Abstract
A unsymmetrical and kink non-coplanar heterocyclic dicarboxylic acid monomer, 4-[4-(4-carboxy phenoxy)-naphthyl]-2-(4-carboxyphenyl)phthalazin-1-one (3) was successfully synthesized with high purity and high yields. A series of novel polyamides containing phthalazinone were prepared from the newly synthesized dicarboxylic acid with various aromatic diamines by means of the phosphorylation polycondensation reaction. Molecular weights of the obtained polyamides were evaluated viscometrically, and the inherent viscosities (ηinh) measured were in the range 0.54–0.69 dL/g. These polyamides were amorphous and readily soluble in many organic solvents, such as N-methyl-2-pyrrolidone, N,N-dimethylacetamide, N,N-dimetheylformamide, dimethyl sulfoxide, pyridine, and m-cresol etc., and they could easily be solution-cast into transparent, flexible films with good mechanical properties, with tensile strength ranging from 63.9 to 81.6 MPa and elongation at break from 7.2 to 11.4%. These polymers still kept good thermal stability with high-glass transition temperatures in the range of 283–338 °C, and the decomposition temperature in nitrogen for a 10% weight-loss temperatures in excess of 490 °C, and char yield at 800 °C in nitrogen ranged from 56 to 63%. Furthermore, the polyamides films were essentially colorless; their cut-off wavelengths were between 365 and 379 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a class of high-performance materials, wholly aromatic polyamides exhibit superior properties, such as very high thermal stability, good mechanical and electrical behavior, as well as low flammability, so they have been widely used in fields of electrical materials, adhesives, composites and membranes [1–4]. However, aromatic polyamides are generally difficult to process due to their poor solubility in organic solvents and high flowing or melting temperature, which are caused by the rigidity of the backbone and present strong hydrogen bonding in macromolecules [5, 6]. Therefore, a great deal of efforts have been made to improve processability and solubility of polyamides without sacrificing their excellent properties via structure modification, typically by introduction of bulky substituents or cardo groups [7–10], asymmetric units [11, 12], and flexible links or bridging functional groups to the main polymer chains [13–15].
Since Hay et al. first found that 1,2-dihydro-4-(4-hydroxyphenyl)(2H) phthalazinones were bisphenol-like monomers [16], then a number of polymers such as poly(phthalazinone ether)s, polyamides, polyimides, and polyesters has been developed from the series of monomer [17–21]. The incorporation of the twisted, non-coplanar structure into the polymer backbone would disrupt inter-chain hydrogen bonding and reduce the chain packing efficiency and crystallinity which could promote solubility. Moreover, introducing heterocyclic aromatic rings into the backbone can retain acceptable thermal properties with high T g through controlled segmental mobility. Furthermore, it has been demonstrated that incorporation both ether and naphthyl units into the polymer backbones is a successful route to improve the solubility of polyamides while retaining high thermal stability too [22, 23]. The combination of the two above effective factors with each other may be proposed as suitable choice for increasing the processability of aromatic polymers without extreme loss of thermal resistance.
In the present article we report the successful synthesis of a new dicarboxylic acid containing phthalazinone (DHPZ) moiety in the main chain. The new dicarboxylic acid was directly polycondensed with various aromatic diamines to produce a series of novel organosoluble polyamides. The properties of these polymers, such as crystallinity, solubility, optical transparency, thermal and mechanical properties of these polymers are also discussed herein.
Experimental
Materials
4-(4-hydroxynaphthalenyl)phthalazin-1-one 1 was synthesized in our laboratory, details of the synthesis and characterization data of the new bisphenol-like compound were described in a separate article [24]. According to a well-established procedure [25], 1,4-bis(4-aminophenoxy)benzene (4c, m.p. 171–172 °C) and 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene (4d, m.p. 133–134 °C) were prepared by the aromatic nucleophilic substitution reaction of hydroquinone with p-chloronitrobenzene and 2-chloro-5-nitrobenzotrifluoride, respectively, in the presence of potassium carbonate, and subsequent reduction of the intermediate dinitro compounds using hydrazine as the reducing agent and palladium as the catalyst. p-Phenylenediamine (4a) were purified by distillation under reduced pressure before use, 4.4′-oxydianiline (4b) were purified by recrystallization from ethanol. N-methyl-2- pyrrolidone (NMP) and pyridine (Py) were purified by distillation under reduced pressure over calcium hydride and stored over 4Å molecular sieves. Triphenyl phosphite (TPP) was purified by distillation under reduced pressure. Commercially obtained anhydrous calcium chloride (CaCl2) was dried under vacuum at 180 °C for 10 h. All other materials were reagent-grade and used without further purification unless otherwise noted.
