Abstract
In this work, cyanuric chloride was reacted with morpholine to obtain 2,4-dichloro-6-morpholino-1,3,5-triazine, which was then reacted with 4-aminobenzoic acid, yielding a new triazine monomer containing dicarboxylic acid. The chemical structure and purity of this monomer was confirmed by different techniques. Direct polycondensations of this diacid with several aromatic diamines were carried out in a molten ionic liquid, tetrabutylammonium bromide. Polyamides (PAs) with moderate inherent viscosities in the range 0.32–0.38 dL g−1 were obtained in high yields. These PAs were characterized by Fourier transform infrared spectroscopy, 1H NMR spectroscopy, X-ray powder diffraction, inherent viscosity measurements, and elemental analysis. All of the PAs were found to be amorphous, to possess outstanding solubilities, and to be easily dissolved in amide-type polar aprotic solvents. The thermal properties of the PAs were evaluated by thermogravimetric analysis and differential scanning calorimetry. These polymers showed good thermal stability with glass transition temperatures (T g) of 223–248°C, and their 10% weight loss temperatures were around 448°C and 460°C, confirming their good thermal stability. The char yields of these polymers were 53–59%, and, given their LOI values of 39–41, these polymers also show good flame retardancy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymers are extensively used in the modern world, and hundreds of millions of tons of synthetic polymers are created every year. They are important materials because of their numerous advantageous properties, such as their light weight, high performance and durability, design and processing flexibility, and high energy efficiency [1, 2]. They are used in the packaging, energy, communications, healthcare, construction, furniture, electronics, and transportation industries (among others). Common organic solvents that are volatile and often flammable, toxic, quite hazardous, and expensive are generally used in the synthesis of these materials [3]. It is important to decrease the amounts of volatile organic solvents used in chemical and industrial processes because these solvents are among the main contributors to air pollution [4, 5]. Such a goal could be achieved by developing new methods of polymer synthesis that reduce or eliminate the environmental problems associated with current polymer manufacturing processes [6, 7].
Chemists attempting to devise more efficient, and simultaneously more eco-friendly, polymer production processes have recently directed considerable attention to a new class of possible solvents: ionic liquids (IL)s, which are associated with so-called green chemistry [8]. ILs are salts that are composed of an organic cation and an organic or inorganic anion and typically have melting points below 100°C. They possess exceptional properties, such as high chemical and thermal stability, high ionic conductivity, negligible vapor pressure, nonflammability, a wide electrochemical window, and broad chemical diversity, owing to the large number of cations and anions that can be combined in an IL [8–11].
ILs can be applied as both solvents and catalysts in green polymerization processes [12]. The use of ILs as solvents in polymerization progressions yields some distinct advantages, such as increased molecular weight and narrower polydispersity, in comparison to when organic solvents are used [13, 14]. On the other hand, the high cost of most conventional room-temperature ILs and concern over their toxicity have led to the investigation of more benign and cheaper salts in the molten state as practical alternatives. Recently, molten tetrabutylammonium bromide (TBAB) was used as a cost-effective IL with low toxicity in a series of useful synthetic transformations [2, 15, 16].
Over the past few decades, increasing demand from the automotive, aerospace, microelectronics, and military industries for new high-performance polymers has provided the driving force for the development of such polymers for use in structural applications [17]. A great deal of effort has been directed into the development of aromatic heterocyclic polymers for use as advanced composite matrices, structural adhesives, or coatings in high-temperature applications [18–20]. Growth in the field of high-performance polymers (HPPs) began in the late 1950s, chiefly to satisfy the needs of the electronics and aerospace industries [20, 21]. Among the HPPs developed, wholly aromatic polyamides (aramids) have been widely used [20]. Aramids are a family of high-performance polymers with good chemical and thermal stability as well as useful mechanical properties [22–24]. They are used in aerospace and military applications, including bulletproof body armor fabric and ballistic composites. Aramids are fibers in which the chain molecules are highly oriented along the fiber axis, so the strength of the chemical bonds in the chains can be exploited [25]. However, aramids have high melt or glass transition temperatures (T g) and their solubilities are limited in common organic solvents due to the rigidity of the aramid backbone and strong hydrogen bonding. Therefore, over the past decade, much research has been conducted into the chemical modification of semi-aromatic polyamides (PA)s in order to improve their processability. Many researchers have focused on developing structurally modified PAs by changing the structures of the bulky, packing-disruptive monomers through the introduction of flexible units and bulky side groups and by breaking the regularity and symmetry of the polymer chain [24–30]. Among the PAs in this category, s-triazine-containing polymers are attracting a great deal of interest due to their excellent thermal resistance and high tensile strengths and moduli at elevated temperatures compared to conventional polymers [28, 31–34].
