Abstract
Mast cells are important immune cells for host defense through activation of innate immunity (via toll-like receptors or complement receptors) and acquired immunity (via FcεRI). Conversely, mast cells also act as effector cells that exacerbate development of allergic or autoimmune disorders. Yet, several lines of evidence show that mast cells act as regulatory cells to suppress certain inflammatory diseases. Here, we review the mechanisms by which mast cells suppress diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cells are derived from hematopoietic stem cells and are distributed in various tissues, especially the airways, skin, and gastrointestinal tract, where various antigens are encountered. Mast cells are thus considered to be sentinel immune cells in host defense. Indeed, mast cells can contribute to host defense against pathogens via innate immune systems such as toll-like receptors or complement receptors [1]. Binding of antigen-specific IgE to FcεRI on the surface of mast cells primes them to release various mediators upon subsequent exposure to the specific Ag [1]. IgE-mediated mast cell activation is important for host defense against certain parasites [2]. Mast cells also influence functions of various types of immune cells such as dendritic cells (DCs), macrophages, T cells, and B cells [3], contributing indirectly to host defense via such cells. However, inappropriate or excessive activation of mast cells is generally known to aggravate development of various diseases such as allergic and autoimmune diseases [4]. On the other hand, mast cells also play a suppressive role in development of certain diseases. Thus, mast cells can act not only as effector cells but also as suppressor cells, in certain immune responses, and those various roles are described in the following sections.
Regulatory role of mast cells in skin allograft rejection
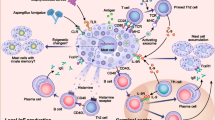
The number of mast cells is increased at the local sites of allografts such as the liver [5], kidney [6], and lung [7] in humans, which suggested that mast cells may contribute to the pathogenesis of allograft rejection. Mast cell-deficient Kit W-sh/W-sh mice were used to elucidate the roles of recipient mast cells in acute and chronic cardiac allograft rejection [8]. In this model, recipient mast cells were not required for acute or chronic cardiac allograft rejection [8]. On the other hand, mast cells played an immune-suppressive role in a murine model of skin allograft transplantation [9]. Compared with in naïve mice, long-term skin allograft survival was observed in mice rendered tolerant to alloantigens by co-injection of allogenic cells and anti-CD154 blocking Abs [9]. In the setting, the numbers of regulatory T cells (Tregs), which are important for tolerance to alloantigens [10], and mast cells were increased in the skin allografts of the tolerized mice compared with the non-tolerized mice [9]. Remarkably, the long-term skin allograft survival seen in tolerized wild-type mice was not observed in tolerized mast cell-deficient Kit W-sh/W-sh mice [9], suggesting that mast cells somehow contribute to Treg-mediated skin allograft tolerance. In addition to Tregs and mast cells, IL-9, which is a potent cytokine that enhances growth and recruitment of mast cells [11–13], was also crucial for skin allograft tolerance in this model [9]. Tregs were a potential source of IL-9 in skin allografts, and treatment of wild-type mice with anti-IL-9 neutralizing Abs resulted in failure of skin allograft tolerance and accumulation of mast cells in the skin allografts [9]. Although direct evidence that Treg-derived IL-9 is involved in skin allograft tolerance was not generated (i.e., by adoptive transfer analysis using IL-9-deficient and IL-9-sufficient Tregs), these observations suggest that Treg-derived IL-9 may control mast cell function (Fig. 1). Likewise, Tregs and mast cells (especially their interaction) were crucial for protection in a Th1- and Th17-dependent model of nephrotoxic serum nephritis [14]. Transfer of wild-type Tregs prevented nephrotoxic serum nephritis and accumulation of mast cells in kidney-draining lymph nodes in wild-type, but not mast cell-deficient Kit W/W-v, recipient mice [14]. On the other hand, transfer of IL-9-deficient Tregs failed to protect wild-type recipient mice against nephrotoxic serum nephritis and mast cell accumulation in kidney-draining lymph nodes [14]. Therefore, these observations in the two models of skin allograft and nephrotoxic serum nephritis suggested that Treg-derived IL-9 promotes mast cell function, and the mast cells somehow subsequently contribute to immune suppression.
