Abstract
Fluorinated polycarbonate-based UV-curable polyurethane acrylate (F-PCUA) was synthesized by incorporating 1H, 1H, 2H, 2H-Perfluoro-1-octanol to the end of polycarbonate-based PUA chains. The structure of F-PCUA was determined by 1H-NMR, 19F-NMR, and FTIR analyses. The physical, surface, and thermal properties of F-PCUA were also examined. The F-PCUA was used as a hydrophobic additive in PUA coatings, and the water and oil wettability of the UV-cured film was investigated by contact angle measurements. The results showed that the coating system had great hydrophobicity. Furthermore, X-ray photoelectron spectroscopy research confirmed that a hydrophobic fluorine-enriched surface was obtained in the coating system. Moreover, the mechanical and chemical properties of the hydrophobic coatings did not show deterioration with the introduction of elemental F.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethanes (PU) have been widely used in many applications such as coatings, adhesives, and thermoplastic elastomers due to their excellent mechanical and physical properties and film-forming ability.1 Their films show an excellent elasticity and toughness. The chemical reaction underlying PU synthesis is polyaddition between the hydroxyl group (–OH) of polyol and the isocyanate group (–NCO).2–5 PU is generally composed of a polyol (soft segment), a diisocyanate (hard segment), and a low-molecular-weight diol component (chain extender, hard segment). Polycarbonate diol (PCDL) polyols have been recently commercialized and are claimed to provide good resistance to hydrolysis, heat aging, oil resistance, weathering, and fungi.6 Synthesis of PCDL-based polyurethane acrylate for UV-curable coatings was meaningful.
Recently, F-modified hydrophobic polymers were extensively studied7–10 for incorporation into UV-curable coatings such as antifrosting coatings,11 self-cleaning coatings,12 and antifingerprint coatings.13 Interactions between molecular chains are reduced when elemental F is introduced into a polymer owing to the low surface energy of F. A film surface enriched with fluorinated species imparts excellent hydrophobic and oleophobic properties on the coating. Miao et al. synthesized a series of fluorinated hyperbranched polyester acrylates and used them as additives for UV-curable coatings, which exhibited increased contact angles ranging from 80° to 120°.14
Because of its better performance relative to that of other diols, polycarbonate diol (PCDL) is used in materials such as antistatic materials,15 biomedical materials,16 shape memory composites,17–20 and electromagnetic interference shielding materials.21 Compared to other polyurethane acrylates (PUAs), PCDL-based PUA (PCUA) exhibits stronger mechanical properties and higher heat resistance, abrasion resistance, oxidation resistance, hydrolysis resistance, and chemical resistance.22–24 Hyeon-Deuk Hwang’s group has performed many studies on PCDL-based waterborne PUAs (WPCUAs), for example, UV-curable low-surface energy fluorinated polycarbonate-based polyurethane and waterborne UV-curable polycarbonate-based polyurethane (meth)acrylate incorporated with polydimethylsiloxane.25–27
In this work, a series of fluorinated PCDL-based PUAs (F-PCUAs) were synthesized using PCDL as the soft segment with different molecular weights (500, 1000, and 2000 g/mol). The F-PCUAs were then used as hydrophobic additives in PUA, yielding hydrophobic UV-curable coatings. FTIR, NMR, TGA, and tensile strength tests were carried out to characterize the properties of the F-PCUAs. In addition, contact angle measurements, XPS, and coating performance tests were performed to examine the UV-cured coatings.
Experimental
Materials
Polycarbonate diols (PCDLs, with molecular weights of 500, 1000, and 2000 g/mol) were supplied by Asahi Kasei Co., Ltd. (Tokyo, Japan). 1H, 1H, 2H, 2H-Perfluoro-1-octanol (PFEL) was supplied by Harbin Xeogia Fluorine-silicon Co., Ltd. (Harbin, China). Isophorone diisocyanate (IPDI) was purchased from Hui Li Chemical Co., Ltd. (Wuxi, China). Pentaerythritol triacrylate (PET3A) was supplied by Eternal Chemical Co., Ltd. (Taiwan, China). Toluene, acetone, N-butyl acetate, sodium hydroxide (NaOH), sulfuric acid (H2SO4), ethanol, oleic acid, and di-butyl hydroxyl toluene (BHT) were all purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 1-Hydroxycyclohexyl-phenyl ketone (Irgacure 184) was provided by Sartomer Chemical Co., Ltd. (Guangzhou, China).