In view of this interest in processable aromatic PAs, we investigated PAs generated through the reaction of a diacid-containing s-triazine with various aromatic diamines under green conditions. First, 4,4-((6-morpholino-1,3,5-triazine-2,4-diyl)bis(azanediyl) dibenzoic acid (2) was prepared through a nucleophilic aromatic substitution reaction between cyanuric chloride, morpholine, and 4-aminobenzoic acid. Then novel PAs with a good balance of thermal resistance and solubility were prepared in a green medium using tetrabutylammonium bromide (TBAB) as a molten IL. The properties of the obtained polymers, such as viscosity, solubility, thermal stability, and morphology, are discussed here.
Experimental
Materials
All materials and solvents were purchased from Merck Chemical Co. (Darmstadt, Germany) and Aldrich Chemical Co. (St. Louis, MO, USA). Cyanuric chloride, tetrahydrofuran (THF), glacial acetic acid, 4-aminobenzoic acid, morpholine, 1,3-phenylene diamine, 4,4′-diaminodiphenyl ether, and 4,4′-diaminodiphenyl sulfone were used as received.
Measurements
Fourier transform infrared (FT-IR; model 680, JASCO, Tokyo, Japan) spectra of the samples were recorded at room temperature in the range 4000–400 cm−1 at a resolution of 4 cm−1. Spectra of solids were obtained using KBr pellets.
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker (Karlsruhe, Germany) Avance DRX 500 or 125 MHz spectrometer, using solutions in deuterated dimethylsulfoxide (DMSO-d6). The proton resonances were categorized into singlets (s) and multiplets (m).
Carbon, hydrogen, and nitrogen contents of the compounds were determined by pyrolysis using a Vario EL elemental analyzer (Elementar, Hanau, Germany).
X-ray diffraction (XRD) patterns were recorded using CuKα radiation on a Bruker D8 Avance diffractometer operating at a current of 100 mA and a voltage of 45 kV. The diffractograms were measured for 2θ in the range 10–80° using the CuKα incident beam (λ = 1.51418 Å).
Thermal stability was measured via thermogravimetry analysis (TGA, using a TGA instrument from TA Instruments, New Castle, DE, USA) from room temperature to 800°C at a heating rate of 10°C min−1 under a continuous flow of nitrogen. Differential scanning calorimetry (DSC) was conducted with a model 110 differential scanning calorimeter from Mettler (Greifensee, Switzerland) at a heating rate of 10°C min−1 under a nitrogen atmosphere.
Inherent viscosities were measured by a standard procedure using a Cannon-Fenske routine viscometer (Cannon, State College, PA, USA) with the sample at a concentration of 0.5 g/dL at 25°C.
Preparation of 2,4-dichloro-6-morpholino-1,3,5-triazine
To 100 mL of dry THF was added 10 mmol of cyanuric chloride and then a stoichiometric amount of morpholine. The reaction mixture was stirred for 3 h at 0°C, and 10 mmol of K2CO3 solution in water were added to the above mixture dropwise. The reaction was monitored by TLC. After product formation was detected, the product was filtered and washed with cold water. The product was a white solid (85%) with M.P. = 138–139°C.
FT-IR (KBr, cm−1): 2983 (m), 2968 (m), 1584 (s), 1485 (m), 1278 (s), 847 (s), 817 (m).
Synthesis of the diacid monomer
4-Aminobenzoic acid (0.1260 g, 0.88 mmol) was added dropwise to the solution of 2,4-dichloro-6-morpholino-1,3,5-triazine (0.1 g, 0.4 mmol) in 5 mL of glacial acetic acid (AcOH). The mixture solution was refluxed for 1 h. The reaction was monitored by TLC. The product was filtered and washed with 100 mL of boiling water to remove solvent and excess p-aminobenzoic acid, yielding 4,4′-((6-morpholino-1,3,5-triazine-2,4-diyl)bis(azanediyl)dibenzoic acid (2) as a white powder with 92% yield and M.P. = 349–351°C.
