Abstract
The recent cloning of the mammalian gene coding for N1-acetylpolyamine oxidase (PAO) provides the opportunity to directly examine the role of human PAO (hPAO) in polyamine homeostasis as well as its potential role in determining cellular response to antitumor polyamine analogues. To facilitate the study of this enzyme, the production, purification, and characterization of the recombinant hPAO is reported. hPAO oxidizes N1-acetylspermidine (Km=2.1 μM, Kcat=15.0 s−1) and has very high affinity for N1-acetylspermine (Km=0.85 μM, Kcat=31.7 s−1). The recombinant hPAO does not efficiently oxidize spermine, thereby demonstrating a significant difference in substrate specificity from the previously described human spermine oxidase PAOh1/SMO. Importantly, hPAO demonstrates the ability to oxidize a subset of antitumor polyamine analogues, suggesting that this oxidase activity could have a significant effect on determining tumor sensitivity to these or similar agents. Transfection of A549 human lung cancer cells with an hPAO-expressing plasmid leads to a profound decrease in sensitivity to those analogues which act as substrates, confirming its potential to alter drug response. One similarity that hPAO shares with human PAOh1/SMO, is that certain oligoamine analogues are potent inhibitors of its oxidase activity. The results of these studies demonstrate how changes in polyamine catabolism may affect drug response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
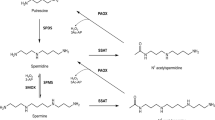
Although the polyamine metabolic pathway in general has been identified as a rational target for antineoplastic therapy [5, 16, 27, 33, 38, 45], considerable interest has recently arisen in the role that polyamine catabolism plays in determining the cellular response to antitumor polyamine analogues. The production of H2O2 and toxic aldehydes resulting from the catabolic oxidation of polyamines has been directly linked to the cytotoxicity observed in response to specific antitumor polyamine analogues [10, 21]. Consequently, interest in the potential of exploiting polyamine catabolism for therapeutic advantage has increased. Until recently, the oxidation of intracellular mammalian polyamines was thought to result solely from the activity of a peroxisomal N1-acetylpolyamine oxidase previously identified as PAO [22, 23, 34–36]. However, initial attempts to molecularly clone this PAO resulted in the discovery of an entirely new enzyme, spermine oxidase (PAOh1/SMO), which demonstrates high affinity for spermine [42, 46, 47]. Although it has been hypothesized that both oxidases have the potential to play a role in the cellular response of cancer cells to the antitumor polyamine analogues, only recently has it been possible to examine the substrate specificity of the individual enzymes for the various natural polyamines and their analogues [8, 43, 47, 50]. Therefore, the goal of the present study was to examine the properties and substrate specificity of the recombinant, classical human N1-acetylpolyamine oxidase, hPAO, and to examine the effects of its expression on cell sensitivity to antitumor polyamine analogues. The results of these studies not only suggest that this enzyme does possess very different substrate specificities for the natural polyamines as compared with PAOh1/SMO, but also demonstrate its ability to oxidize specific antitumor polyamine analogues and significantly alter the response of tumor cells to these agents.
Materials and methods
Chemicals and reagents
N1,N11-Bis(ethyl)norspermine (BENSpm) was provided by Parke-Davis (Ann Arbor, Mich.). N1-Ethyl-N11-(cyclopropyl)methyl-4,8-diazaundecane (CPENSpm), N1-ethyl-N11-(cycloheptyl)methyl-4,8-diazaundecane (CHENSpm), (S)-N1-(2-methy-1-butyl)-4,8-diazaundecane (IPENSpm), SL-11093, SL-11144, SL-11150, SL-11158, SL-11156, and the polyamine oxidase inhibitor N1,N4-bis(2,3-butadienyl)-1,4-butanediamine (MDL 72,527) were synthesized as previously described [3, 30, 31, 39, 40, 48] (Fig. 1). Spermine, spermidine, N1-acetylspermidine, N8-acetylspermidine, Sephadex G-100, and luminol were purchased from Sigma Chemical Co. (St. Louis, Mo.). N1-Acetylspermine was purchased from Fluka (Switzerland). Horseradish peroxidase was from Roche Molecular Biochemicals (Indianapolis, Ind.). Restriction and DNA-modifying enzymes were purchased from New England Biolabs (Beverly, Mass.), Invitrogen (Carlsbad, Calif.) and Sigma. Invitrogen custom oligomers were used for PCR as indicated.