Synthesis of fluorinated PCDL-based PUA (F-CPUA)
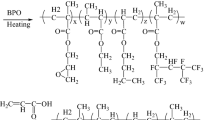
The synthesis route of F-PCUA is shown in Fig. 1. A flame-dried 500-mL three-necked round-bottom flask equipped with a magnetic stirrer and a thermometer was charged with 0.2 mol of IPDI. Then, 0.1 mol of PCDL with an appropriate amount of butyl acetate and DBTDL (300 ppm) was added to IPDI dropwise over a period of 2.5 h at 40°C and maintained for 6 h, yielding polyurethane prepolymer. PFEL was then added dropwise to the urethane prepolymer in a mole ratio of NCO:OH = 1:0.5. The mixture was heated to 70°C and maintained for 6 h. Then PET3A with BHT (0.15 wt%) was added dropwise to the mixture. Completion of the reaction was confirmed by FTIR with the disappearance of the NCO absorption peak. F-500-PCUA, F-1000-PCUA, and F-2000-PCUA, corresponding to PCDL molecular weights of 500, 1000, and 2000 g/mol, respectively, were obtained.
Preparation of F-CPUA films
The optimized composition of F-PCUA was mixed with the appropriate amounts of photoinitiator and n-butyl acetate in a beaker. The mixture was poured into glass molds, and bubbles were removed by magnetic stirring. After 2 days, F-PCUA films measuring approximately 1 mm in thickness took shape in an oven heated to 40°C. The F-PCUA films were exposed to UV radiation with an energy density of 1500 mJ/cm2 under a UV lamp for curing. The tensile strength and thermal properties of the films were examined.
Preparation of hydrophobic PUA Coatings
To examine the hydrophobicity of F-PCUA as a hydrophobic additive, nonfluorinated PCUA-2000 was used as the matrix resin. PCUA-2000 was synthesized in a manner similar to that used to synthesize F-2000-PCUA, except that both ends of the polymer were capped by PET3A. F-2000-PCUA with different mass fractions was used as the hydrophobic additive and mixed with PCUA-2000 and the appropriate amounts of photoinitiator and n-butyl acetate in a beaker. The mixture was coated on PC plates with a film roller measuring approximately 120 μm in thickness and then prebaked at 50°C for 20 min in an oven. Lastly, the coatings were exposed to 1500 mJ/cm2 of UV radiation for curing. A series of coatings with different fluorine contents (0, 0.15, 0.31, 0.46, 0.62, 0.77, and 1.54%) were denoted as PCUA, F0.15-PCUA, F0.31-PCUA, F0.46-PCUA, F0.62-PCUA, F0.77-PCUA, and F1.54-PCUA, respectively. The F-PCUA contents and F content ratios of the coatings are shown in Table 1.
Characterization
The PCDL and oligomers were characterized by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance spectroscopy (1H-NMR and 19F-NMR). The FTIR spectra of these resins were recorded with an FTLA2000-104 (ABB BOMEN, Canada) using a KBr cell. 1H-NMR and 19F-NMR spectra were obtained by a Bruker Spectrospin MSL-300 MHz using deuterated chloroform (CDCl3) as a solvent and tetramethylsilane (TMS) as an internal standard.
The tensile strength and elongation at break of the films were investigated using an LRX single-column universal testing machine. The samples were cut into dumbbell shape and submitted to tensile stress–strain measurements. The cross-head speed was set to 10 mm min−1.
The thermal degradation behavior of the F-PCUAs was investigated by thermal gravity analysis (TGA/1100SF, METTLER). In each case, a 10 mg sample was examined under a N2 flow rate of 5 × 10−5 m3 min−1 at a heating rate of 10°C min−1 from room temperature to 600°C.