FTIR (KBr, cm−1): 2600–3300 (m, br), 1693 (s), 1602 (s), 1544 (m), 1513 (w), 1311 (m), 1241 (s), 794 (m).
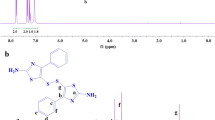
1H NMR (500 MHz, DMSO-d6, ppm): 12.4–12.6 (2H, COOH), 9.81–9.83 (2H, NH), 7.81–7.94 (d, 4H, Ar–H, J = 6.5 Hz), 7.85–7.87 (d, 4H, Ar–H,), 3.81–3.82 (t, 2H, CH2, J = 3.6), 3.70–3.72 (t, 2H, CH2, J = 3.5).
13C NMR (125 MHz, DMSO-d6, ppm): 169 (1CH), 166 (2C=O amide), 164 (2CH), 120–150 (8CH, aromatic), 67 (2CH2), 45 (2CH2).
Anal. calcd. for C21H20N6O5 (436.43 g/mol): C, 57.79%; H, 4.62%; N, 19.26%. Found: C, 57.43%; H, 4.37%; N, 18.35%.
Preparation of aromatic PAs
The PAs were prepared by the following general procedure (using the preparation of PA 4a as an example). A mixture of 0.100 g (0.458 mmol) of diacid 2 and 0.590 g (1.832 mmol) of TBAB was ground until a powder was formed, which was then transferred into a 25-mL round-bottom flask and 0.049 g (0.458 mmol) of 1,3-phenylene diamine were added to the mixture. This was heated until a homogeneous solution was formed and then 0.436 g (1.832 mmol) of triphenyl phosphate (TPP) were added. After that, the solution was stirred for 16 h at 130°C and the viscous solution was precipitated in 30 mL of methanol. The white solid was filtered off and dried to give 0.146 g (95%) of white polymer. The other polymers (PAs 4b–4d) were prepared by a similar procedure.
PAa
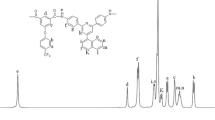
FT-IR peaks (KBr, cm−1): 3420 (br), 3074 (w), 2987 (m), 2917 (w), 1678 (m), 1576 (w), 1439 (w), 1271 (m), 1158 (w) and 576 (w). 1H NMR (500 MHz, DMSO-d 6, δ, ppm): 10.20 (s, 2H, NH), 9.60 (s, 2H, NH), 7.92–7.97 (4H, Ar–H), 7.46–7.48 (2H, Ar–H), 7.22–7.24 (2H, Ar–H), 3.79 (t, 2H, CH2), 3.70 (t, 2H, CH2). Elemental analysis: calcd. for C27H24N8O3 (508.54 g/mol)n: C, 63.77%; H, 4.76%; N, 22.03%. Found: C, 62.86%; H, 4.23%; N, 21.12%.
PAb
FT-IR peaks (KBr, cm−1): 3354 (br), 3090 (w), 2921 (m), 2851 (w), 1680 (m), 1583 (w), 1441 (w), 1307 (w), 1148 (w). 1H NMR (500 MHz, DMSO-d 6, δ, ppm): 1H NMR (500 MHz, DMSO-d 6 , δ, ppm): 10.50 (s, 2H, NH), 9.50 (s, 2H, NH), 7.7–8.3 (16H, Ar–H), 3.70 (t, 2H, CH2), 3.80 (t, 2H, CH2). Elemental analysis: calcd. for C33H28N8O3S (648.70 g/mol)n: C, 61.11%; H, 4.35%; N, 17.27%; S, 4.94%. Found: C, 60.40%; H, 3.88%; N, 16.67%; S, 4.05%.
PAc
FT-IR peaks (KBr, cm−1): 3372 (br), 3060 (w), 2963 (m), 2916 (w), 1673 (m), 1593 (w), 1217 (w). 1H NMR (500 MHz, DMSO-d 6, δ, ppm): 10.30 (s, 2H, NH), 9.65 (s, 2H, NH), 7.1–8.2 (16H, Ar–H), 3.90(t, 2H, CH2), 4.10 (t, 2H, CH2). Elemental analysis: calcd. for C33H28N8O4 (600.64 g/mol)n: C, 65.99%; H, 4.70%; N, 18.66%. Found: C, 64.72%; H, 4.17%; N, 17.45%.