Construction of the bacterial hPAO expression vector
Published sequences [43, 50] were used to clone human PAO cDNAs by RT-PCR using total cellular RNA prepared from HEK-293, NCI-A549, or NCI-H157 cells using Trizol RNA reagent according to the supplied protocol (Invitrogen). PAO cDNAs were amplified by nested PCR using two sets of primer pairs, 5′-CGAGAGCTCCAGACCTCCCGGCTA with 5′-GCATCTGGTGTCTCAGCTCAAGTC, and 5′-CAGAAGCCCTCGGACTGCCCGGAC with 5′-GGCCAGACTCCAATAACAGACACA. The resulting PCR products were then TA-cloned into the pCR2.1 vector (Invitrogen) according to the manufacturer’s supplied protocol.
To construct the bacterial expression vector pET15b/hPAO-1, the hPAO-1 cDNA was amplified by PCR from the respective pCR2.1 clone. Primers used for PCR contained NdeI (upstream, 5′-TCGGCGCCATATGGAG TCGACCGGCAGCGTCGG) or XhoI (downstream, 5′-ATTACTCGAGAACTGA GAAGGTGGCCCTGGTTA) restriction sites that were used to clone the hPAO-1 PCR product in-frame into the pET15b vector (Novagen, Madison, Wis.) in the same restriction sites.
Purification of the recombinant hPAO-1
The pET15b/hPAO-1 plasmid was transformed into the BL21(DE3) strain of Escherichia coli and selected on LB agar with 100 μg/ml ampicillin. The expression of hPAO-1 was induced in LB medium by 1 mM IPTG treatment for 4 h at 37°C. The cell lysate was prepared under denaturing conditions with 8 M urea, and hPAO-1 protein was isolated from the cell lysate by Ni-NTA resin, according to the supplied protocol. The resulting denatured protein was renatured by dialysis in buffer (250 mM NaCl, 50 mM Tris-HCl, 0.1 mM EDTA, 1 mM DTT, 0.2 μM FAD) with decreasing concentrations of urea as previously described [47]. The renatured hPAO-1 protein was further purified by gel filtration chromatography. Sephadex G-100 matrix was preswollen in dialysis buffer and packed into a 1×60-cm glass column. A 1-ml aliquot of the renatured protein sample was loaded onto the column and eluted with dialysis buffer (70 μl/min). Fractions (350 μl/tube) were assayed for enzyme activity and the protein was visualized by Coomassie staining after separation by 10% SDS-PAGE.
Determination of PAO enzyme activity
hPAO-1 activity was determined using a modification of the chemiluminescence analysis reported by Fernandez et al. and Rogers et al. as previously described for PAOh1/SMO activity measurements [18, 32, 47]. Briefly, enzyme activity was assayed in 83 mM glycine buffer, pH 8.0, 5 nmol luminol, 20 μg horseradish peroxidase, 0.2 mM 2-bromoethylamine (catalase inhibitor), 15 μM deprenyl (copper-containing amine oxidase inhibitor), 0.15 mM clorgyline (mitochondrial oxidase inhibitor), and the substrate as indicated. Where indicated, inhibitors were added at the specified concentrations 2 min prior to the addition of substrate.
The apparent initial rate constants for Km and Kcat of the enzyme preparations were estimated using Lineweaver-Burk transformation of the Michaelis-Menten kinetic equation at pH 8.0 in atmospheric oxygen with the indicated substrate. The pH of 8.0 was chosen since this represents the best approximation of pH in human peroxisomes measured to date [14, 15].
Stable expression of hPAO-1 in A549 cell line
A mammalian hPAO-1 expression vector was constructed by restricting pCR2.1/hPAO-1 with EcoRV and HindIII, followed by ligation of the resulting fragment into the pcDNA3.1(-) vector (Invitrogen) using the same restriction sites. For stable transfections, A549 human non-small-cell lung carcinoma cells were cultured in 100-mm plates to 70% confluence in RPMI 1640 medium with 10% calf serum. Lipofectamine-mediated (Invitrogen) transfection was performed with 10 μg pcDNA3.1/hPAO-1 or empty pcDNA3.1 vector (used as control) for 5 h. Transfected cells were selected 48 h after transfection with medium containing 400 μg/ml G-418 for 3 weeks and clones were screened for hPAO-1 activity.
