiazole-containing poly(amide imide)s with high refractive indices and low birefringences. Polym Int 64(4):486–495. https://doi.org/10.1002/pi.4815
Javadi A, Najjar Z, Bahadori S, Vatanpour V, Malek A, Abouzari-Lotf E, Shockravi A (2015) High refractive index and low-birefringence polyamides containing thiazole and naphthalene units. RSC Adv 5(111):91670–91682. https://doi.org/10.1039/C5RA18898A
Mallakpour S, Dinari M (2009) Preparation of thermally stable and optically active organosoluble aromatic polyamides containing l-leucine amino acid under green conditions. Polym Bull 63(5):623. https://doi.org/10.1007/s00289-009-0113-4
Winnacker M (2017) Polyamides and their functionalization: recent concepts for their applications as biomaterials. Biomater Sci 5(7):1230–1235. https://doi.org/10.1039/C7BM00160F
Jiang J-W, Pei X-L, Sheng S-R, Wu X-Y, Liu X-L, Song C-S (2011) Novel soluble fluorine-containing polyamides derived from 2-(4-trifluoromethylphenoxy)terephthalic acid and trifluoromethyl-substituted aromatic bis(ether amine)s. Polym Bull 67(2):263–274. https://doi.org/10.1007/s00289-010-0414-7
Fahmy MM, Al-Ghamdi RF, Mohamed NA (2011) Synthesis, characterization, and thermal stability of novel wholly para-oriented aromatic poly(ether-amide-hydrazide)s bearing pendant groups and their corresponding poly(ether-amide-1,3,4-oxadiazole)s. Polym Bull 66(5):609–625. https://doi.org/10.1007/s00289-010-0296-8
Kolahdoozan M, Mirsafaei R, Mallakpour S (2012) Synthesis and properties of new highly soluble poly(amide–ester–imide)s containing poly(ethylene glycol) as a soft segment. Polym Bull 68(5):1239–1254. https://doi.org/10.1007/s00289-011-0601-1
Park SH, Lee JW, Suh DH (1999) Synthesis and the properties of novel cycloaliphatic-aromatic polyamides having pendent N,N′-diphenyl imido groups. Polym Bull 43(4):311–318. https://doi.org/10.1007/s002890050616
Aharoni SM (1993) Synthesis of comb-like graft copolyamides with rigid aromatic main-chains and long flexible side-chains. Polym Bull 30(2):149–153. https://doi.org/10.1007/bf00296843
Mahajan SS, Sarwade BD, Maldar NN (1990) Synthesis and characterization of aromatic–alipathic polyamides containing tetraphenylthiophene units into the backbone. Polym Bull 24(2):143–149. https://doi.org/10.1007/bf00297310
Damaceanu M-D, Rusu R-D, Nicolescu A, Bruma M, Rusanov AL (2011) Organosoluble asymmetric aromatic polyamides bearing pendent phenoxy groups. Polym Int 60(8):1248–1258. https://doi.org/10.1002/pi.3070
Tan J, Wang C, Peng W, Li G, Jiang J-M (2009) Synthesis, characterization, and properties of novel aromatic polyamides containing phthalazinone moiety. Polym Bull 62(2):195–207. https://doi.org/10.1007/s00289-008-0013-z
Glatz FP, Mülhaupt R (1993) Syntheses and properties of soluble poly(arylene thioether imide)s and the corresponding poly(arylene sulfone imide)s. Polym Bull 31(2):137–143. https://doi.org/10.1007/bf00329958
Daccord G, Sillion B (1982) α, ω-Bifunctional poly(p-phenylene sulfide) oligomers. Polym Bull 6(8):477–484. https://doi.org/10.1007/bf00255809
Cheung M-F, Plummer HK (1991) Tensile fracture morphology of polysulfone-poly(phenylene sulfide) blends. Polym Bull 26(3):349–356. https://doi.org/10.1007/bf00587980
S Xu, Yang J, S Long, Y Chen, G Li (2005) Synthesis and characterization of poly(p-phenylene sulfide sulfone/ketone) copolymer. Polym Bull 54(4):251–261. https://doi.org/10.1007/s00289-005-0392-3
Javadi A, Shockravi A, Koohgard M, Malek A, Shourkaei FA, Ando S (2015) Nitro-substituted polyamides: a new class of transparent and highly refractive materials. Eur Polym J 66:328–341. https://doi.org/10.1016/j.eurpolymj.2015.02.032
Javadi A, Shockravi A, Kamali M, Rafieimanesh A, Malek AM (2013) Solution processable polyamides containing thiazole units and thioether linkages with high optical transparency, high refractive index, and low birefringence. J Polym Sci Part A Polym Chem 51(16):3505–3515. https://doi.org/10.1002/pola.26752
Rezania J, Shockravi A, Ehsani M, Vatanpour V (2017) Novel polyimides based on diamine containing thiazole units with thioether linkage and pyridine as pendent group: synthesis and characterization. High Perform Polym. https://doi.org/10.1177/0954008317732397
Acknowledgements
The authors gratefully acknowledge the financial support of Kharazmi University, Polymeric Membrane Research Core and thank Iran Polymer and Petrochemical Institute (IPPI), for their financial and other supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezania, J., Hayatipour, M., Shockravi, A. et al. Synthesis and characterization of soluble aromatic polyamides containing double sulfide bond and thiazole ring. Polym. Bull. 76, 1547–1556 (2019). https://doi.org/10.1007/s00289-018-2441-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2441-8