PAd
FT-IR peaks (KBr, cm−1): 3415 (br), 3030 (w), 2960 (m), 2930 (w), 1685 (m), 1593 (w), 14,998 (w), 1234 (w), 1112 (w). 1H NMR (500 MHz, DMSO-d 6, δ, ppm): 10.30 (s, 2H, NH), 9.80(s, 2H, NH), 7.0–8.2 (20H, Ar–H), 3.43 (t, 2H, CH2), 3.92 (t, 2H, CH2), 3.80 (s, 3H, CH3). Elemental analysis: calcd. for C40H35N9O4 (705.78 g/mol)n: C, 68.07%; H, 5.00%; N, 17.86%. Found: C, 66.76%; H, 5.24%; N, 16.38%.
Results and discussion
Preparation of the monomer and the organosoluble PAs
At low temperature (0°C), only one site on the triazine can be substituted, whereas two sites can participate in substitution reactions at room temperature (25°C), and all three sites on the triazine can react at elevated temperatures (>70°C). The first chlorine atom of cyanuric chloride was replaced with morpholine at low temperature to form 2,4-dichloro-6-morpholino-1,3,5-triazine [35]. The other two chlorine atoms of cyanuric chloride were then replaced via substitution reactions of 4-aminobenzoic acid with 2,4-dichloro-6-morpholino-1,3,5-triazine to give the s-triazine 2 in refluxed glacial acetic acid, as shown in Scheme 1. The purity of the s-triazine monomer was checked by different techniques.
Polycondensation is typically conducted at relatively high temperatures, so the nonvolatility and thermal stability of ILs make them suitable solvents for polycondensation processes [2, 11, 13]. In the current investigation, in order to extend the utilization of ILs in polymer synthesis, molten TBAB was used as the solvent in the formation of several novel organosoluble and thermally stable PAs by the direct polymerization of the diacid monomer with different aromatic diamines (Scheme 2). The combination of an ionic medium (TBAB) with an activating agent (TPP) allowed us to carry out polymer synthesis under relatively mild conditions. When the acid group of TPP is activated, it becomes a good leaving group, which is necessary for attack by the amino group. The above polyamidation will not occur in the absence of either TPP or TBAB, as they act as catalyst and solvent.
The effect of duration of heating was examined to determine the optimum reaction conditions. In order to investigate the effect of duration of heating on the yield and inherent viscosities of each polymer, an initial polymerization reaction of diacid 2 with 1,3-phenylene diamine (3a) was carried out for different durations (Table 1). The best results were obtained for a duration of heating at 120°C of 16 h. With longer durations, lower yields of the dark yellow product were obtained; on the other hand, with short durations, low inherent polymer viscosities were achieved. So, a duration of heating at 120°C of 16 h was used in the preparation of other PAs. The results are summarized in Table 2. The inherent viscosities of the obtained PAs 4a–4d under the optimized condensation conditions were 0.32–0.38 dL/g, and yields were 87–95%.
Characterization
FT-IR study
Figure 1 shows FT-IR spectra of 2,4-dichloro-6-morpholino-1,3,5-triazine (1) and the diacid monomer (2). As shown in Fig. 1a, a characteristic C=N stretching band was observed for s-triazine in the range 1590–1620 cm−1. A strong absorption band due to C=N stretching in the triazine moiety also appeared in the range 1200–1400 cm−1. Moreover, peaks at 1117–1121 cm−1 were attributed to C–O–C stretching in the morpholine ring. In the FTIR spectrum of diacid 2, a broad and strong peak at 2600–3600 cm−1 was noted, which was assigned to the COOH groups (Fig. 1b). A strong peak from carbonyl in carboxylic acids occurred at 1693 cm−1.
Figure 2 displays FT-IR spectra of the synthesized PAs. All of the polymers exhibited characteristic absorption bands of an amide group at around 3354–3425 cm−1 (N–H stretching) and 1673–1385 cm−1 (C=O stretching). Other characteristic bands originating from C=N, C=C, and C–O were also present in the FT-IR spectra of all the PAs. The PA 4b showed absorption bands at 1251 and 1150 cm−1 arising from the sulfone moiety (SO2 stretching). These FT-IR spectra thus confirm the formation of new aromatic PAs.