Cell proliferation assay
The high hPAO-1 expressing clone, A18-5, or control vector-transfected A549 cells were seeded at 2000 cells/50 μl medium/well in 96-well plates. After 24 h, the polyamine analogues in a volume of 50 μl medium were added into each well at the indicated concentrations. After a 72-h exposure, 20 μl MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] solution from a One Solution Cell Proliferation Assay Kit (Promega, Madison, Wis.) was added and the absorbance at 490 nm was recorded after a 90-min incubation at 37°C.
Northern blotting assay
Total cellular RNA was extracted from cells with Trizol RNA reagent (Invitrogen) according the manufacturer’s protocol. RNA samples, 15 μg for each lane, were separated by 1.5% agarose/formaldehyde gel electrophoresis, transferred onto Zeta-Probe nylon membrane (Bio-Rad, Hercules, Calif.), and UV-crosslinked. The membrane was then hybridized to a random primer-labeled probe specific for the hPAO-1 cDNA, and reprobed with 18S ribosomal cDNA as a loading control.
Results
Activity of recombinant hPAO-1
It should be noted that in our cloning of the human PAO using RT-PCR methods similar to those used to discover PAOh1/SMO splice variants [28, 46], cDNAs corresponding to 12 splice variants of hPAO were identified (sequence and the cell lines from which they were cloned have been submitted to GenBank and assigned the accession numbers AY541513–AY541523) from multiple human tumor cell lines. However, due to the large number of splice variants, a single cDNA, hPAO-1, corresponding to the cDNAs reported by Wu et al. and Vujcic et al. [43, 50] and represented in all cell lines examined, was chosen for further study here.
High expression of hPAO-1 protein was achieved using pET15b/hPAO-1-transformed BL21 (DE3) E. coli cells after induction with 1 mM IPTG for 4 h at 37°C. The denatured protein was enriched by Ni-NTA column chromatography, followed by renaturation in decreasing concentrations of urea. However, Ni-NTA chromatography of the bacterial lysate consistently resulted in two bands on SDS-PAGE, one corresponding to the expected size for the recombinant protein (about 59 kDa) and a smaller band (about 35 kDa). Therefore, Sephadex column chromatography was used to further enrich the recombinant protein. Fractions 51–55 demonstrated high PAO activity and were pooled for further study (Fig. 2a). An aliquot corresponding to 0.5 μg of the pooled protein resolved as a single band of about 59 kDa by SDS-PAGE analysis (Fig. 2b).
Purification of recombinant hPAO-1. a Activity of fractions eluted from a Sephadex-G100 column. After Ni-NTA column chromatography and renaturation, protein was added to the Sephadex-100 column and eluted with dialysis buffer. Fractions were collected and assayed for activity and are presented as relative light units (RLU) using the standard assay system as described. Fractions 51–55 were pooled for further analysis. b Protein (0.5 μg) was separated by 10% SDS-PAGE and stained with Coomassie brilliant blue. Lane 1 purified protein after Sephadex-G100 chromatography, lane 2 markers with sizes as indicated
To determine the apparent initial Km and Kcat values for the hPAO-1 protein preparation, concentrations of N1-acetylspermine and N1-acetylspermidine ranging from 0.5 μM to 25 μM were used. The Km, Kcat, and Kcat/Km values of hPAO-1 were determined by to be 0.85 μM, 31.7 s−1, and 3.72×107 M−1 s−1, respectively, for N1-acetylspermine and 2.1 μM, 15.0 s−1, and 7.14×106 M−1 s−1 with N1-acetylspermidine as the substrate.
When the natural polyamines and their acetylated counterparts were examined for their ability to serve as substrates for the recombinant hPAO-1 (Fig. 3a) the order of preference was found to be: N1-acetylspermine>N1-acetylspermidine>N1,N12-diacetylspermine. However, under the same conditions N8-acetylspermidine, spermidine, and spermine were not found to be efficiently oxidized.
