Abstract
A series of UV-curable polyurethane acrylates containing fluorinated acrylic monomer (heptadecafluorodecyl methacrylate, PFA, 6 wt%)/vinyltrimethoxysilane (VTMS, 0–9 wt%) [FPUA6/0, FPUA6/3, FPUA6/6 and FPUA6/9, where the numbers indicate the wt% of PFA/VTMS] were synthesized from a reactive urethane oligomer (40 wt%) and diluents (60 wt%). This study examined the effect of bulky VTMS (0–9 wt%)/bulky IBOA (34–25 wt%) weight ratio on the properties of the UV-curable polyurethane acrylates for transparent anti-fouling coating materials. In the wavelength range of 400–800 nm, the transmittance % of the FPUA film samples increased markedly up to nearly 100 % with increase in the VTMS content up to 9 wt%. As the VTMS content increased, the storage modulus/tensile modulus/hardness of the UV-cured film samples increased significantly and the tensile strength/glass transition temperature increased a little; however, the elongation at break decreased significantly. XPS showed that the film–air surface of the UV-cured polyurethane acrylate film had a higher fluorine content than the film–dish interface indicating the gradient concentration of fluorine in the structure of the film from the film–air surface to the film–glass interface. As the VTMS content increased from 0 to 9 wt%, the surface tension of the UV-cured urethane acrylate films decreased from 17.2 to 15.9 mN/m, whereas the water/methylene iodide contact angles of the film–air surface increased significantly from 107.9/80.9° to 114.2.9/84.2°. These results suggest that the UV-curable polyurethane acrylate containing 9 wt% of VTMS has strong potential as a coating material for transparent antifouling applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The UV-curable polyurethane acrylate system is typically composed of reactive polyurethane acrylate oligomer (prepolymer), reactive diluents and a photoinitiator. Various liquid reactive diluents miscible with the oligomer can be used to decrease the viscosity of the viscose oligomer and to control the properties of UV-cured materials. Their high production rate/energy efficiency and versatility of properties have made them ideal materials for use in a wide range of applications, including thin film coatings [1–4], protective coatings for optical fibers [5, 6] and photolithography [7].
Fluorinated polymers have many useful and desirable features, such as unique surface properties (low surface tension, high soil resistance), good insulating/gas barrier properties and high resistance to thermal/chemical/weather attack, owing to the low polarizability, strong electronegativity of fluorine atoms and high strength of the C–F bond. The most common commercially available fluorine polymers (fluoropolymers) are based on the monomers of tetrafluoroethylene, vinylidene fluoride and chlorotrifluoroethylene. Polytetrafluoroethylene is a polymeric solid with a very low surface energy. The surface tension/water contact angle of polytetrafluoroethylene which consisted of closed-packed perfluoroalkyl-CF2 group was 18.5 mNm/108° [8]. The lower critical surface energy of many polymers containing fluorocarbon side chains has been attributed to the higher content of CF3 groups on their surfaces [9–12]. Fluoroalkyl acrylate homopolymers with long side chains have very low critical surface tension, ranging from 10 to 11 mN/m [9]. Generally, the surface energy (tension)/contact angle of a material is used as a criterion for the antifouling property.
The main disadvantage of the fluorinated monomer is the relatively high cost. Therefore, the content of fluorinated monomers should be minimized while maintaining a reasonable surface energy (water/oil repellency). Acrylic copolymers containing perfluoroalkyl side chains have been the focus of many studies, because of the good reactivity of perfluorinated acrylate with fluorine-free acrylate and good adhesion to the matrices [13–15]. The fluorinated side groups in fluorinated acrylic copolymers have emerged preferentially at the coating–air surface [16–20]. Fluorinated acrylic copolymer films not only retain the original properties of polyacrylate films, such as good adhesion to the matrix, but also have better durability as well as good antifouling property. Therefore, water-based acrylic copolymer dispersions containing perfluoroalkyl groups have been available for some time and are used widely in coatings for textiles/carpets [21] and leathers [22].