The surface-free energy of the UV-cured films was evaluated from the static contact angles using a contact angle analyzer (OCA40, DATA physics) at 25°C. The surface-free energy was calculated from the contact angles based on equations (1) and (2):28
X-ray photoelectron spectroscopy (XPS) was carried out to analyze the surface composition of the fluorinated UV-cured films. The experiments were carried out on an RBD upgraded PHI-5000C ESCA system (Perkin Elmer) with Mg Kα radiation (hν = 1253.6 eV) or Al Kα radiation (hν = 1486.6 eV). Briefly, the X-ray anode was operated at 250 W and a high voltage of 14.0 kV with a detection angle of 54°. The pass energy was fixed to 23.5, 46.95, or 93.90 eV to ensure sufficient resolution and sensitivity. The base pressure of the analyzer chamber was approximately 5 × 10−8 Pa. To compare the F content at different depths of the coatings, a coating sample was chosen to run at different take-off angles of 30° and 90°.
To evaluate the oil resistance of the samples, coated panels were immersed in oleic acid for 24 h at room temperature. To examine their chemical resistance, samples were immersed in 0.5 mol L−1 H2SO4, 1 mol L−1 NaOH, and 50% ethanol.
Results and discussion
FTIR
The FTIR spectra obtained for PCDL, PCDL-IPDI, PCDL-IPDI-F, and F-PCUA are shown in Fig. 2. It is known that N–H stretching occurs at 3380 cm−1, C–N stretching and δ N–H bending occur at 1526 cm−1, C–H stretching occurs from 2780 to 3045 cm−1, and C=O stretching occurs from 1643 to 1865 cm−1. Compared to PCDL-IPDI and PCDL-IPDI-F, the peak at 2265 cm−1 disappeared, whereas a new peak at 1635 cm−1 appeared due to the double bond of the acrylate group, suggesting that the hydroxyl group on the PCDL segment and PET3A had been consumed due to its reaction with –NCO on IPDI. In addition, the absorption peak at 1243 cm−1 assigned to C–F stretching vibration can be observed in the F-PCUA curve, indicating that F-PCUA was successfully end-capped with PET3A and perfluoroalkyl groups.
1H-NMR and 19F-NMR
A typical 1H-NMR survey spectrum of F-PCUA is shown in Fig. 3. The peaks at 6.80 and 7.31 ppm are assigned to the hydrogen atom of secondary amine, which was connected to the carbonate ester group of PCDL and NCO groups of IPDI. The split peaks at 2.3–2.6 ppm are characteristic peaks of the hydrogen atoms of methylene connected to perfluoroalkyl groups, which reveal the successful synthesis of PCDL-IPDI-F. Two characteristic peaks at 5.70–6.40 ppm are attributed to the hydrogen atom of double bonds, which indicates that the methacrylate group was successfully connected to the end of the molecular chain. In addition, peaks between 0.78 and 1.80 ppm are assigned to the methyl and methylene groups in the molecular structure.
The 19F-NMR spectrum obtained for F-PCUA is shown in Fig. 4. There are four peaks in Fig. 4 corresponding to the chemical shift of F atoms in four different positions. The ratio of the peak areas is 2:2:3:6, which coincides with the number of F atoms that occur at different positions. FTIR, 1H NMR, and 19F-NMR analyses confirm that the F-PCUAs were successfully synthesized as designed.
Tensile strength
Tensile strength tests are carried out to study the basic mechanical properties of polymeric materials, including Young’s modulus, tensile strength, elongation at break, and toughness. Figure 5 shows the strain curves of F-1000-PET3A and F-2000-PET3A, and the tensile strength, elongation at break, and Young’s modulus of the cured films are listed in Table 2. The effects of the molecular weight of PCDL on the tensile behavior of the synthesized F-PCUA resins were also demonstrated. With increasing molecular weight of PCDL, the maximum tensile strength and initial modulus decreased (F-1000-PET3A > F-2000-PET3A); however, the elongation at break increased (F-1000-PET3A < F-2000-PET3A). The maximum stresses of F-1000-PET3A and F-2000-PET3A were 14.15 and 4.76 MPa, respectively. However, in terms of elongation, the opposite trend to that of the stress appeared. The elongation of the coatings increased sharply with the increasing molecular weight of PCDL. In fact, the F-500-PET3A film was too hard and fragile to stretch. This behavior can be explained by considering the fact that a network with a high crosslink density exhibits higher resistance to an external extension force, leading to higher tensile strength and lower elongation at break. Moreover, as the molecular weight of PCDL increased, the mass fraction of the hard segment decreased and the mass fraction of the soft segment increased, which also caused the film to exhibit better ductility and flexibility.