Substrate specificity of purified recombinant hPAO-1. Purified protein was incubated in the presence of 250 μM of the indicated substrate: (a) natural polyamines, or (b) polyamine analogues. The data are from a representative experiment performed in triplicate with error bars indicating the standard deviation
Oxidation of specific polyamine analogues by hPAO-1
There is evidence that some antitumor polyamine analogues are substrates for cellular oxidases, including PAO [26, 37, 43, 50]. Therefore, the ability of recombinant hPAO-1 to oxidize various antitumor polyamine analogues that are in, or are being considered for, clinical trials was examined. The symmetrically substituted analogue BENSpm was demonstrated by Wu et al. and Vujcic et al. [43, 50] to be oxidized by PAO. Here we examined the unsymmetrically substituted analogues CPENSpm, CHENSpm, and IPENSpm, the oligoamines, SL-11144, SL-11150, and SL-11158, the tetra-amine SL-11156 and its conformationally restricted analogue, SL-11093, as potential substrates for hPAO-1. Each of the analogues was incubated at a concentration of 250 μM with hPAO-1 (Fig. 4). Consistent with our previous findings suggesting that resistance to CHENSpm was mediated by oxidase activity [26], CHENSpm was found to be oxidized by the recombinant enzyme. Similarly, CPENSpm and IPENSpm also served as substrates. It is noteworthy that none of the oligoamine compounds or the aminobutyl-containing tetra-amines were found to be substrates for hPAO-1. HPLC analyses of the reaction products of recombinant hPAO-1 and each of the analogues indicate that in addition to H2O2, CPENSpm, CHENSpm, and IPENSpm are each catabolized by hPAO-1 resulting in at least two major amine-containing products (data not shown). For each compound, one product has a retention time similar to that of mono(ethyl)norspermidine [43], while the other has a retention time shorter than that of mono(ethyl)norspermidine, longer than that of spermidine, and unique to the specific analogue. Further analysis is required to confirm the identity of these products.
Expression of hPAO-1 in transfected A549 lung cancer cells. a Northern blot analysis of the A18-5 clone demonstrating increases expression of hPAO-1. Total cellular RNA (15 μg) was analyzed from vector control-transfected (lane 1) and hPAO-1-transfected (lane 2) cells. hPAO-1 mRNA was expressed as a single band of about 2.0 kb. b Cell lysates from the A18-5 or vector control clones were assayed for oxidase activity using both N1-acetylspermine (N1-ASPM) and spermine (SPM). The results are from a representative experiment performed in triplicate and are presented as a mean of triplicate determinations with the error bars indicating standard deviation
Overexpression of hPAO-1 decreases the sensitivity of human A549 non-small-cell lung cancer cells to specific polyamine analogues
To confirm that high PAO activity has pharmacodynamic relevance, human A549 lung cancer cells were transfected with an hPAO-1-expressing vector. Clone A18-5 was found to have significantly increased expression of hPAO-1 at both the mRNA and enzyme levels and was chosen for further study (Fig. 5). It is important to note that the increased expression of hPAO-1 did not alter the ability of the transfected A549 cells ability to oxidize spermine, thus suggesting that in situ hPAO-1 maintains its preference for N1-acetylated polyamines.
Expression of hPAO-1 in A549 cells decreases sensitivity to specific polyamine analogues. The hPAO-1-overexpressing A549 clone A18-5 was exposed to increasing concentrations of the indicated polyamine analogue for 72 h. Growth of treated cells was estimated by MTS assay. Each point represents three determinations with the error bars indicating standard deviation falling within the symbol. Note that overexpression of hPAO-1 does not alter the response of A549 cells to SL-11156
The A18-5 clone was then exposed to increasing concentrations of the three analogues that were oxidized by the recombinant hPAO-1 in vitro to determine if increased expression of hPAO-1 altered cellular sensitivity to the analogues (Fig. 6). The results of these experiments clearly indicated that the increased expression of hPAO-1 greatly decreased the sensitivity of A549 cells to CHENSpm, CPENSpm, and IPENSpm, each of which were demonstrated to be substrates for the enzyme. By contrast, the sensitivity of A549 to SL11156, an analogue that is not a substrate for the enzyme, was not altered by overexpression of hPAO-1.