Generally, longer alkyl and perfluoroalkyl moieties increase the tendency for microphase separation in the bulk and surfaces [23, 24]. Comb-shaped polymers containing fluorinated side chain are used as surface modification agents, such as water and oil repellents, and soil release on different substrates, such as textiles, paper, leather and carpets, as well as nonwoven and building materials [25]. The extent to which the polymer surface energy is reduced depends not only on the surface coverage by fluorocarbon segments, but also on the degree of ordering on the surface layer.
On the other hand, hydrophobic vinylalkoxysilanes are some of the functional groups that can introduce self-cross-linking structure during the film formation process [26, 27]. Some studies have reported the synthesis and properties of self-cross-linkable fluorinated acrylate copolymer emulsions [21, 28, 29], but there has been little research on the optimum content of hydrophobic bulky alkoxysilanes for improving the water/oil repellency (low surface tension) and mechanical properties. In an earlier investigation of the synthesis and surface properties of self-cross-linking core–shell acrylic copolymer emulsions containing fluorine/silicone in the shell, we found that the optimum vinyltriethoxysilane (VTES) content was about 6 wt% to obtain a high water/oil repellent coating material [30].
Acrylic polymers are generally considered to be inexpensive with good water and weathering resistance, proper mechanical properties and gloss, but exhibit poor elasticity and abrasion resistance. Polyurethane has attracted particular attention in coatings, because it is easy to control its properties, such as toughness, flexibility, adhesion on substrate and abrasion resistance, etc. As a result, various formulators have examined ways of combining the advantages of waterborne polyurethane and acrylic polymer. Polyurethane/polyacrylate hybrid emulsions have been studied [31–35].
A combination of polyurethane with an acrylic copolymer containing fluorine is expected to be effective in increasing the performance of the resulting materials. These materials combine some of the virtues of polyurethane and fluorinated acrylate/acrylate polymer, such as high thermal stability, good chemical resistance, low water absorptivity (water resistance), attractive surface properties, excellent flexibility, good wearability and high weatherability [12, 36–40].
Some studies have examined the UV-curable waterborne polyurethane/polyurethane acrylate [41–43], but there are few reports on UV-curable fluorinated poly(urethane acrylate)s using fluorinated urethane [44, 45]. However, there has been little work on UV-curable copolymers of urethane acrylate oligomer with acrylate monomer/acrylate monomer possessing perfluoroalkyl groups. Moreover, studies on UV-curable polyurethane acrylate using PFA and VTMS have not been published. In our earlier study on the preparation and properties of a series of UV-curable polyurethane acrylates containing perfluoroalkyl groups, we found that the surface energy of the UV-cured film samples improved markedly with increase in the PFA content, but their transmittance % and mechanical properties decreased markedly [46].
In this study, the bulky monomer MMA was used to increase the hardness of the material, the rigid bulky cycloalkyl group contained monomer IBOA to increase the toughness, the bulky alkoxysilane group contained monomer VTMS to increase the transparency and surface/mechanical properties and the long perfluoroalkyl group contained monomer perfluoroalkyl acrylate (PFA) to increase surface tension. This study focused on the effect of VTMS/IBOA weight ratio on properties such as surface tension, transparency and mechanical properties of UV-cured polyurethane acrylate films. The objective of this study is to enhance these properties by using VTMS containing bulky side groups. Therefore, in this study, the effect of the VTMS content on the properties of UV-cured fluorinated polyurethane acrylates with a fixed urethane prepolymer content (40 wt%) and a fixed PFA content (6 wt%) was investigated to find the optimum content of VTMS for good transparent antifouling coatings having low surface tension.
Experimental
Materials
Poly(tetramethylene glycol) (PTMG Mn = 2000 g/mol; Aldrich Chemical, Milwaukee, WI) was dried at 90 °C under 1–2 mmHg for 3 h before use. 4,4′-Dicyclohexymethanediisocyanate (H12MDI, Aldrich Chemical, Milwaukee, WI), dibutyltin dilaurate (DBTDL, Aldrich Chemical, Milwaukee, WI), 2-hydroxyethyl methacrylate (HEMA, Aldrich Chemical, Milwaukee, WI), 3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecyl methacrylate (PFA, Aldrich Chemical, Milwaukee, WI), isobornyl acrylate (IBOA, Aldrich Chemical, Milwaukee, WI), methyl methacrylate (MMA, Aldrich Chemical, Milwaukee, WI), 1-hydroxycyclohexyl acetophenone (Irgacure 184, Aldrich Chemical, Milwaukee, WI) and vinyltrimethoxysilane (VTMS, Aldrich Chemical, Milwaukee, WI) were used as received.