NMR study
The 1H NMR spectrum of the new diacid-containing monomer is shown in Fig. 3. Two triplet peaks at 3.7–3.8 ppm relate to the CH2 protons on the morpholine ring. Aromatic protons appear in the range 7.6–7.9 ppm. A singlet peak at 9.81–9.83 ppm was assigned to the NH groups in this compound. A broad peak at around 12.6–12.8 ppm relates to carboxylic acid protons. The 13C NMR spectrum of this dicarboxylic acid also shows features due to both aromatic and aliphatic carbon atoms (Fig. 4). The results of elemental analyses of this compound were close to the corresponding calculated values, demonstrating that the expected compound was obtained.
The structures of the synthesized PAs were clarified by 1H NMR (the results of this are presented in the “Experimental” section). In the 1H NMR spectra of these polymers, the appearance of signals due to amide N–H protons at around 10.20–10.5 ppm and due to amine N–H protons at 9.5–9.8 ppm indicate the presence of these groups in the polymer chain. Signals due to aromatic proton resonance appear in the range 7.0–8.3 ppm. Signals arising from aliphatic protons are seen in the range 3.4–4.1 ppm. 1H NMR spectra of the PAs 4a and 4d are shown in Fig. 5. The results of elemental analysis of the PEs were also found to be in good agreement with the calculated percentages of carbon, hydrogen, and nitrogen in the polymers.
Solubilities of the polymers
In general, aromatic PAs possess poor solubilities and are generally insoluble in organic solvents. However, the incorporation of nonlinear chain segments or polar groups into a PA tends to significantly improve its solubility. Also, the solubility behavior of a PA depends on its chain-packing ability and intermolecular interactions [2, 13]. The solubilities of the PAs obtained in this work were tested in different solvents. The solubilities were determined by adding 0.05 g of each polymer to 1 mL of each solvent (Table 3). The results showed that the newly synthesized PAs were highly soluble in aprotic polar solvents such as N-methyl-2-pyrrolidinone (NMP), N,N-dimethylacetamide (DMAc), DMSO, and N,N-dimethylformamide (DMF) at room temperature. Their good solubilities and amorphous nature may be due to the presence of the pendent morpholinium groups, which disrupt interactions among polymer chains as they increase polymer chain separation, thereby leading to a decrease in crystallinity and an increase in solubility. However, the polymers were also found to be insoluble in general organic solvents such as CH3OH, CHCl3, and acetone, and to be partially soluble in THF. The PA 4d showed better solubility than the other polymers due to the presence of a bulky pendant group.
X-ray diffraction
To investigate the crystallinity of the polymers, wide-angle X-ray powder diffractometry of the polymers was performed in the region 2θ = 10–80° at room temperature. As shown in Fig. 6, a set of broad diffraction peaks were observed, which indicated that the PAs had amorphous morphologies. This can be attributed to the presence of the morpholinium pendant groups as well as flexible linkages (–O– and –SO2–) in the main chain of the polymer, which reduce interactions among chains and result in poorer chain packing, leading to amorphous PAs with improved solubility and processability. This agrees with the general rule that solubility decreases with increasing crystallinity.
Thermal properties
The thermal properties of the prepared polymers were investigated by means of DSC and TGA under a nitrogen atmosphere at a heating rate of 10°C/min. An endothermic melting peak was not observed in the DSC curves of the PAs, emphasizing the amorphous nature of these polymers. The glass-transition temperature (T g) was taken as the midpoint of the change in slope of the DSC curve baseline. According to the results obtained, these polymers exhibit T g values ranging from 223 to 248°C (Table 4 and Fig. 7). As a general rule, the incorporation of bulky and rigid groups onto the polymer backbone restricts the free rotation of the macromolecular chain, leading to higher T g values (see PAs 4a and 4d). On the other hand, the presence of flexible bonds such as ether and sulfone linkages in the main chain of the polymer reduces the rigidity of the backbone, and thus decreases T g (see PAs 4b and 4c). Therefore, the T g values of these polymers are affected by these two opposing factors: restricted bond rotation due to the presence of bulky pendant groups and increased flexibility of the main chain due to the inclusion of ether and sulfone linkages.