Inhibition of hPAO-1 activity by polyamine analogues
MDL 72,527 was originally designed as a specific inhibitor of PAO, the enzyme that preferentially oxidizes acetylated polyamines as its substrates [3, 34]. This inhibitor, as expected, potently inhibited the hPAO-1 enzyme preparation with an IC50<0.1 μM (Fig. 6). Since oxidation of polyamines appears to be a mediator of analogue cytotoxicity with specific compounds, the determination of which analogues act as inhibitors of hPAO-1 may be instructive with regard to understanding the mechanism of action of the individual analogues. Therefore, 10 μM of each was examined for its ability to inhibit the oxidation of N1-acetylspermine (250 μM) (Fig. 7). Of the analogues examined, three oligoamine analogues, SL-11144, SL-11150, and SL-11158, were found to be inhibitors of the recombinant hPAO-1 enzyme, with SL-11150 and SL-11158 completely inhibiting hPAO-1 activity at the concentration of 10 μM tested. Although additional work is required to determine the mechanism of inhibition, these data demonstrate that polyamine analogues in addition to MDL 72,527 are capable of inhibiting hPAO-1, and this information should be useful in the design of future analogues.
Inhibition of hPAO-1 activity by polyamine analogues. Recombinant protein was incubated in the presence of 250 μM N1-acetylspermine and 10 μM of the indicated analogue. It should be noted that the analogues were added to the reaction mixture containing the recombinant protein 2 min prior to the addition of substrate. The data are from a representative experiment performed in triplicate with error bars indicating the standard deviation. Control indicates the activity (22 μmol/mg protein/min) of hPAO-1 using 250 μM N1-acetylspermine as a substrate with no inhibitor
Discussion
The recent cloning of the mammalian FAD-dependent peroxisomal PAO [43, 50] provides the opportunity to fully examine the role that polyamine catabolism plays both in polyamine homeostasis and in response to antitumor polyamine analogues. The previous cloning and characterization of the other two mammalian enzymes controlling polyamine catabolism, SSAT [6, 7, 11–13, 19, 20, 29, 44, 51] and PAOh1/SMO [9, 17, 28, 42, 46, 47], and the elucidation of their regulation will eventually allow full examination of the interplay among the catabolic enzymes.
The current study was undertaken to determine the activity and substrate specificity of recombinant human PAO. Using previously published sequences we were able to adapt the method of recombinant protein production and purification used previously to produce human PAOh1/SMO [46] to rapidly produce sufficient human PAO protein for analysis. The unanticipated finding that the human PAO gene codes for at least 12 splice variants provides fertile ground for further study in the regulation of the polyamine catabolic pathway. However, for practical purposes we limited our initial studies to the splice variant designated here as hPAO-1, that codes for a 511 amino acid protein identical to that reported by Vujcic et al. [43]. Our results demonstrate that hPAO-1 has the highest affinities for N1-acetylspermine (Km about 0.85 μM) and N1-acetylspermidine (Km about 2.1 μM). These apparent Km values, derived in atmospheric oxygen, are different than those reported by Wu et al. [50] for the mouse homologue, suggesting that the human recombinant protein has a higher affinity for both spermidine and spermine. However, it is important to note that the rate constants reported here are initial rate constants rather than the steady-state rate constants reported previously [50]. The only other natural polyamine derivative that was found to be readily oxidized using the recombinant human protein was N1,N12-diacetylspermine. However, the physiological relevance of the diacetyl substrate is questionable since it is not normally found in cells [41].