Preparation of UV-curable polyurethane acrylates and their films
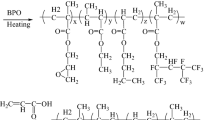
Scheme 1 shows the preparation process of UV-curable polyurethane acrylates. PTMG was placed in a four-neck, round-bottom flask equipped with a thermometer, mechanical stirrer, condenser with a drying tube, an inlet for dry nitrogen and a heating jacket, and was degassed in a vacuum at 90 °C for 1 h. PTMG was allowed to cool to 45 °C with moderate stirring (125–150 rpm). H12MDI was dropped slowly into the flask, and the reaction mixture was allowed to react at 85 °C until the theoretical NCO content was reached. The change in NCO value during the reaction was determined using the standard dibutylamine back-titration method (ASTM D 1638). The reaction mixture of the NCO-terminated urethane prepolymer was cooled to 45 °C and HEMA was added dropwise. To obtain the vinyl-terminated urethane prepolymer, the capping reaction of the NCO-terminated urethane prepolymer with HEMA was continued until the NCO content reached zero, as evidenced by the disappearance of the IR NCO peak (2,270 cm−1). The UV-curable fluorinated urethane acrylate mixtures were formulated from viscous prepolymer (40 wt%), diluents and photoinitiator Irgacure 184 (1.5 wt% based on the prepolymer/diluents). A series of UV-curable fluorinated urethane acrylates mixtures (prepolymer/diluents/photoinitiator) were formulated by adding the acrylate diluents [methyl methacrylate (MMA, 20 wt%)/isobornyl acrylate (IBOA, 34–25 wt%)/heptadecafluorodecyl methacrylate (PFA, 6 wt%)/vinyltrimethoxysilane (VTMS, 0–9 wt%), total diluents: 60 wt%] and photoinitiator Irgacure 184 (1.5 wt%) to the viscose urethane acrylate prepolymer (40 wt%) and mixing them at 45 °C for 3 h. Table 1 lists the sample designation and composition of the UV-curable fluorinated urethane acrylate containing various contents of VTMS.
The UV-cured fluorinated urethane acrylate films (thickness: 0.3 mm) were prepared by casting the above-formulated mixture onto a glass plate at room temperature followed by curing using a medium-pressure mercury lamp (80 W/cm). Radiation curing was carried out using UV light with a main wavelength of 365 nm. The distance between lamp and sample was 20 cm. Sufficient UV-curing time of 3 min was used for complete curing in this study.
Characterization
The chemical components of the UV-cured fluorinated polyurethane acrylate film samples containing various contents of VTMS were confirmed by Fourier transform infrared (FT-IR, NICOLET iS5, Thermo scientific, USA) spectroscopy. The FT-IR spectra of the samples were recorded in the range 4000–650 cm−1 using an ATR (ZnSe crystal) apparatus at a resolution of 4 cm−1 and 32 scans. A constant compression load was applied to the samples.
Surface analysis was performed by X-ray photoelectron spectroscopy (XPS, Theta Probe AR-XPS System, Thermo Fisher Scientific, U. K) equipped with a monochromated Al Ka X-ray source (15 kV, 150 W) and a spot size of 400 μm. The samples for XPS were prepared by casting the polymer onto a clean glass disc. The disc was placed in an oven at 60 °C for 12 h and 60 °C for 6 h under vacuum.
The water and methylene iodide contact angles were measured at 25 °C using a contact angle goniometer (Erma Contact Angle Meter, Japan), and the results reported are the mean of five values. The contact angle, which is a measure of the surface wettability, was used to determine surface tension. The surface tension of the solid film can be determined using the following equation:
where γs is the surface energy of the solid film, \( \gamma_{\text{s}}^{\text{d}} \) is the dispersion force and \( \gamma_{\text{s}}^{\text{p}} \) is the polarity force. The testing liquids used were water (L1) and methylene iodide (L2), and their \( \gamma_{\text{L1}}^{\text{d}} \), \( \gamma_{\text{L1}}^{\text{p}} \), \( \gamma_{\text{L2}}^{\text{d}} \) and \( \gamma_{\text{L2}}^{\text{p}} \) were 21.8, 51.9, 1.3 and 49.5 mN/m, respectively [47].