Thermogravimetric analysis (TGA)
The thermal stability of the UV-cured films was measured by TGA at temperatures ranging from 25 to 600°C as shown in Fig. 6. The first and second stages were due to the degradation of the hard and soft segments of polyurethane linkages.29,30 The maximum thermal degradation of the hard and soft segments of polyurethane occurs at 235–245 and 355–375°C, respectively. The third peak is associated with degradation of the fluorine connected to the end of the F-PCUA chain at approximately 430–450°C.25 The molecular weight of the F-PCUA was increased with the molecular weight of PCDL, resulting in a decrease in the density of double bonds. In addition, the content of the hard segment decreased at the same time. Therefore, the thermal stability of the fluorine-modified PUAs was decreased with the increased molecular weight of PCDL.
Contact angle
The contact angles of the UV-cured films with different concentrations of F-PCUA are shown in Fig. 7. It is clear that the water and oil wettability of the cured films depend on the concentration of F-PCUA and that the contact angle increased with the addition of F-PCUA. Without adding F-PCUA, the water contact angle was 78°. By adding F-PCUA, the contact angle increased to above 100°, even though the F content was very low, exhibiting great hydrophobicity. We attribute these results to the presence of hydrophobic tail-like fluorine-substituted chains on the surfaces of the PCUAs.26 However, no appreciable increase in hydrophobicity was observed at F contents above 0.6%. Similar trends were observed for the oil test.
Surface-free energy
The calculated surface tensions (γ s) of the UV-cured films are listed in Table 3. With the incorporation of only 0.15 wt% F, the surface-free energy decreased from 44.0 to 26.3 mN m−1. Only a very small amount of F-PCUA affected the surface-free energy significantly. These results demonstrate that the CF2 groups migrated to the outermost surface and provided UV-cured films with a hydrophobic fluorine-enriched surface.31
X-ray photoelectron analysis (XPS)
Figure 8 shows the XPS spectra of the UV-cured films according to the F-PCUA content. Figure 9 shows the XPS spectra obtained at different take-off angles for the same sample (F1.54-PCUA). Signals of fluorine, oxygen, nitrogen, and carbon can easily be observed from the survey scan in the region corresponding to 0–800 eV, with the bonding energies of 694, 536, 401, and 290 eV, respectively. Figure 9 shows that the fluorine content at a take-off angle of 30° was higher than that at 90°. This result was observed because the detecting depth of the film at 30° was smaller than that at 90°, closer to the surface. This finding proves that the coating surface was enriched with fluorine. Table 5 also supports this conclusion.
The surface enrichment factor (SF) was defined as the ratio of the fluorine content measured experimentally at the top surface of a dried film (F surface) to the estimated atomic % fluorine in the film solids, assuming a uniform and random distribution (F bulk), as indicated in equation (3)32:
Table 4 lists the fluorine contents of the experimental formulation and at the interface of the coating films, which indicates the enrichment factor of the fluorine element. The fluorine surface enrichment ranged from 19- to 46-fold with respect to the bulk composition. All of the samples containing F-PCUAs showed a higher fluorine atomic content at the surface than that estimated in the bulk. Therefore, the fluorine chain can easily tailor the surface owing to the thermodynamic driving force of the PFEL segments associated with their very low surface energy.33
Coating performance
To evaluate the adhesion of the UV-cured coatings on PC substrates and the samples’ pencil hardness, coated PC panels were tested by conventional methods. The samples were then immersed in 0.5 mol L−1 sulfuric acid, 1 mol L−1 sodium hydroxide, 50% ethanol, and oleic acid for 24 h to evaluate the chemical resistance of the coatings. The results are shown in Table 5. The results indicate that the addition of F-PCUA did not adversely affect the coating performance. The coatings still exhibited good adhesion on PC and good chemical resistance.
Conclusion
Fluorinated PCDL-based PUAs (F-PCUAs) were successfully synthesized by incorporating perfluoroalkyl to the end of PCUA chains. The contact angle of the UV-cured coatings could be increased to 110° even with a very low content of F-PCUA, indicating good hydrophobicity. XPS demonstrated the enrichment of elemental fluorine on the coating surfaces. The hydrophobic coatings exhibited good chemical resistance and adhesion on PC panels.