Since there is considerable interest in developing polyamine analogues as antineoplastic agents, it has been a concern as to whether such analogues would serve as substrates for amine oxidases, including the polyamine oxidases. The first-generation analogues, including BENSpm and BESpm, were synthesized for their capacity to block the oxidation of the primary amines through the addition of alkyl groups [1]. Although this strategy prevents significant oxidation by the copper-containing serum amine oxidase, previous studies indicated [2, 34] and recent results demonstrate that these analogues are, in fact, substrates for the acetylpolyamine oxidase [43, 50]. These results are in contrast to results with PAOh1/SMO, which did not oxidize any of the polyamine analogues examined [47]. The results presented here with the unsymmetrically substituted analogues CHENSpm, CPENSpm, and IPENSpm are consistent with our previous report suggesting that CHO cells are resistant to CHENSpm treatment as a result of oxidase activity [26]. Although resistance could be overcome by the addition of the oxidase inhibitor MDL 72,527 these results did not conclusively implicate PAO activity, since MDL 72,527 inhibits both PAO and PAOh1/SMO [43, 46].
In addition to confirming it is the acetylpolyamine oxidase, PAO, and not PAOh1/SMO, that is responsible for cellular resistance to unsymmetrically substituted analogues, the current findings also support the hypothesis that hPAO-1 represents a more promiscuous enzyme than PAOh1/SMO. The significant differences in substrate specificity between the human polyamine oxidases and other closely related polyamine oxidases, including maize PAO, are interesting since they all are approximately the same size and share considerable domain homology. The basis for substrate specificity awaits crystallization and structure solution to better define the active site of the mammalian polyamine oxidases, as has been accomplished for maize PAO [4]. It is important to note that the ability of hPAO-1 to oxidize antitumor polyamine analogues has physiological relevance, as clearly demonstrated by the decreased sensitivity to CHENSpm, CPENSpm, and IPENSpm in A549 cells overexpressing the oxidase.
Neither PAOh1/SMO [47] nor hPAO-1 appear capable of oxidizing the oligoamine or the tetra-amine analogues that are composed of aminobutyl repeats. This may be a result of the terminal aminobutyl moieties common to these compounds as opposed to the terminal aminopropyl moieties found in both the symmetrically and unsymmetrically substituted analogues. Although these oligoamines are not substrates for the human polyamine oxidases, three of the compounds tested here are inhibitors of both hPAO-1 and PAOh1/SMO [47] and still possess significant antitumor activity [25, 24], indicating that the production of H2O2 through polyamine catabolism may not be necessary for the antitumor activity of the oligoamines as has been implicated for other analogues [10, 21]. However, it should be noted that the mechanism by which the oligoamines inhibit the oxidases is not currently known.
In summary, this is the first reported study to examine the recombinant human acetylpolyamine oxidase protein, hPAO. The clear demonstration that specific analogues are substrates for the human PAO suggests one possibility as to why the results of clinical trials with the polyamine analogues have been less than desirable [49]. However, the identification of analogue substrates and inhibitors of this important polyamine catabolic enzyme and understanding the structural requirements surrounding these properties should be helpful in the further design of more effective agents targeting polyamine metabolism.
Abbreviations
- hPAO:
-
Human N1-acetylpolyamine oxidase
- PAOh1/SMO:
-
Human spermine oxidase
- SSAT:
-
Spermidine/spermine N1-acetyltransferase
- BENSpm:
-
N1,N11-bis(ethyl)norspermine
- CPENSpm:
-
N1-ethyl-N11-(cyclopropyl)methyl-4,8-diazaundecane
- CHENSpm:
-
N1-ethyl-N11-(cycloheptyl)methyl-4,8-diazaundecane
- IPENSpm:
-
(S)-N1-(2-methyl-1-butyl)-N11-ethyl-4,8-diazaundecane
- MDL 72,527:
-
N1,N4-bis(2,3-butadienyl)-1,4-butanediamine
References
Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JR, Bortell R, Ingeno MJ (1988) Synthetic polyamine analogues as antineoplastics. J Med Chem 31:1183