The dynamic mechanical properties of film samples were measured at 3 Hz using dynamic mechanical thermal analyzer (DMA, TA-Q800; TA Instrument, USA) with a heating rate of 10 °C/min in the temperature range from −100 to 100 °C.
The tensile properties were measured at room temperature using a Universal Testing Machine (UTM, WL2100, WITHLAB). A cross-head speed of 10 mm/min was used throughout these investigations to determine the ultimate tensile strength, modulus and elongation at break. The hardness was measured using a shore A type durometer (Asker, Kobunshi Keiki, Japan) according to ASTM D 2240. The films were overlapped and used in the test. The values quoted are the mean of five measurements. The UV–visible spectra were obtained using a UV–visible spectrophotometer (T70 + UV/VIS Spectrometer, PG Instruments, England).
Results and discussion
Composition of UV-curable polyurethane acrylate (prepolymer/diluents mixture)
Table 1 lists the sample designation, composition of prepolymer and prepolymer/diluents of UV-curable polyurethane acrylates [prepolymer (40 wt%/diluents (60 wt%)/photoinitiator (1.5 wt%)]. In this study, the oligomer (prepolymer) was kept at a constant molar ratio, and the UV-curable urethane acrylate had a fixed weight ratio of oligomer (prepolymer)/diluents (40/60 wt%) and MMA/PFA/(20/6 wt%.), whereas the weight ratio of IBOA/VTMS was 34/0, 31/3, 28/6, 25/9 and 25/9 wt%. The reaction process is shown in Scheme 1. In this study, the effect of VTMS content (0–9 wt%) on the transparency, surface properties and mechanical properties of UV-cured polyurethane acrylate film was investigated to find the optimum content of VTMS for transparent antifouling coating materials.
Identification of chemical structure of UV-cured film samples
Most commonly, the process of photopolymerization reactions of acrylic materials was followed by monitoring the disappearance of the reactive acrylic groups present in the monomers and oligomers. Figure 1 shows the FT-IR spectra of the UV-cured FPUA6/0 FPUA6/9 film samples. The UV-cured PUA6/0 and FPUA6/9 film samples had no C=C peak at 1630–1640 cm−1, indicating the complete reaction of all vinyl groups. The ester carbonyl (C=O) group of the PUA6/0 and FPUA6/9 films was identified by the characteristic peaks at approximately 1724 cm−1. The stretching vibrations of the –CF2 group at 1162 cm−1 and –CF3 group at 1,241 cm−1 were all detected in the FPUA6/0 and FPUA6/9 film samples. The characteristic bands at approximately 1100 cm−1 and 792 cm−1 confirm the Si–O and Si–C groups of the FPUA6/9 film sample. The stretching vibrations of the urethane group (N–H) at 3300–3500 cm−1 and methylene/methyl group (C–H) at 2853–2962 cm−1 were all detected in the FPUA6/0 and FPUA6/9 film samples. On the other hand, many peaks of ether/urethane/acrylate/fluorine/silicone groups overlapped with each other.