Regulatory role of mast cells in skin allograft rejection. Mast cell-derived TNF and GM-CSF, respectively, enhance migration of dendritic cells (DCs) from the skin to draining lymph nodes and survival of DCs in draining lymph nodes. Then, DCs induce regulatory T cell (Treg) expansion in draining lymph nodes, after which the Tregs move to the skin allograft and produce IL-9, resulting in enhanced growth and recruitment of mast cells. Tregs and mast cells somehow suppress CD8+ T cell-mediated allograft rejection
Certain subsets of DCs, so-called tolerogenic DCs, are known to be crucial for induction of Tregs in draining lymph nodes, thereby contributing to allograft tolerance [15, 16]. Indeed, long-term skin allograft survival seen in wild-type mice tolerized by co-injection of allogenic cells and anti-CD154 blocking Abs was diminished in tolerized DC-depleted mice as well as mast cell-deficient Kit W-sh/W-sh mice [9, 16]. In the setting, mast cell-deficient Kit W-sh/W-sh mice showed impaired DC migration from the skin grafts to draining lymph nodes [16]. Mast cell-derived TNF and GM-CSF were, respectively, crucial for migration of DCs from skin grafts to draining lymph nodes and survival of DCs in draining lymph nodes [16] (Fig. 1). Taken together, mast cells control tolerogenic DCs, resulting in induction of Tregs in draining LNs during skin allograft tolerance.
Regulatory role of mast cells in graft-versus-host disease
Mast cells may contribute to the pathogenesis of acute graft-versus-host disease (GVHD) in humans [17]. Acute GVHD induced in irradiated DBA/2 mice (H-2d) transplanted with T cells and bone marrow cells from B10.D2 mice (H-2d) by mismatching minor histocompatibility antigens [18] was suppressed by treatment with peptide antagonists of binding of IgE to FcεR1 [19], suggesting that IgE/Ag-stimulated mast cells can enhance development of acute GVHD. In addition, the onset of acute GVHD in irradiated WBB6F1-Kit W/W-v mast cell-deficient mice was significantly delayed compared with in irradiated WBB6F1-Kit +/+ mice (H-2ja/b) after transplantation of CD8+ T cells and T cell-depleted bone marrow cells from C3H.SW mice (H-2b) [20]. These observations suggest that mast cells act as effector cells in the development of acute GVHD induced by mismatching minor histocompatibility antigens in mice. Conversely, the development of acute GVHD by mismatching MHC antigens was significantly exacerbated in C57BL/6J mast cell-deficient Kit W-sh/W-sh mice (H-2b) transplanted with T cell-depleted bone marrow cells from FVB/N mice (H-2q) compared with C57BL/6J wild-type mice transplanted with T cell-depleted bone marrow cells from FVB/N mice [21], suggesting that mast cells play an immunoregulatory role in this setting. The exacerbated acute GVHD seen in C57BL/6J mast cell-deficient Kit W-sh/W-sh recipient mice was independent of Tregs, because the number, frequency, and suppressive function of Tregs were normal in the liver, spleen, and lymph nodes of those mice during the acute GVHD [21]. The exacerbated acute GVHD recovered to the level seen in the wild-type recipient mice when wild-type, but not IL-10-deficient, mast cells were administered [21]. These observations suggest that mast cell-derived IL-10 is crucial for inhibition of acute GVHD due to mismatched MHC antigens, independently of Tregs in mice, although it remains unknown what triggers mast cells to produce IL-10 in the setting.
Regulatory role of mast cells in contact hypersensitivities
Delayed-type hypersensitivity (DTH), which is experimentally elicited in mice by immunization with exogenous antigens such as cells (i.e., sheep red blood cells [SRBC] and allogenic splenocytes), protein antigens (i.e., ovalbumin [OVA], methylated bovine serum albumin [mBSA], and keyhole limpet hemocyanin), and pathogens (Mycobacterium, Leishmania, and viruses), is considered to be a Th1 cell-mediated cellular immune response [22]. Mast cells were not essential for development of DTH induced by immunization of mice with methylated human serum albumin (mHSA) emulsified in complete Freund’s adjuvant (CFA) or SRBC emulsified in incomplete Freund’s adjuvant (IFA) or CFA [23]. On the other hand, mast cell-deficient Kit W/W-v mice and Kit Wf/Wf mice showed reduced DTH when immunized with mHSA emulsified in IFA [23], SRBC without adjuvant [24], or OVA emulsified in CFA [25]. Therefore, mast cells must function as effector cells in such models of DTH.
Classically, contact hypersensitivity (CHS), which is induced by epicutaneous exposure to haptens, was considered to be a form of DTH reaction. However, studies using gene-deficient mice showed that the molecular mechanism of development of CHS differs from that of DTH [26]. Mast cells are involved [24, 27–33] or not involved [34–39] in development of acute CHS (Table 1). This apparent discrepancy in the contribution of mast cells to DTH and acute CHS may have been due to different experimental protocols. Likewise, the role of mast cells in development of OVA-induced allergic airway inflammation differed between immunization protocols (i.e., in the presence or absence of adjuvant) [40, 41]. On the other hand, mast cells play a regulatory role in development of certain CHS models in mice. It is known that ultraviolet B (UVB) irradiation suppresses systemic immune responses including CHS [42]. After exposure to UVB, induction of acute CHS by 2,4,6-trinitrochlorobenzene (TNCB) was suppressed in Kit +/+ mice, but not in mast cell-deficient Kit W-f/W-f mice [43]. In this model, UVB-induced production of histamine by mast cells is considered to be important for UVB-induced immune suppression during acute CHS [43].