The TGA curves of the aromatic PAs are shown in Fig. 8, and Table 4 shows the thermal properties of the PAs, including the temperature at which a weight loss of 5% occurred (T 5%), the temperature at which a weight loss of 10% occurred (T 10%), and the residual weight at 800°C (char yield). All of the polymers presented similar thermal stabilities and exhibited no weight loss up to 370°C. As shown in Table 4, these polymers had T 5% and T 10% values of 418–496°C and 448–502°C, respectively. The highest thermal stability at 486°C was exhibited by 4a, which is in accordance with its high molecular weight and rigid aromatic backbone. The amount of carbonized residues (the char yield) at 800°C under a nitrogen atmosphere was in the range 53–59 wt% for all the PAs. The high char yields of these PAs can be attributed to the high aromatic content in the main chain. The char yield is useful for estimating the limiting oxygen index (LOI) of each PA via the van Krevelen and Hoftyzer equation [36]:
Here, CR is the char yield. The LOI values of the PAs are in the range 39–41, indicating high flame retardancy. LOI values above a threshold value of 26 indicate that the polymer is self-extinguishing and can therefore be used in various applications requiring good flame resistance. The synthesized PAs show LOI values of greater than 26, confirming their good flame retardancy.
Conclusions
Novel s-triazine-containing PAs were successfully synthesized via the direct polycondensation of 4,4-(6-morpholine-1,3,5-triazine-2,4-diyl)bis(azanediyl)dibenzoic acid with different aromatic diamines under green conditions, with molten TBAB employed to remove harmful materials from the reaction. The present one-pot synthesis of organosoluble PAs offers several advantages: it is safe, green, eco-friendly, and cost-effective. The structures of the monomer and the various polymers obtained were clarified by FT-IR, 1H NMR, and elemental analysis. The polymers were found to be readily soluble in highly polar solvents and exhibited high thermal stability (T 10%, 450–500°C) and good flame retardancy (LOI values of 39–41). These results suggest a new synthetic pathway to novel PA-based materials, as it allows the synthesis of new polymers with well-defined molecular structures and advanced functional properties.
References
Erdmenger T, Guerrero-Sanchez C, Vitz J, Hoogenboom R, Schubert US (2010) Recent developments in the utilization of green solvents in polymer chemistry. Chem Soc Rev 39:3317–3333
Mallakpour S, Dinari M (2012) Novel nanostructure amino acid-based poly(amide–imide)s enclosing benzimidazole pendant group in green medium: fabrication and characterization. Amino Acids 43:1605–1613
Azapagic A, Emsley A, Hamerton I (2003) Polymers: the environment and sustainable development. Wiley, New York
Nising P, Zeilmann T, Meyer T (2003) On the degradation and stabilization of poly(methyl methacrylate) in a continuous process. Chem Eng Technol 26:599–604
Tsarevsky NV, Matyjaszewski K (2006) Environmentally benign atom transfer radical polymerization: towards “green” processes and materials. J Polym Sci A Polym Chem 44:5098–5112
Tamada M, Hayashi T, Ohno H (2007) Improved solubilization of pyromellitic dianhydride and 4,4-oxydianiline in ionic liquid by the addition of zwitterion and their polycondensation. Tet Lett 48:1553–1557
Livi S, Duchet-Rumeau J, Gerard JF, Pham TN (2015) Polymers and ionic liquids: a successful wedding. Macromol Chem Phy 216:359–368
Greaves TL, Drummond CJ (2015) Protic ionic liquids: evolving structure–property relationships and expanding applications. Chem Rev 115:11379–11448
lsbosch J, De Vos DE, Binnemans K, Ameloot R (2016) Biobased ionic liquids: solvents for a green processing industry? ACS Sustain Chem Eng 4:2917–2931
Jadhav AH, Lim AC, Thorat GM, Jadhav HS, Seo JG (2016) Green solvent ionic liquids: structural directing pioneers for microwave-assisted synthesis of controlled MgO nanostructures. RSC Adv 6:31675–31686
Mallakpour S, Dinari M (2012) Green solvents II: properties and applications of ionic liquids. Springer, New York
Kubisa P (2009) Ionic liquids as solvents for polymerization processes—progress and challenges. Prog Polym Sci 34:1333–1347
Mallakpour S, Dinari M (2010) High performance polymers in ionic liquids: a review on prospects for green polymer chemistry. Part I: Polyamides. Iran Polym J 19:983–1004