XPS is a surface chemical analysis technique for analyzing the surface chemistry of a material in the as-received state or after some treatment. Figure 2 presents the XP spectra of (a) film surface (film–air interface) of PUA6/0, (b) film–dish interface of FPUA6/0, (c) film–air surface of FPUA6/3, (d) film–air surface of FPUA6/6, (e) film–air surface of FPUA6/9 and (f) film–dish interface of FPUA6/9. The atomic concentration of film samples is shown in Table 2. The surface peak intensity of F1s at 69 eV increased with increase in the VTMS content in FPUAs. By comparing the peak intensities of F1s for film–air surfaces of FPUA 6/0(a) and FPUA6/9(e), and for film–dish interfaces of FPUA6/0 (b)FPUA6/9(f), the relative peak intensity of F1s in the film–air surface was higher than that in the film–dish interface. By curve fitting analysis, our previous studies that covered this subject showed the same result [30, 34]. This suggests that the long perfluoralkyl group of the PFA component was introduced mainly to the surface layer of the fluorinated material as expected. The curing process should encourage the emergence of PFA/VTMS components (long alkyl/perfluoroalkyl groups) on the coating–air surface. The peak intensity of F1s in the film–air surface increased significantly with increase in the VTMS content. This indicated that the VTMS component containing bulky trimethoxysilane groups turned out be significantly more effective than the IBOA component containing bulky trimethylcycloalkyl group in promoting the emergence of PFA component onto the surface layer. This should be due to the presence of less rigid VTMS containing silicone which makes it easy for fluorine components to migrate over the surface. On the other hand, the peak at 152 eV assigned to Si2p and the peak at 101 eV assigned to Si2p were not observed in the FPUA6/0 film sample. The concentration of Si component in the film–air surface was found to be higher than that in the film–dish interface. The peak intensity/concentration of the Si component for film samples (FPUA6/3, 6/6 and 6/9 samples) were found to increase with increase in the VTMS content.
The transmittance % of UV-cured film samples
Figure 3 shows the UV–visible spectra of the UV-cured film samples (FPUA6/0, FPUA6/3, FPUA6/6, FPUA6/9 and FPUA6/12). The FPUA6/0 film without VTMS showed a low transmittance % of approximately 82 % in the visible range (400–800 nm). The transmittance % of the UV-cured film samples increased markedly up to near 100 % with increase in the VTMS content up to 9 wt%. The increase in transmittance % should be due to the increases of amorphous region of film samples. The increase of amorphous region was caused by the incorporation of bulky VTES component into ordered region of polyol PTMG and acrylate components, which led to the decrease of the total ordered region. From these results, it was found that the FPUA6/9 film sample had near-perfect glassy (amorphous) morphology.
DMA properties of UV-cured film samples
Figure 4 shows the storage modulus and tan delta of the UV-cured film samples. The film sample containing a higher VTMS content had a higher storage modulus. As the VTMS content increased, the Tgα of the film sample increased from 41.0 to 46.2 °C, whereas the broad peak at approximately −50 °C assigned to the soft segment Tg was almost unchanged. The increase of Tgα also might be due to the bulky trimethoxysilane group of VTMS. This suggests that the VTMS in vinyl monomers mainly affects the alpha amorphous region.
Mechanical properties and hardness of UV-cured polyurethane acrylate film samples
Figure 5 shows the stress–strain curves of UV-cured acrylate film samples. Table 3 lists the Young modulus, tensile strength, elongation at break and hardness of the film samples. As VTMS content increased, the tensile modulus and hardness increased significantly and the tensile strength increased a little; however, the elongation at break decreased significantly. These results might be due to not only the increase of entanglement of the bulky trimethoxysilane side group, but also the increase of self-cross-linking of trimethoxysilane group exposed to water vapor during sample preparing/UV-curing processes under room environment containing a certain amount of water vapor. The specific influence of water vapor on the self-cross-linking of alkoxysilane will be studied in a future research.
Surface properties of UV-cured polyurethane acrylate film samples
Wettability of solid surface with liquid (water/oil) in air is a very important phenomenon in our daily life as well as in various industrial processes. Generally, wettability is governed by two factors: the chemical factor of the solid/liquid (attractive force) and the geometrical factor of the solid surface (morphology: surface roughness). Table 3 and Fig. 6 show the water/methylene iodide contact angles and the surface tension of UV-cured polyurethane acrylates containing a range of VTMS contents (0–9 wt%). The water/methylene iodide contact angles of the UV-cured film samples FPUA6/0, FPUA6/3, FPUA6/6 and FPUA6/9 were 107.9/80.9°, 110.1/82.1°, 112.4/83.4° and 114.2/84.2°, respectively, whereas the surface tensions of these film samples were 17.2, 16.7, 16.2 and 15.9 mN/m, respectively. The surface tension and contact angles changed significantly with increase in the VTMS content. This might be due to the increase of migration of the hydrophobic PFA component to the film surface layer by increase in the bulky trimethoxysilane groups of VTMS, as mentioned in XPS analysis results. Generally, a self-assembled micro-domain with the fluorinated side chain standing up on the uppermost surface has been proposed for polyurethane with higher fluorinated component content. Atomic force microscopy (AFM) is a powerful technique for characterizing a surface. Usually, the higher root mean square roughness (RMS) values determined using AFM implies surface change from smooth to rough. In our previous study, we found that the RMS value (surface roughness) increased with increase in fluorinated component content in fluorinated polyurethanes [48]. Therefore, the increase of contact angles/decrease of surface tension of film samples prepared here with increasing VTMS content should be attributed to not only the lower attractive force of fluorine/silicone components in the uppermost surface, but also the surface roughness.