Chronic CHS is induced by repeated epicutaneous exposure to haptens. Development of chronic CHS induced by 2,4-dinitrofluorobenzene (DNFB) or urushiol was exacerbated in mast cell-deficient Kit W-sh/W-sh and Kit W/Kit W-v mice compared with Kit +/+ mice, suggesting that mast cells suppress the development of chronic CHS [44]. The exacerbated DNFB-induced chronic CHS was attenuated by intradermal engraftment of bone marrow cell-derived cultured mast cells from wild-type, but not IL-10-deficient, mice [44]. In this model, IL-10 production by IgG1/FcγR-mediated mast cells is crucial for suppression of chronic CHS [44] (Fig. 2). Likewise, development of chronic CHS induced by oxazolone was exacerbated in mast cell-deficient Kit W-sh/W-sh mice compared with Kit +/+ mice [45]. The exacerbated oxazolone-induced chronic CHS was similarly attenuated by engraftment of bone marrow cell-derived cultured mast cells from wild-type, but not IL-2-deficient, mice [45]. These results suggested that IL-2 production by mast cells in response to IgE/Ag in the spleen, but not local skin, enhances Treg expansion in the inflamed skin, but not in the spleen, following suppression of skin inflammation during chronic CHS by Tregs [45] (Fig. 2).
Regulatory role of mast cells in contact hypersensitivities. In chronic contact hypersensitivity (CHS) induced by DNFB, IgG/hapten-dependent mast cell-derived IL-10 suppresses skin inflammation independently of Tregs. In chronic CHS induced by oxazolone, mast cells produce IL-2 in response to IgE/hapten in the spleen, and IL-2 subsequently enhances expansion of Tregs in the skin, resulting in suppression of CHS
By contrast, mast cell-depleted mice (diphtheria toxin (DT)-injected Mcpt5-Cre+ iDTR+ mice or Mcpt5-Cre+ Rosa-DTA+ mice, in which connective tissue-type mast cells are depleted but mucosal-type mast cells are present) showed attenuated development of DNFB-induced chronic CHS as well as DNFB- and/or FITC-induced acute CHS [32], suggesting that mast cells are potent effector cells in induction of acute and chronic CHS. The reason for the discrepancy between mast cell-deficient Kit mutant mice and mast cell-depleted mice remains unclear, but it may be due to mast cell-independent Kit signaling in mast cell-deficient Kit mutant mice (Kit W-sh/W-sh mice, Kit W/Kit W-v mice, etc.) or some effect of mucosal-type mast cells in mast cell-depleted mice (DT-injected Mcpt5-Cre+ iDTR+ mice and Mcpt5-Cre+ Rosa-DTA+ mice). Also, since a commercial database (NextBio, Illumina Inc.) indicates that Mcpt5 mRNA is expressed in certain types of macrophages, B cells and NK cells, and mast cells, we can not rule out the possibility that depletion of such cells as well as mast cells also influences the phenotypes seen of DT-injected Mcpt5-Cre+ iDTR+ mice and Mcpt5-Cre+ Rosa-DTA+ mice.