Bergeron RJ, Weimar WR, Luchetta G, Streiff RR, Wiegand J, Perrin J, Schreier KM, Porter C, Yao GW, Dimova H (1995) Metabolism and pharmacokinetics of N1,N11-diethylnorspermine. Drug Metab Dispos 23:1117
Bey P, Bolkenius FN, Seiler N, Casara P (1985) N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem 28:1
Binda C, Coda A, Angelini R, Federico R, Ascenzi P, Mattevi A (1999) A 30-angstrom-long U-shaped catalytic tunnel in the crystal structure of polyamine oxidase. Struct Fold Des 7:265
Casero RA, Woster PM (2001) Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem 44:1
Casero RA Jr, Celano P, Ervin SJ, Applegren NB, Wiest L, Pegg AE (1991) Isolation and characterization of a cDNA clone that codes for human spermidine/spermine N1-acetyltransferase. J Biol Chem 266:810
Casero RA Jr, Mank AR, Xiao L, Smith J, Bergeron RJ, Celano P (1992) Steady-state messenger RNA and activity correlates with sensitivity to N1,N12-bis(ethyl)spermine in human cell lines representing the major forms of lung cancer. Cancer Res 52:5359
Cervelli M, Polticelli F, Federico R, Mariottini P (2003) Heterologous expression and characterization of mouse spermine oxidase. J Biol Chem 278:5271
Cervelli M, Bellini A, Bianchi M, Marcocci L, Nocera S, Polticelli F, Federico R, Amendola R, Mariottini P (2004) Mouse spermine oxidase gene splice variants. Nuclear subcellular localization of a novel active isoform. Eur J Biochem 271:760
Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW (2001) Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res 61:6437
Coleman CS, Pegg AE (2001) Polyamine analogues inhibit the ubiquitination of spermidine/spermine N1-acetyltransferase and prevent its targeting to the proteasome for degradation. Biochem J 358:137
Coleman CS, Huang H, Pegg AE (1995) Role of the carboxyl terminal MATEE sequence of spermidine/spermine N1-acetyltransferase in the activity and stabilization by the polyamine analog N1,N12-bis(ethyl)spermine. Biochemistry 34:13423
Coleman CS, Huang H, Pegg AE (1996) Structure and critical residues at the active site of spermidine/spermine-N1-acetyltransferase. Biochem J 316:697
Dansen TB, Wirtz KW, Wanders RJ, Pap EH (2000) Peroxisomes in human fibroblasts have a basic pH. Nat Cell Biol 2:51
Dansen TB, Pap EHW, Wanders RJ, Wirtz KW (2001) Targeted fluorescent probes in peroxisome function. Histochem J 33:65
Davidson NE, Hahm HA, McCloskey DE, Woster PM, Casero RA Jr (1999) Clinical aspects of cell death in breast cancer: the polyamine pathway as a new target for treatment. Endocr Relat Cancer 6:69
Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA (2003) Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother Pharmacol 52:383
Fernandez C, Sharrard RM, Talbot M, Reed BD, Monks N (1995) Evaluation of the significance of polyamines and their oxidases in the aetiology of human cervical carcinoma. Br J Cancer 72:1194
Fogel-Petrovic M, Kramer DL, Ganis B, Casero RA Jr, Porter CW (1993) Cloning and sequence analysis of the gene and cDNA encoding mouse spermidine/spermine N1-acetyltransferase—a gene uniquely regulated by polyamines and their analogs. Biochim Biophys Acta 1216:255
Fogel-Petrovic M, Vujcic S, Miller J, Porter CW (1996) Differential post-transcriptional control of ornithine decarboxylase and spermidine-spermine N1-acetyltransferase by polyamines. FEBS Lett 391:89
Ha HC, Woster PM, Yager JD, Casero RA Jr (1997) The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci U S A 94:11557
Holtta E (1977) Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry 16:91
Holtta E (1983) Polyamine oxidase (rat liver). Methods Enzymol 94:306
Huang Y, Hager ER, Phillips DL, Hacker A, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Davidson NE (2002) Conformationally constrained polyamine analogues and oligoamines inhibit growth and induce apoptosis in human breast cancer cells. Proc Am Assoc Cancer Res 43:90
Huang Y, Hager ER, Phillips DL, Dunn VR, Hacker A, Frydman B, Kink JA, Valasinas AL, Reddy VK, Marton LJ, Casero RA Jr, Davidson NE (2003) A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin Cancer Res 9:2769
Lawson KR, Marek S, Linehan JA, Woster PM, Casero RA Jr, Payne CM, Gerner EW (2002) Detoxification of the polyamine analogue N1-ethyl-N11-[(cycloheptyl)methy]-4,8-diazaundecane (CHENSpm) by polyamine oxidase. Clin Cancer Res 8:1241
Marton LJ, Pegg AE (1995) Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 35:55