Conclusion
UV-curable polyurethane acrylates containing fluorinated acrylic monomer (heptadecafluorodecyl methacrylate, PFA, 6 wt%)/vinyltrimethoxysilane (VTMS, 0–9 wt%) [FPUA6/0, FPUA6/3, FPUA6/6 and FPUA6/9, where the numbers indicate the wt% of PFA/VTMS] were synthesized from a reactive oligomer [4,4′-dicyclohexymethanediisocyanate (H12MDI)/poly(tetramethylene glycol)(PTMG)/2-hydroxyethyl methacrylate (HEMA): 2/1/2 molar ratio, prepolymer: 40 wt%] and diluents [methyl methacrylate (MMA, 20 wt%)/PFA (6 wt%)/isobornyl acrylate (IBOA, 34–25 wt%)/VTMS (0–9 wt%), total diluents: 60 wt%]. The effect of bulky VTMS/IBOA weight ratio on the properties of the UV-cured polyurethane acrylates was assessed for potential transparent antifouling coating applications. The transmittance % of UV-cured FPUA6/0 film sample containing 6 wt% PFA without VTMS was about 81.7 %; however, the transmittance % of the UV-cured FPUA6/9 film sample containing 9 wt% of VTMS was found to be near 100 %, indicating almost perfect transparency over the wavelength range of 400–800 nm (visible light). The storage modulus and hardness increased significantly with increasing VTMS content, but the elongation at break decreased. By XPS analysis, it was found that the film–air surface of the UV-cured polyurethane acrylate film samples had a higher fluorine content than the film–dish interface. As the VTMS content increased from 0 to 9 wt%, the water/methylene iodide contact angles of UV-cured film samples increased from 107.9/80.9° to 114.2/84.2°, whereas the surface tension of these film samples decreased significantly from 17.2 to 15.9 mN/m. These results point to the strong potential of UV-curable polyurethane acrylate (FPUA6/9) with PFA/VTMS contents (6/9 wt%) as a coating material with the lowest surface energy (the highest contact angles) and the highest transparency. Overall, the FPUA6/0, FPUA6/3 and FPUA6/6 and FPUA 6/9 samples have better antifouling properties than polytetrafluoroethylene.
References
Kim BK, Lee KH, Kim HD (1996) Preparation and properties of UV-curable polyurethane acrylates. J Appl Polym Sci 60:799–805
Yoo HJ, Lee YH, Kwon JY, Kim HD (2001) Comparison of the properties of UV-cured polyurethane acrylates containing different diisocyanates and low molecular weight diols. Fibers Polymers 2(3):122–128
Kwon JY, Yoo HJ, Kim HD (2001) Effect of chemical structure on the properties of UV-cured polyurethane acrylates films. Fibers Polymers 2(3):141–147
Xu HP, Qiu FX, Wang YY, Wu WL, Yang DY, Guo Q (2012) UV-curable waterborne polyurethane-acrylate: preparation, characterization and properties. Prog Org Coat 73:47–53
Kim HD, Kang SG, Ha CS (1992) Properties of UV-curable polyurethane acrylates for primary optical fiber coating. J Appl Polym Sci 46:1339–1351
Koshiba M, Hwang KS, Foley SK, Yarusso DJ, Cooper SL (1982) Properties of ultra-violet curable polyurethane acrylates. J Material Sci 17:1447–1458
Ono H, Kawatsuki N (1994) Electrooptical properties of poly(vinyl alcohol)/liquid crystal composite films with added photocured polymers. Jpn J Appl Phys 33:6268–6272
Fox HW, Zisman WA (1950) The spreading of liquids on low energy surfaces, I. polytetrafluoroethylene. J Colloid Sci 5:514–531
Bernett MK, Zisman WA (1962) Wetting properties of acrylic and methacrylic polymers containing fluorinated side chains. J Phys Chem 66:1207–1208
Hare EF, Shafrin EG, Zisman WA (1954) Properties of films of adsorbed fluorinated acids. J Phys Chem 58:236–239