Regulatory role of mast cells in innate-type allergic airway inflammation
It is thought that, in the sensitization process to allergens during allergic airway inflammation such as asthma, the allergens have to invade hosts beyond the epithelial cell barrier in the airway. House dust mites (HDMs) are considered as a major source of allergens in various allergic diseases such as atopic asthma, dermatitis, and rhinitis [46]. HDM-derived cysteine proteases such as Der p1 and Der f1 can disrupt the tight junctions between epithelial cells [47–49], allowing invasion of allergens into hosts. In addition to disrupting the tight junctions, such proteases also induce necrosis of epithelial cells, following induction of antigen-non-specific inflammation by damage-associated molecular patterns (DAMPs). Supporting this, inhalation of Der p1 and papain, which is a plant-derived cysteine protease and homologous to Der p1/Der f1 and human cathepsin B [50], in mice resulted in induction of airway inflammation in the absence of acquired immune systems [51, 52]. In the setting, papain damaged airway epithelial cells, after which epithelial cell-derived DAMP “IL-33,” which is a member of the IL-1 cytokine family and binds to IL-33R (a heterodimer of ST2 and IL-1R accessory protein), activated group 2 innate lymphoid cells (ILC2) to secrete IL-5 and IL-13 in the lung, leading to development of eosinophilic airway inflammation [51–53] (Fig. 3). IL-33 can activate both mast cells and basophils even in the absence of IgE/Ag-FcεRI cross-linking [54–56]. During the papain-induced innate-type airway inflammation, IL-33-dependent basophil-derived IL-4 was important for type 2 cytokine production by ILC2, indicating that basophils are potent effector cells [52] (Fig. 3). On the other hand, mast cell-deficient Kit W-sh/W-sh mice showed exacerbated development of papain-induced innate-type airway inflammation, suggesting that mast cells normally play a suppressive role [57]. In the setting, IL-33-dependent mast cell-derived IL-2 induced expansion of Tregs, after which Treg-derived IL-10 inhibited ILC2 proliferation and type 2 cytokine production, resulting in suppression of ILC2-mediated papain-induced innate-type airway inflammation [57] (Fig. 3).
Regulatory role of mast cells in innate-type allergic airway inflammation. After inhalation of protease antigens, epithelial cells release IL-33, followed by activation of group 2 innate lymphoid cells (ILC2) and basophils. IL-33-stimulated and/or IL-33-stimulated basophil-derived IL-4-dependent ILC2 produce type 2 cytokines such as IL-5 and IL-13, resulting in induction of airway eosinophilia in the absence of antigen-specific T cells and B cells. On the other hand, IL-33 stimulates mast cells to produce IL-2, after which IL-2 induces expansion of Tregs. Then, Treg-derived IL-10 suppresses ILC2-mediated airway eosinophilia
References
Voehringer D (2013) Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 13:362–375
Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR et al (2004) IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol 172:1139–1145
Abraham SN, St John AL (2010) Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10:440–452
Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ (2007) Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev 217:304–328
El-Refaie AM, Burt AD (2005) Mast cells and c-Kit expression in liver allograft rejection. Histopathology 47:375–381
Goto E, Honjo S, Yamashita H, Shomori K, Adachi H, Ito H (2002) Mast cells in human allografted kidney: correlation with interstitial fibrosis. Clin Transplant 16(Suppl 8):7–11
Jungraithmayr W (2015) The putative role of mast cells in lung transplantation. Am J Transplant 15:594–600
Itoh S, Nakae S, Velotta JB, Kosuge H, Connolly A, Tsai M et al (2010) The role of recipient mast cells in acute and chronic cardiac allograft rejection in C57BL/6-KitW-sh/W-sh mice. J Heart Lung Transplant 29:401–409
Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K et al (2006) Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442:997–1002
Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR et al (2001) IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol 166:3789–3796
Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA (2000) IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13:573–583
Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E et al (1990) Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9). Eur J Immunol 20:1413–1416
Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K (2003) IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol 170:3461–3467
Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN et al (2011) IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol 186:83–91
Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F et al (2006) Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7:652–662
de Vries VC, Pino-Lagos K, Nowak EC, Bennett KA, Oliva C, Noelle RJ (2011) Mast cells condition dendritic cells to mediate allograft tolerance. Immunity 35:550–561
Wu KN, Emmons RV, Lisanti MP, Farber JL, Witkiewicz AK (2009) Foxp3-expressing T regulatory cells and mast cells in acute graft-versus-host disease of the skin. Cell Cycle 8:3601–3605
Schroeder MA, DiPersio JF (2011) Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech 4:318–333
Korngold R, Jameson BA, McDonnell JM, Leighton C, Sutton BJ, Gould HJ et al (1997) Peptide analogs that inhibit IgE-Fc epsilon RI alpha interactions ameliorate the development of lethal graft-versus-host disease. Biol Blood Marrow Transplant 3:187–193
Murphy GF, Sueki H, Teuscher C, Whitaker D, Korngold R (1994) Role of mast cells in early epithelial target cell injury in experimental acute graft-versus-host disease. J Invest Dermatol 102:451–461
Leveson-Gower DB, Sega EI, Kalesnikoff J, Florek M, Pan Y, Pierini A et al (2013) Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood 122:3659–3665
Iwakura Y, Nakae S, Saijo S, Ishigame H (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226:57–79