Measurement
IR spectra were recorded in the range 4,000–500 cm−1 by Nicolet Magna 470 spectrometer. Elemental analyses were made on a Carlo-Erba1106 instrument. The inherent viscosities of all polymers were determined at 0.5 g/dL concentration with Ubbelohde viscometer at 30 °C. NMR spectra were recorded using Bruker AM 400 MHz instrument. Differential scanning calorimetric analysis was performed on differential scanning calorimeter (TA 2900M) at a heating rate of 20 °C/min. Thermogravimetric data were obtained on a DuPont 2000 SDT-2960 under nitrogen flowing condition at a rate of 50 cm3/min and a heating rate of 20 °C/min. Dynamic mechanical analysis (DMA) was performed on thin films (30-mm long, 10-mm wide, 50–60 μm thick) on a DMTA-4 Analyzer (America Rheogoniometer Co.) in the tensile mode at frequency of 1 Hz over the temperature range 30–400 °C at a heating rate of 5 °C/min in nitrogen. Ultraviolet-visible (UV-vis) spectra of the polymer films and the dilute N,N-dimetheylformamide (DMF) solution were recorded on a Lambda 35 (Perkin Elmer) spectrophotometer. The emission spectra were measured with a Perkin Elmer LS50B luminescence spectrometer in a dilute DMF solution. Wide-angle X-ray diffraction (WAXD) patterns were obtained at room temperature on a Rigaku D/MAX 2500 powder diffractometer with a scanning speed of 4°/min, and the patterns were recorded in the 2θ range of 5°–40°. Mechanical properties of the films were measured on a Instron model 1,130 tensile tester with a 5 kg load cell at a crosshead speed of 5 cm/min on strips approximately 35–60 μm thick and 0.5 cm wide with a 2 cm gauge length.
Monomer synthesis
4-[4-(4-cyano phenoxy)-naphthyl]-2-(4-cyano phenyl)phthalazin -1-one (2)
In a 250 mL round-bottomed flask equipped with a nitrogen inlet, a Dean-Stark trap and a condenser, 13.8 g (0.1 mol) of potassium carbonate were suspended in a solution of bisphenol-like compounds 1 11.53 g (0.04 mol) in a mixture of 70 mL of N,N-dimethylacetamide (DMAc) and 50 mL toluene, then the reactive system was heated with stirring at reflux temperature for 5 h. After the toluene has been removed completely, the mixture was cooled and then 11.01 g (0.08 mol) of p-chlorobenzonitrile was added. The reaction was carried out at 150 °C for 15 h, and then the reaction mixture was poured into 800 mL of cold water. The products was filtered and recrystallized from ethanol/DMAc (V/V = 1:3) and was dried to afford 13.10 g (yield 69%) of yellowish powder, mp. 197–198 °C (by DSC). IR (KBr, cm−1): 2,226 (C≡N), 1,675 (C=O), 1,243 (C–O–C).1H NMR(DMSO-d6, ppm) (Scheme 1): δ 8.50 (d, 1H, 1-H), 8.07 (d, 1H, 7-H), 7.99 (m,4H, 12-H,14-H), 7.95 (t, 1H, 2-H), 7.88 (m, 4H, 3-H,10-H,11-H), 7.78 (d, 1H, 5-H), 7.62 (t, 1H, 8-H), 7.55 (t, 1H, 9-H), 7.41 (d, 1H, 6-H), 7.32 (d, 1H, 4-H), 7.27 (d, 2H, 13-H), 13C NMR(DMSO-d6, ppm): 161.26, 158.30, 151.05, 146.51, 145.31, 134.71, 134.10, 133.42, 132.64, 132.45, 129.87, 128.97, 128.55, 127.96, 127.75, 127.06, 127.03, 126.83, 126.48, 126.17, 126.03, 121.55, 118.58, 118.42, 118.08, 115.50, 109.70, 105.46. Anal. Calcd for C32H18N4O2: C, 78.36%; H, 3.70%; N, 11.42%. Found: C, 78.11%; H, 3.68%; N, 11.57%.