Bernett MK, Zisman WA (1960) Wetting properties of tetrafluoroethylene and hexafluoropropylene copolymers. J Phys Chem 64:1292–1294
Li H, Zhang ZB, Hu CP, Wu SS, Ying SK (2004) Surface composition and property of film prepared with aqueous dispersion of polyurethaneurea-acrylate including fluorinated block copolymer. Euro Polym J 40:2195–2201
Mawson S, Johnston KP, Betts DE, McClain JB, Desimone JM (1997) Stabilized polymer microparticles by precipitation with a compressed fluid antisolvent. 1. Poly(fluoro acrylates). Macromolecules 30:71–77
Park IJ, Lee SB, Choi CK (1998) Surface properties of the fluorine-containing graft copolymer of poly((perfluoroalkyl)ethyl methacrylate)-g-poly(methyl methacrylate). Macromolecules 31:7555–7558
Cheng S, Chen Y, Chen ZJ (2002) Core–shell latex containing fluorinated polymer rich in shell. J Appl Polym Sci 85:1147–1153
Krupers M, Slangen PJ, Moller M (1998) Synthesis and properties of polymers based on oligo(hexafluoropropene oxide) containing methacrylates and copolymers with methyl methacrylate. Macromolecules 31:2552–2558
Hopken J, Moller M (1992) Low surface energy polystyrene. Macromolecules 25:1461–1467
Bouteiller V, Garnault AM, Teyssie D, Boileau S, Moller M (1999) Synthesis, thermal and surface characterization of fluorinated polystyrenes. Polym Int 48:765–772
Schmidt DL, Coburn CE, DeKoven BM, Potter GE, Meyers GF, Fischer DA (1994) Water-based non-stick hydrophobic coatings. Nature 368:39–41
Elman JF, Johs BD, Long TE, Koverstein JT (1994) A neutron Reflectivity investigation of surface and interface segregation of polymer functional end groups. Macromolecules 27:5341–5349
Boutevin B, Diaf KO, Pietrasanta Y, Taha M (1986) Synthesis of block cotelomers involving a perfluorinated chain and a hydrophilic chain Part 1. Use of fluorinated telogens with trichloromethyl end groups. J Polym Sci Part A Polym Chem 24:3129–3137
Bonardi C (1991) Telomers acryliques fluores et application au traitement hydrofuge et oleofluge de substrats divers. Eur Pat Appl EP 426530
Wang JG, Mao GP, Ober CK, Kramer EJ (1997) Liquid crystalline, semifluorinated side grou block copolymers with stable low energy surfaces: synthesis, liquid crystalline structure, and critical surface tension. Macromolecules 30:1906–1914
Komber DH, Voigt D, Jehgnichen D, Haubler L, Gottwald A et al (2003) Synthesis and characterization of semifluorinated polymers and block copolymers. Macromol Symp 199:173–186
Corpart JM, Girault S, Juhue D (2001) Structure and surface properties of liquid crystalline fluoroalkyl polyacrylates: Role of the spacer. Langmuir 17:7237–7244
Tingting Y, Hui P, Shiyuan C, Park IJ (2005) Surface immobilization of perfluorinated acrylate copolymers by self-crosslinking. J Fluorine Chem 126:1570–1577
Marcu I, Daniels ES, Dimonie VL, Hagiopol C, Roberts JE, El-Aasser MS (2003) Incorporation of alkoxysilane into mode latex systems: vinyl copolymerization of vinyltriethoxysilane and n-butyl acrylate. Macromolecules 36:328–332
Cheng X, Chen Z, Shi T, Wang H (2007) Synthesis and characterization of core–shell LIPN-fluorine-containing polyacrylate latex. Colloids Surf A292:119–124
Zhang Y, Miao L, Yang C, Lu M (2013) Synthesis of ambient temperature self-crosslinking VTES-based core–shell polyacrylate emulsion via modified micro-emulsion polymerization process. Polym Bull 70:1631–1645
Kim DH, Lee YH, Park CC, Kim HD (2014) Synthesis and surface properties of self-crosslinking core–shell acrylic copolymer emulsions containing fluorine/silicone in the shell. Colloid Polym Sci 292:173–183