Long TE, Elabd YA, Yuan J (2016) Ionic liquids in polymer design. Macromol Rapid Commun 37:1105
Mallakpour S, Dinari M (2010) A study of the ionic liquid mediated microwave heating for the synthesis of new thermally stable and optically active aromatic polyamides under green procedure. Macromol Res 18:129–136
Mallakpour S, Dinari M (2013) Straightforward and green method for the synthesis of nanostructure poly(amide-imide)s-containing benzimidazole and amino acid moieties by microwave irradiation. Polym Bull 70:1049–1064
Mittal LK (2001) Polyimides and other high temperature polymers: synthesis, characterization and applications. CRC Press, Boca Raton
Yang HH (1989) Aromatic high-strength fibers. Wiley, New York, pp 66–289
Jian X, Chen P, Liao G, Zhang S, Wang J (2003) Syntheses and properties of novel high performance series poly(aromatic ethers) polymers containing phthalazinone moieties. Acta Polym Sin 4:469–475
Garcia JM, Garcia FC, Serna F, de la Pena JL (2010) High-performance aromatic polyamides. Prog Polym Sci 35:623–686
Mallakpour S, Dinari M (2011) High performance polymers in ionic liquid: a review on prospects for green polymer chemistry. Part II: Polyimides and polyesters. Iran Polym J 20:259–279
Knijnenberg A, Bos J, Dingemans TJ (2010) The synthesis and characterization of reactive poly(p-phenylene terephthalamide)s: a route towards compression stable aramid fibers. Polymer 51:1887–1897
Hsiao SH, Lin KH (2016) A comparative study on the properties of aromatic polyamides with methyl- or trifluoromethyl-substituted triphenylamine groups. J Fluorine Chem 188:33–42
Khademinejad S, Mehdipour-Ataei S, Ziaee F, Abbasi F (2016) Poly(ether ether sulfone amide)s as a new category of processable heat-resistant polymers. Des Monomers Polym 19:553–559
Deshmukh YS, Wilsens CHRM, Verhoef R, Hansen MR, Dudenko D, Graf R, Klop EA, Rastogi S (2016) Conformational and structural changes with increasing methylene segment length in aromatic−aliphatic polyamides. Macromolecules 49:950–962
Liou GS, Fang YK, Yen HJ (2014) Synthesis and properties of noncoplanar rigid-rod aromatic polyamides containing phenyl or naphthyl substituents. J Polym Res 7:147–155
Amininasab SM, Rashidi A, Taghavi M, Shami Z (2016) Preparation and characterization of novel thermostable polyamides bearing different photoactive pendent architectures with antibacterial properties. Chin J Polym Sci 34:766–776
Hajibeygi M, Shabanian M, Khodaei-Tehrani M (2016) New heat resistant nanocomposites reinforced silicate nanolayers containing triazine rings based on polyamide: synthesis, characterization, and flame retardancy study. Polym Compos 37:188–198
Zou F, Wen H, Yan T, Cai M (2016) Synthesis and properties of novel soluble aromatic polyamides containing 4-aryl-2,6-diphenylpyridine moieties and pendant fluorinated phenoxy groups. J Polym Res 23:225–234
Zhang G, Yan GM, Ren HH, Li Y, Wang XJ, Yang J (2016) Effects of a trans- or cis-cyclohexane unit on the thermal and rheological properties of semi-aromatic polyamides. Polym Chem 7:44–53
Matsuo S (1994) Synthesis and properties of poly(arylene ether phenyl-s-triazine)s. J Polym Sci Part A Polym Chem 32:2093–2098
Fink R, Frenz C, Thelakkat M, Schmidt HW (1997) Synthesis and characterization of aromatic poly(1,3,5-triazine-ether)s for electroluminescent devices. Macromolecules 1997(30):8177–8181
Balasubramanian R, Kumutha K, Sarojadevi M (2016) Mechanical, thermal and electrical properties of polyimides containing 1,2,3-triazole ring prepared by click reaction. Polym Bull 73:309–330
Grate JW, Mo KF, Daily MD (2016) Triazine-based sequence-defined polymers with side-chain diversity and backbone–backbone interaction motifs. Angew Chem 128:3993–3998
Manohar S, Khan SI, Rawat DS (2010) Synthesis, antimalarial activity and cytotoxicity of 4-aminoquinoline-triazine conjugates. Bioorg Med Chem Lett 20:322–325
Krevelen DW, Hoftyzer PJ (1976) Properties of polymers, their estimation and correlation with chemical structure. Elsevier, Amsterdam
Acknowledgements
We wish to express our gratitude to the Research Affairs Division, Isfahan University of Technology (IUT), Isfahan, for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Dinari, M., Haghighi, A. Synthesis and characterization of new heat-resistant polyamides bearing an s-triazine ring under green conditions. J Polym Res 24, 29 (2017). https://doi.org/10.1007/s10965-017-1184-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1184-9