Torii I, Morikawa S, Harada T, Kitamura Y (1993) Two distinct types of cellular mechanisms in the development of delayed hypersensitivity in mice: requirement of either mast cells or macrophages for elicitation of the response. Immunology 78:482–490
Askenase PW, Van Loveren H, Kraeuter-Kops S, Ron Y, Meade R, Theoharides TC et al (1983) Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol 131:2687–2694
Villa I, Skokos D, Tkaczyk C, Peronet R, David B, Huerre M et al (2001) Capacity of mouse mast cells to prime T cells and to induce specific antibody responses in vivo. Immunology 102:165–172
Kimber I, Dearman RJ (2002) Allergic contact dermatitis: the cellular effectors. Contact Dermatitis 46:1–5
van Loveren H, Meade R, Askenase PW (1983) An early component of delayed-type hypersensitivity mediated by T cells and mast cells. J Exp Med 157:1604–1617
Geba GP, Ptak W, Anderson GM, Paliwal V, Ratzlaff RE, Levin J et al (1996) Delayed-type hypersensitivity in mast cell-deficient mice: dependence on platelets for expression of contact sensitivity. J Immunol 157:557–565
Webb EF, Tzimas MN, Newsholme SJ, Griswold DE (1998) Intralesional cytokines in chronic oxazolone-induced contact sensitivity suggest roles for tumor necrosis factor alpha and interleukin-4. J Invest Dermatol 111:86–92
Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G et al (2000) Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med 192:1441–1452
Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC (2004) Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity 20:381–392
Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A et al (2011) Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34:973–984
Otsuka A, Kubo M, Honda T, Egawa G, Nakajima S, Tanizaki H et al (2011) Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS One 6, e25538
Thomas WR, Schrader JW (1983) Delayed hypersensitivity in mast-cell-deficient mice. J Immunol 130:2565–2567
Galli SJ, Hammel I (1984) Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science 226:710–713
Mekori YA, Galli SJ (1985) Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol 135:879–885
Mekori YA, Weitzman GL, Galli SJ (1985) Reevaluation of reserpine-induced suppression of contact sensitivity. Evidence that reserpine interferes with T lymphocyte function independently of an effect on mast cells. J Exp Med 162:1935–1953
Ha TY, Reed ND, Crowle PK (1986) Immune response potential of mast cell-deficient W/Wv mice. Int Arch Allergy Appl Immunol 80:85–94
Mekori YA, Chang JC, Wershil BK, Galli SJ (1987) Studies of the role of mast cells in contact sensitivity responses. Passive transfer of the reaction into mast cell-deficient mice locally reconstituted with cultured mast cells: effect of reserpine on transfer of the reaction with DNP-specific cloned T cells. Cell Immunol 109:39–52
Williams CM, Galli SJ (2000) Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med 192:455–462
Oboki K, Ohno T, Saito H, Nakae S (2008) Th17 and allergy. Allergol Int 57:121–134
Streilein JW, Taylor JR, Vincek V, Kurimoto I, Richardson J, Tie C et al (1994) Relationship between ultraviolet radiation-induced immunosuppression and carcinogenesis. J Invest Dermatol 103:107S–111S
Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ (1998) Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med 187:2045–2053
Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ (2007) Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol 8:1095–1104
Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A et al (2011) Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity 35:562–571
Gregory LG, Lloyd CM (2011) Orchestrating house dust mite-associated allergy in the lung. Trends Immunol 32:402–411
Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ et al (1995) Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol 12:369–378
Nakamura T, Hirasawa Y, Takai T, Mitsuishi K, Okuda M, Kato T et al (2006) Reduction of skin barrier function by proteolytic activity of a recombinant house dust mite major allergen Der f1. J Invest Dermatol 126:2719–2723
Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ et al (1999) Der p1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest 104:123–133
Chua KY, Stewart GA, Thomas WR, Simpson RJ, Dilworth RJ, Plozza TM et al (1988) Sequence analysis of cDNA coding for a major house dust mite allergen, Der p1. Homology with cysteine proteases. J Exp Med 167:175–182
Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A et al (2010) IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A 107:18581–18586
Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA et al (2014) Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 40:758–771
Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM et al (2014) Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40:425–435
Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M et al (2007) IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol 82:1481–1490
Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y et al (2007) IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest 87:971–978
Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A et al (2008) An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol 181:5981–5989
Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A et al (2015) An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity 43:175–186
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (B) (K.M.); a grant from Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency (S.N.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan; a Health Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan (K.M.); and a grant from Banyu Life Science Foundation International (H.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
This article is a contribution to the special issue on Basophils and Mast Cells in Immunity and Inflammation - Guest Editor: Hajime Karasuyama
Rights and permissions
About this article
Cite this article
Morita, H., Saito, H., Matsumoto, K. et al. Regulatory roles of mast cells in immune responses. Semin Immunopathol 38, 623–629 (2016). https://doi.org/10.1007/s00281-016-0566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-016-0566-0