Chen YJ, Zhang CC, Chen XX (2006) Emulsifier-free latex of fluorinated acrylate copolymer. Euro Polym J 42:694–701
Hirose M, Kadowaki F, Zhou J (1997) The structure and properties of core–shell type acrylic–polyurethane hybrid aqueous emulsions. Prog Org Coat 31:157–169
Xin H, Shen YD, Li XR (2011) Synthesis and properties of cationic polyurethane-fluorinated acrylic hybrid latexes by emulsifier-free emulsion polymerization and the solvent-free method. Polym Bull 67:1849–1863
Shin MS, Lee YH, Mohammad MR, Kim HD (2013) Synthesis and properties of waterborne fluorinated polyurethane-acrylate using a solvent-/emulsifier-free method. Polymer 54:4873–4882
Lee SW, Lee YH, Park H, Kim HD (2013) Effect of total acrylic/fluorinated acrylic monomer contents on the properties of waterborne polyurethane/acrylic hybrid emulsions. Macromol Res 21:709–718
Tanaka H, Suzuki Y, Yoshino F (1999) Synthesis and coating application of waterborne fluoroacrylic–polyurethane composite dispersions. Coll Surf A 153:597–601
Zhang CY, Zhang XY, Dai JB et al (2008) Synthesis and properties of PDMS modified waterborne polyurethane-acrylic hybrid emulsion by solvent-free method. Prog Org Coat 63:238–244
Tang YW, Santerre JP, Labow RS, Taylor DG (1996) Synthesis of surface-modifying macromolecules for use in segmented polyurethanes. J Appl Polym Sci 62:1133–1145
Park IJ, Lee SB, Choi CK (1994) Surface properties for poly(perfluoroalkylethyl methacrylate. J Appl Polym Sci 54:1449–1454
Kano Y, Akiyama S (1996) Estimation of surface tension and surface segregation of poly(ethyl acrylate)/poly(vinylidene fluoride-co-hexafluoroacetone) blends. Polymer 37:4497–4503
Xu HP, Qiu FX, Wang YY, Wu WL, Yang DY, Guo Q (2012) UV-curable waterborne polytrethane-acrylate:preparation, characterization and properties. Prog Org Coat 73:47–53
Hyeon HD, Kim HJ (2011) UV-curable low surface energy fluorinated polycarbonate-based polyurethane dispersion. J Colloid Interface Sci 362:274–284
Liu T, Pan X, Wu Y, Zhang T, Zheng Z, Ding X, Peng Y (2012) Synthesis and characterization of UV-curable waterborne polyurethane acrylate possessing perfluorooctanoate side-chain. J Polym Res 19:9741–9748
Lin YH, Liao KH, Chou NK, Wang SS, Chu SH, Hsieh KH (2008) UV-curable low-surface-energy fluorinated poly(urethane-acrylate)s for biomedical applications. Euro Polym J 44:2927–2937
Canak TC, Serhati IE (2013) Synthesis of fluorinated urethane acrylate based UV-curable coatings. Prog Org Coat 76:388–399
Park JM, Lee YH, Park H, Kim HD (2014) Preparation and properties of UV-curable fluorinated polyurethane acrylate. J App Polym Sci 131:40603
Kaelble DH, Moacanin J (1977) A surface energy analysis of bioadhesion. Polymer 18:475–482
Rahman MM, Lee IW, Chun HH, Kim HD, Park H (2014) Properties of waterborne polyurethane-fluorinated marine coatings: the effect of different types of diisocyanates and tetrafluorobutanediol chain extender content. J Appl Polym Sci 131:39905
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) through GCRC-SOP (No. 2011-0030013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.M., Jeon, J.H., Lee, Y.H. et al. Synthesis and properties of UV-curable polyurethane acrylates containing fluorinated acrylic monomer/vinyltrimethoxysilane. Polym. Bull. 72, 1921–1936 (2015). https://doi.org/10.1007/s00289-015-1380-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1380-x