4-[4-(4-carboxy phenoxy)-naphthyl]-2-(4-carboxy phenyl)phthalazin-1-one (3)
The dicarboxylic acid 3 was obtained by alkaline hydrolysis of the dinitrile compound 2. In a 250 mL round-bottomed flask, a suspension of dinitrile 2 (9.80 g, 0.02 mol) in an water/ethanol (75 mL/75 mL) containing dissolved 22.40 g (0.4 mol) of potassium hydroxide was boiled under reflux with nitrogen atmosphere. Reflux was continued until the evolution of ammonia had ceased. The result clear solution was cooled, and the PH value was adjusted by dilute hydrochloric acid to near 3. The offwhite precipitate was collected by filtration and washed thoroughly with distillated water and methanol, then dried in vacuum, to give 10.25 g (yield 97%) offwhite powder without further purification, mp. 166–168 °C (by DSC). IR (KBr, cm−1): 3,070 (broad, C(O)O–H), 1,692 (C=O), 1,239 (C–O–C). 1H NMR(DMSO-d6, ppm) (Scheme 2): δ 12.93 (s, 2H, COOH), 8.51 (d, 1H, 1-H), 8.14 (d, 1H, 7-H), 8.07 (d, 2H, 12-H), 8.00 (d, 2H, 14-H), 7.96 (t, 1H, 2-H), 7.88 (m, 4H, 3-H,10-H,11-H), 7.77 (d, 1H, 5-H), 7.60 (t, 1H, 8-H), 7.56 (t, 1H, 9-H), 7.34 (d, 1H, 6-H), 7.31 (d, 1H, 4-H), 7.21 (d, 2H, 13-H), 13C NMR (DMSO-d6, ppm): 166.68, 166.64, 161.05, 158.25, 151.82, 146.26, 145.13, 133.97, 133.38, 132.33, 131.71, 129.89, 129.59, 129.54, 128,56, 128.47, 127.99, 127.69, 126.95, 126.86, 126.78, 126.18, 125.91, 125.73, 125.71, 121.69, 117.30, 114.75. Anal. Calcd for C32H18N4O2: C, 72.72%; H, 3.81%; N, 5.30%. Found: C, 72.49%; H, 3.77%; N, 5.45%.
Polymers synthesis
The synthesis of 5d is given as a typical example for the preparation of the polyamides. A mixture of 0.7927 g (1.5 mmol) of diacid 3, 0.6425 g (1.5 mmol) of 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene (4d), 0.30 g dried calcium chloride, 1.2 mL of Py, 1.8 mL of triphenyl phosphite, and 3.0 mL of NMP was heated with stirring at 110 °C until the magnetic stirrer could no longer work. The polymer solution was poured slowly into 300 mL of stirring methanol giving rise to a fiber-like precipitate which was washed thoroughly with hot water and methanol, collected by filtration, and dried under vacuum at 120 °C. The yield (99.6%) was quantitative. Other polyamides were synthesized by an analogous method. The film preparation was performed via the procedure of Hsiao’s experience [14], then the obtained films were used for X-ray diffraction measurements, solubility test, tensile test, and thermal analyses.
Results and discussion
Monomer synthesis
As shown in Scheme 3, the new phthalazione-containing aromatic dicarboxylic acid was synthesized by alkaline hydrolysis of the dinitrile compound resulting from the nucleophilic substitution reaction of 4-(4-hydroxynaphthalenyl)phthalazin-1-one 1 with p-chlorobenzonitrile. All synthesized compounds were characterized by elemental analysis, FT-IR and NMR spectroscopy with satisfied results. Figure 1 shows the IR spectra of dinitrile and diacid compound. The characteristic bands representative of the cyano functionality were identified in the IR spectrum at 2,228 cm−1, where it disappeared in IR spectrum of diacid 3, and instead a broad O–H absorption appeared in the region of 2,600–3,500 cm−1. Furthermore, in the IR spectrum of diacid, the characteristic absorption band at 1,689 cm−1 signed to C=O stretching became wider than that of dinitrile compound, implying more presence of carbonyl group existing in diacid.