References
Guan, J, Song, Y, Lin, Y, et al., “Progress in Study of Non-isocyanate Polyurethane.” Ind. Eng. Chem. Res., 50 (11) 6517–6527 (2011)
Liu, N, Zhao, Y, Kang, M, et al., “The Effects of the Molecular Weight and Structure of Polycarbonatediols on the Properties of Waterborne Polyurethanes.” Prog. Org. Coat., 82 46–56 (2015)
Nam, KH, Seo, K, Seo, J, et al., “Ultraviolet-Curable Polyurethane Acrylate Nanocomposite Coatings Based on Surface-Modified Calcium Carbonate.” Prog. Org. Coat., 85 22–30 (2015)
Lucio, B, de la Fuente, JL, et al., “Rheological Cure Characterization of an Advanced Functional Polyurethane.” Thermochim. Acta, 596 6–13 (2014)
Hwang, HD, Park, CH, Moon, JI, et al., “UV-Curing Behavior and Physical Properties of Waterborne UV-Curable Polycarbonate-Based Polyurethane Dispersion.” Prog. Org. Coat., 72 663–675 (2011)
Liu, J, Liu, Q, Zheng, X, et al., “Synthesis of UV-Curable Polycarbonate Diols (PCDL)-Based Polyurethane Acrylate for Negative Photoresist.” Polym. Bull., 73 647–659 (2016)
Ji, H, Côté, A, Koshel, D, et al., “Hydrophobic Fluorinated Carbon Coatings on Silicate Glaze and Aluminum.” Thin Solid Films., 405 (1) 104–108 (2002)
Cortese, G, Martina, F, Vasapollo, G, et al., “Modification of Micro-channel Filling Flow by Poly (dimethylsiloxane) Surface Functionalization with Fluorine-Substituted Aminonaphthols.” J. Fluor. Chem., 131 (3) 357–363 (2010)
Futamata, M, Gai, X, Itoh, H, “Improvement of Water-Repellency Homogeneity by Compound Fluorine–Carbon Sprayed Coating and Silane Treatment.” Vacuum., 73 (3) 519–525 (2004)
Yan, Z, Liu, W, Gao, N, et al., “Synthesis and Characterization of a Novel Difunctional Fluorinated Acrylic Oligomer Used for UV-Cured Coatings.” J. Fluor. Chem., 147 49–55 (2013)
Wang, H, Tang, L, Wu, X, et al., “Fabrication and Anti-frosting Performance of Super Hydrophobic Coating Based on Modified Nano-sized Calcium Carbonate and Ordinary Polyacrylate.” Appl. Surf. Sci., 253 (22) 8818–8824 (2007)
Moon, IJ, Lee, YH, Kim, HJ, et al., “Investigation of the Peel Test for Measuring Self-Cleanable Characteristic of Fluorine-Modified Coatings.” Polym. Test., 31 (3) 433–438 (2012)
Bongiovanni, R, Medici, A, Zompatori, A, et al., “Perfluoropolyether Polymers by UV Curing: Design, Synthesis and Characterization.” Polym. Int., 61 (1) 65–73 (2012)
Miao, H, Cheng, L, Shi, W, “Fluorinated Hyperbranched Polyester Acrylate Used as an Additive for UV Curing Coatings.” Prog. Org. Coat., 65 (1) 71–76 (2009)
Kwon, JY, Kim, HD, “Preparation and Properties of Acid-Treated Multiwalled Carbon Nanotube/Waterborne Polyurethane Nanocomposites.” J. Appl. Polym. Sci., 96 (2) 595–604 (2005)
Verdejo, R, Jell, G, Safinia, L, et al., “Reactive Polyurethane Carbon Nanotube Foams and Their Interactions with Osteoblasts.” J. Biomed. Mater. Res. Part. A., 88 (1) 65–73 (2009)
Cho, JW, Kim, JW, Jung, YC, et al., “Electroactive Shape-Memory Polyurethane Composites Incorporating Carbon Nanotubes.” Macromol. Rapid Commun., 26 (5) 412–416 (2005)
Deka, H, Karak, N, Kalita, RD, et al., “Biocompatible Hyperbranched Polyurethane/Multi-Walled Carbon Nanotube Composites as Shape Memory Materials.” Carbon, 48 (7) 2013–2022 (2010)
Meng, Q, Hu, J, “Self-organizing Alignment of Carbon Nanotube in Shape Memory Segmented Fiber Prepared by In Situ Polymerization and Melt Spinning.” Compos. Part. A Appl. S., 39 (2) 314–321 (2008)