Murray-Stewart T, Wang Y, Devereux W, Casero RA Jr (2002) Cloning and characterization of multiple human polyamine oxidase splice variants that code for isoenzymes with different biochemical characteristics. Biochem J 368:673
Pegg AE, Stanley BA, Wiest L, Casero RA Jr (1992) Nucleotide sequence of hamster spermidine/spermine-N1-acetyltransferase cDNA. Biochim Biophys Acta 1171:106
Reddy VK, Valasinas A, Sarkar A, Basu HS, Marton LJ, Frydman B (1998) Conformationally restricted analogues of N1,N12-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J Med Chem 41:4723
Reddy VK, Sarkar A, Valasinas A, Marton LJ, Basu HS, Frydman B (2001) cis-Unsaturated analogues of 3,8,13,18,23-pentaazapentacosane (BE-4-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cell lines. J Med Chem 44:404
Rogers MS, Yim SF, Li KC, Wang CC, Arumanayagam M (2002) Cervical intraepithelial neoplasia is associated with increased polyamine oxidase and diamine oxidase concentrations in cervical mucus. Gynecol Oncol 84:383
Schipper RG, Deli G, Deloyer P, Lange WP, Schalken JA, Verhofstad AA (2000) Antitumor activity of the polyamine analog N1,N11-diethylnorspermine against human prostate carcinoma cells. Prostate 44:313
Seiler N (1995) Polyamine oxidase, properties and functions. Prog Brain Res 106:333
Seiler N, Bolkenius FN, Knodgen B, Mamont P (1980) Polyamine oxidase in rat tissues. Biochim Biophys Acta 615:480
Seiler N, Bolkenius FN, Rennert OM (1981) Interconversion, catabolism and elimination of the polyamines. Med Biol 59:334
Seiler N, Duranton B, Vincent F, Gosse F, Renault J, Raul F (2000) Inhibition of polyamine oxidase enhances the cytotoxicity of polyamine oxidase substrates. A model study with N(1)-(n-octanesulfonyl)spermine and human colon cancer cells. Int J Biochem Cell Biol 32:703
Thomas T, Thomas TJ (2001) Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci 58:244
Valasinas A, Sarkar A, Reddy VK, Marton LJ, Basu HS, Frydman B (2001) Conformationally restricted analogues of N1,N14-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J Med Chem 44:390
Valasinas A, Reddy VK, Blokhin AV, Basu HS, Bhattacharya S, Sarkar A, Marton LJ, Frydman B (2003) Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg Med Chem 11:4121
Vujcic S, Halmekyto M, Diegelman P, Gan G, Kramer DL, Janne J, Porter CW (2000) Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J Biol Chem 275:38319
Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW (2002) Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J 367:665
Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW (2003) Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J 370:19
Wallace HM, Nuttall ME, Coleman CS (1988) Polyamine recycling enzymes in human cancer cells. Adv Exp Med Biol 250:331
Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376:1
Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA Jr (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 61:5370
Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA (2003) Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun 304:605
Webb HK, Wu Z, Sirisoma N, Ha HC, Casero RA Jr, Woster PM (1999) 1-(N-alkylamino)-11-(N-ethylamino)-4,8-diazaundecanes: simple synthetic polyamine analogues that differentially alter tubulin polymerization. J Med Chem 42:1415
Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, Casero RA Jr, Davidson NE (2003) A phase II study of the polyamine analog N1, N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin Cancer Res 9:5922
Wu T, Yankovskaya V, McIntire WS (2003) Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, n1-acetylated polyamine oxidase. J Biol Chem 278:20514
Xiao L, Casero RA Jr (1996) Differential transcription of the human spermidine/spermine N1-acetyltransferase (SSAT) gene in human lung carcinoma cells. Biochem J 313:691
Acknowledgements
This research was supported by National Institutes of Health grants CA51085, CA85509, CA88843, and CA98454.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Ben Frydman who died on 25 September 2004.
Rights and permissions
About this article
Cite this article
Wang, Y., Hacker, A., Murray-Stewart, T. et al. Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother Pharmacol 56, 83–90 (2005). https://doi.org/10.1007/s00280-004-0936-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0936-5