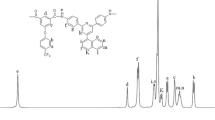
The chemical structures compound 2 and monomer 3 were also confirmed by 1H NMR spectroscopy. Figure 2 shows 1H NMR spectrum of monomer 3, in which all the protons were assigned as shown in scheme 2. In addition, the corrected elemental analysis values were identical with the calculated ones.
Polymer synthesis
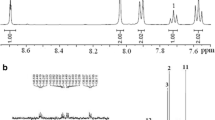
Polymerization of diacid 3 with stoichiometric amount of different aromatic diamines was carried out in dry freshly distilled NMP and Py outlined in Scheme 4. The direct polycondensation of aromatic dicarboxylic acid with diamines using a mixture of triphenyl phosphite and Py as condensing agent is a convenient method on a laboratory scale [26]. All polyamidations proceeded in a homogeneous system throughout the reaction, and the polyamides were isolated as tough fibers in quantitative yields. The synthesis conditions and inherent viscosities of the resulting polyamides are summarized in Table 1. Inherent viscosities of the polyamides ranged from 0.54–0.69 dL/g, indicative of the formation of high molecular weights. Among these polymers, the one (5c) showed the highest inherent viscosity maybe the highest reactivity of 4c during the diamines. The structures of these polyamides were confirmed by IR spectroscopy. For example, polymer 5d (Fig. 1) showed characteristic absorptions of amide group occurred around 3,310 and 1,668 cm−1, peculiar to N–H stretching and carbonyl stretching, respectively. The 1H NMR spectrum of 5d was showed in Fig. 3. As it can be seen, the absorption peak at 10.6 ppm was assigned to the N–H protons. Meanwhile, no absorption was detected in the range greater than 10.6 ppm, and this indicated the completely polymerization. The total protons in the spectrum are consistent with the proposed chemical structure of polymer 5d.
Thermal properties
The thermal behavior and glass transitions temperature (T g) of the polymers were evaluated with TGA and DSC, respectively (Fig. 4), and the characteristic data are summarized in Table 2. The T g value of 5a–d were in the range of 283–338 °C. As anticipated, the T g values of these polyamides depended on the structure of the diamine component and decreased with increasing flexibility of diamine employed, the 5a show the highest T g up to 338 °C because of the highest rigidity of diamine used. We also measured all polyamides by DMA, the tan δ peak at 1 Hz of these polymers was used to calculate the T g, and the result followed the same trend as the DSC. All polyamides showed high thermal stability. The 5 and 10% weight-loss temperature in nitrogen were in the range of 440–470 °C and 490–513 °C, respectively. The amount of residue of all polyamides at 800 °C in nitrogen was higher than 56%. Their excellent thermal stabilities may be due to the presence of very rigid aromatic heterocyclic backbone.
Solubility and crystallinity
The solubility behavior of the new aromatic polyamides was determined at concentration of 5% (W/V) in a number of solvents and the results were tabulated in Table 3. Almost all prepared polyamides exhibited excellent solubility in polar aprotic solvents such as NMP, DMF, DMAc, dimethyl sulfoxide (DMSO), and even in less polar solvents like Py and m-cresol, but showed poorer solubility in THF and chloroform due to the low value of dielectric constant of the solvent. Their high solubility is attributed to the presence of twist and noncoplanar aromatic heterocyclic moiety in the polymer backbone, which interrupt the regular packing of polymer chains. In addition, the bulky CF3 side group and more flexible ether group were introduced in the polymer backbone are especially effective for the high solubility. As expected, the WAXD studies of polyamides 5a–d indicated that all of these polymers were essentially amorphous. It appears that the kink noncoplanar together with ether unit in the polymer backbone lead to loose chain packing.