Sahoo, NG, Jung, YC, Yoo, HJ, et al., “Influence of Carbon Nanotubes and Polypyrrole on the Thermal, Mechanical and Electroactive Shape-Memory Properties of Polyurethane Nanocomposites.” J. Compos. Sci. Technol., 67 (9) 1920–1929 (2007)
Ma, CCM, Huang, YL, Kuan, HC, et al., “Preparation and Electromagnetic Interference Shielding Characteristics of Novel Carbon-Nanotube/Siloxane/Poly-(urea urethane) Nanocomposites.” J. Polym. Sci. Part B Polym. Phys., 43 (4) 345–358 (2005)
Kojio, K, Nonaka, Y, Masubuchi, T, et al., “Effect of the Composition Ratio of Copolymerized Poly (carbonate) Glycol on the Microphase-Separated Structures and Mechanical Properties of Polyurethane Elastomers.” J. Polym. Sci. Part B Polym. Phys., 42 (24) 4448–4458 (2004)
Špírková, M, Pavličević, J, Strachota, A, et al., “Novel Polycarbonate-Based Polyurethane Elastomers: Composition-Property Relationship.” Eur. Polym. J., 47 (5) 959–972 (2011)
Feng, YX, Yin, N, Li, QF, et al., “Environmentally Benign Route for the Synthesis of Polycarbonate Diols (PCDLs)-Calcined MgAl Hydrotalcites as Heterogeneous Catalysts.” Ind. Eng. Chem. Res., 47 (7) 2140–2145 (2008)
Hwang, HD, Kim, HJ, “UV-Curable Low Surface Energy Fluorinated Polycarbonate-Based Polyurethane Dispersion.” J. Colloid Interface Sci., 362 (2) 274–284 (2011)
Hwang, HD, Kim, HJ, “Enhanced Thermal and Surface Properties of Waterborne UV-Curable Polycarbonate-Based Polyurethane (meth) Acrylate Dispersion by Incorporation of Polydimethylsiloxane.” J. React. Funct. Polym., 71 (6) 655–665 (2011)
Hwang, HD, Park, CH, Moon, JI, et al., “UV-Curing Behavior and Physical Properties of Waterborne UV-Curable Polycarbonate-Based Polyurethane Dispersion.” Prog. Org. Coat., 72 (4) 663–675 (2011)
Wu, S, “Calculation of Interfacial Tension in Polymer Systems.” J. Polym. Sci. Part C., 34 (1) 19–30 (1971)
Asif, A, Shi, W, “UV Curable Waterborne Polyurethane Acrylate Dispersions Based on Hyperbranched Aliphatic Polyester: Effect of Molecular Structure on Physical and Thermal Properties.” Adv. Technol., 15 (11) 669–675 (2004)
Yang, J, Wang, Z, Zeng, Z, et al., “Chain-Extended Polyurethane-Acrylate Ionomer for UV-Curable Waterborne Coatings.” J. Appl. Polym. Sci., 84 (10) 1818–1831 (2002)
Lin, Y, Liao, K, Chou, N, et al., “UV-Curable Low-Surface-Energy Fluorinated Poly (Urethane-Acrylate)s for Biomedical Applications.” Eur. Polym. J., 44 (9) 2927–2937 (2008)
Levine, F, La Scala, J, Kosik, W, “Properties of Clear Polyurethane Films Modified with a Fluoropolymer Emulsion.” Prog. Org. Coat., 69 (1) 63–72 (2010)
Fabbri, P, Messori, M, Montecchi, M, et al., “Perfluoropolyether-Based Organic–Inorganic Hybrid Coatings.” Polymer, 47 (4) 1055–1062 (2006)
Acknowledgments
We acknowledge the financial support from the National Science Foundation of China (No. 51403082), the National Nature Science Foundation of Jiangsu Province (No. BK20130153), and the Fundamental Research Funds for the Central Universities (JUSRP11514).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Wang, B., Yuan, Y. et al. Synthesis of fluorinated polycarbonate-based polyurethane acrylate for UV-curable coatings. J Coat Technol Res 14, 233–241 (2017). https://doi.org/10.1007/s11998-016-9847-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-016-9847-8