Mechanical properties
As mentioned above, all polyamides could be readily processed into flexible, creasable films by solution casting from DMAc. The mechanical properties of polyamide films are listed in Table 4. These films showed tensile strengths of 63.9–81.6 MPa, elongations at break of 7.2–11.4%, and tensile modulus of 1.71–2.27 GPa. It was noted that the tensile strength became higher values as more ether units were introduced in the polymer chains. These results indicated that the series of polyamides are strong and tough materials, which could be applied practically.
Optical properties
Our previous study about poly(arylene ether)s containing kink noncoplanar heterocyclic structure exhibited interesting optical properties [24], therefore the absorption and the emission features of polyamides in the DMF solution were investigated. The typical UV-visible spectra and emission spectra of polyamides in DMF solution were given in Fig. 5, the UV-visible absorption showed maxima in the range of 293–312 nm, and the peak absorption showed a slight blue shift from 5a to 5c. It’s probably due to the introduction of ether unit along the chains, which could disrupt the conjugation, and subsequently increase the electronic bandgap of the polymers. The fluorescence spectra of polyamides showed emission maxima around 355 nm, whereas 5d exhibited an additional peak at 430 nm, when excited at corresponding maximum absorption wavelength. Moreover, the optical transparency of polyamide films were also measured using UV-vis spectroscopy and the UV-visible spectra of the polyamide films are showed in Fig. 6. The cutoff wavelengths (λ0) of all polyamides ranged in 365–379 nm. It revealed that the polymers were light colored with high transparency in visible light region, and it was noticed that the 5d with CF3 group revealed a shorter λ0 than 5c, which is accorded with the Yang’s results [27, 28].
Conclusions
A novel unsymmetrical diacid from bisphenol-like 4-(4-hydroxy-naphthalenyl) phthalazin-1-one 1 was obtained in two steps. And a series of polyamides 5a–d containing phthalazione were synthesized from prepared diacid with various aromatic diamines by the Yamazaki phosphorylation method. The results indicated that adding asymmetrical and noncoplanar heterocyclic unit into polymer backbone improved the solubility of polyamides with the retention of the thermostability. The polyamide film showed good mechanical properties and excellent transparency. The solution in DMF of all polyamides were UV-fluorescent. These properties make these polyamides attractive for practical applications such as processable high-performance polymeric materials.
References
Cassidy PE (1980) Thermally stable polymers, synthesis and properties. Marcel Dekker, New York
Bichlmeir BR, Rappaport JB, Salamone JC (1996) Aramid fibers and nonwovens. CRC Press Polym Mater Encyclo
Spiliopoulos IK, Mikroyannidis JA, Tsivgoulis GM (1998) Rigid-rod polyamides and polyimides derived from 4,3″-diamino-2′6′-diphenyl- or di(4-biphenylyl)-p-terphenyl and 4-amino-4″-carboxy-2′,6′-diphenyl- p-terphenyl. Macromolecules 31:522
Shiraishi K, Kanbara T, Yamamoto T, Groenendaal LB (2001) Preparation of a soluble and neutral alkyl derivative of poly(3,4-ethylene-dioxythiophene) and its optical properties. Polymer 42:7229
Yang HH (1989) Aromatic high-strength fibers. Wiley, New York
Yang HH (1992) Kevlar aramid fibers. Wiley, New York
Liaw DJ, Liaw BY, Yang CM (2001) Synthesis and characterization of new soluble cardo aromatic polyamides bearing diphenylmethylene linkage and norbornyl group. Macromol Chem Phys 202:1866
Ayala V, Maya EM, Garcia JM, Campa JG, Lozano AE, Abajo JD (2005) Synthesis, characterization, and water sorption properties of new aromatic polyamides containing benzimidazole and ethylene oxide moieties. J Polym Sci Part [A] 43:112
Hsiao SH, Yang CP, Chen CW, Liou GS (2005) Synthesis and properties of novel poly(amide-imide)s containing pendent diphenylamino groups. Eur Polym J 41:511
Sava I, Bruma M (2006) Aromatic polyamides containing pendent acetoxybenzamide groups. Macromol Symp 239:36
Microyannidis JA (1997) Soluble and fusible polyamides and polyimides containing pyrazoline moieties and their crosslinking to heat-resistant resins. J Polym Sci [A] 35:1353
Cheng L, Jian XG (2004) Synthesis of new soluble aromatic poly(amide imide)s from unsymmetrical extended diamine containing phthalazinone moiety. J Appl Polym Sci 92:1516
Hsiao SH, Chen WT (2003) Syntheses and properties of novel fluorinated polyamides based on a bis(ether-carboxylic acid) or a bis(ether amine) extended from bis(4-hydroxyphenyl)phenyl-2,2,2-trifluoroethane. J Polym Sci Part [A] 41:420
Hsiao SH, Chang YH (2004) New soluble aromatic polyamides containing ether linkages and laterally attached p-terphenyls. Eur Polym J 40:1749
Mallakpour S, Kowsari E (2005) Synthesis and characterization of new optically active poly(amide-imide)s containing epiclon and L-methionine moieties in the main chain. Polym Adv Technol 16:732
Berard N, Hay AS (1993) Polymers from hydroxyphenylphthalazionones. Polymer Prepr 34:148
Wang JY, Liao GX, Liu C, Jian XG (2004) Poly(ether imide)s derived from phthalazinone-containing dianhydrides. J Polym Sci Part [A] 42:6089
Cheng L, Wu H, Ying L, Yang XL, Jian XG (2004) New soluble aromatic polyamides from non-symmetrical extended diacid containing a phthalazinone moiety. Des Monomers Polym 7:225
Li XH, Hay AS (2005) Synthesis of a high molecular weight fluorinated poly(phthalazinone ether) by self-condensation of an AB-type monomer. Macromolecules 39:3714
Zhang XH, Chen S, Min YQ, Qi GR (2006) Synthesis of novel bisphenol containing phthalazinone and azomethine moieties and thermal properties of cured diamine/bisphenol/DGEBA polymers. Polymer 47:1785
Cheng L, Ying L, Feng J, Wang CY, Li JL, Xu Z (2007) Novel heterocyclic poly(arylene ether ketone)s: synthesis and polymerization of 4-(4-hydroxyaryl)(2H)phthalazin-1-ones with methyl groups. J Polym Sci Part [A] 45:1525
Yang CP, Hsiao SH, Wu KL (2003) Organosoluble and light-colored fluorinated polyimides derived from 2,3-bis(4-amino-2-trifluoromethylphenoxy)naphthalene and aromatic dianhydrides. Polymer 44:7067
Reddy DS, Chou CH, Shu CF, Lee GH (2003) Synthesis and characterization of soluble poly(ether imide)s based on 2,2′- bis(4-aminophenoxy)-9,9′-spirobifluorene. Polymer 44:557
Wang CY, Tan JH, Peng WY, Li G, Jiang JM (2008) High glass transitions and fluorescence of novel organosoluble poly(arylene ether)s containing kink noncoplanar heterocyclic structures. Polymer Bull 61:299
Yang CP, Chen YP, Woo EM (2004) Fluorinated polyamides and poly(amide imide)s based on 1, 4-bis(4-amino-2-trifluromethylphenoxy)benzene, aromatic dicarboxylic acids, and various monotrimellitimides and bistrimellitimides: syntheses and properties. J Polym Sci Part [A] 42:3116
Yamazaki N, Matsumoto M, Higashi F (1975) Studies on reactions of the N-phosphonium salts of pyridines. XIV. Wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salts. J Polym Sci Polym Chem Ed 13:1373
Yang CP, Su YY, Hsiao FZ (2004) Synthesis and properties of organosoluble polyimides based on 1,1-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]cyclohexane. Polymer 45:7529
Yang CP, Su YY (2006) Novel organosoluble and colorless poly(ether imide)s based on 3,3-bis[4-(3,4-dicarboxyphenoxy)phenyl]phthalide dianhydride and aromatic bis(ether amine)s bearing pendent trifluoromethyl groups. J Polym Sci Part [A] 44:3140
Acknowledgments
We thank the financial support provide by the National Natural Science Foundation (50673017) of the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, J., Wang, C., Peng, W. et al. Synthesis, characterization, and properties of novel aromatic polyamides containing phthalazinone moiety. Polym. Bull. 62, 195–207 (2009). https://doi.org/10.1007/s00289-008-0013-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-008-0013-z