Abstract
Intracellular mammalian polyamine catabolism occurs through two distinct pathways, both of which culminate in oxidation reactions that generate highly reactive, potentially toxic by-products. In the back-conversion to spermidine, spermine can either undergo direct oxidation by spermine oxidase (SMOX) or be acetylated by spermidine/spermine N 1-acetyltransferase (SSAT), followed by subsequent oxidation by acetylpolyamine oxidase (APAO). Spermidine undergoes acetylation and oxidation back to putrescine through this same SSAT/APAO pathway. Polyamines are absolutely essential for cell viability and proliferation, and polyamine biosynthesis and intracellular concentrations are frequently upregulated in hyperproliferative conditions such as cancer. As a result, many studies have successfully focused on the induction of polyamine catabolism as a rational target for antiproliferative chemotherapeutic intervention. However, it is also becoming apparent that chronically elevated levels of polyamine catabolism in nontumorigenic cells can have disease implications. A variety of stimuli, including microbial pathogens, inflammatory signals, and tissue injury, have now been identified to induce the polyamine catabolic enzymes. In addition to the back-conversion of polyamines, these reactions also release the reactive oxygen species precursor hydrogen peroxide as well as potentially toxic aldehydes. These metabolites as well as the reduction in spermine and spermidine levels can have deleterious physiological effects resulting in the manifestation and promotion of multiple pathologies. This chapter focuses on recent discoveries in the regulation of the mammalian polyamine catabolic enzymes and the pathophysiological effects of this upregulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aldehyde
- Epigenetic
- Inflammation
- Reactive oxygen species
- Spermidine/spermine N 1-acetyltransferase
- Spermine oxidase
1 Introduction
Putrescine, spermidine, and spermine constitute the naturally occurring mammalian polyamines. As in all cells, the mammalian polyamines are absolutely essential for viability through their contributions to critical cellular functions, including nucleic acid and protein synthesis, transcriptional and translational regulation, and macromolecular structural integrity (Pegg 1988, 2009; Saini et al. 2009; Park et al. 2010). Spermine, in particular, has also been shown to provide significant protection against oxidative damage (Ha et al. 1998a, b; Rider et al. 2007). For these functional interactions, polyamine homeostasis must be tightly regulated: an excess of intracellular polyamines becomes rapidly toxic (Tabor and Rosenthal 1956), whereas highly upregulated polyamine catabolism reduces the natural intracellular polyamines and generates toxic by-products (Wang and Casero 2006).
2 The Mammalian Polyamine Catabolic Enzymes and Their Metabolites
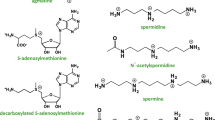
In mammalian cells, the catabolism of spermine to spermidine occurs via one of two distinct pathways. As a substrate for spermidine/spermine N 1-acetyltransferase (SSAT), spermine can be converted to N 1-acetylspermine, which is subsequently oxidized by the FAD-dependent acetylpolyamine oxidase (APAO) to form spermidine. Conversely, spermine can be directly oxidized by spermine oxidase (SMOX) to form spermidine. Spermidine is then back-converted to putrescine through the two-step SSAT/APAO reaction that includes an N 1-acetylspermidine intermediate (Fig. 5.1).
The mammalian polyamine catabolic pathway. Spermine (Spm) is back-converted to spermidine (Spd) by either spermine oxidase (SMOX) or spermidine/spermine N 1-acetyltransferase (SSAT) followed by acetylpolyamine oxidase (APAO). Spermidine is further back-converted to putrescine (Put) through the same SSAT/APAO mechanism. Both oxidation reactions generate the reactive oxygen species (ROS) precursor H2O2 and aldehydes as by-products. The resulting reduction in spermine and spermidine pools implies diminished antioxidant and antiinflammatory functions
2.1 Spermidine/Spermine N 1-Acetyltransferase and N1-Acetylpolyamine Oxidase
SSAT is the rate-limiting enzyme of the polyamine catabolic pathway that catalyzes the transfer of an acetyl group from acetyl coenzyme A to the N 1 position of spermine or spermidine (Casero and Pegg 1993; Pegg 2008; Matsui et al. 1981). The resulting molecule has a reduced positive charge that alters its binding affinity for cellular macromolecules and facilitates its export from the cell. Additionally, N1-acetylated spermine or spermidine can be oxidized by APAO, resulting in spermidine or putrescine, respectively, hydrogen peroxide (H2O2), and 3-acetamidopropanal (Holtta 1977; Wu et al. 2003; Wang et al. 2005b). H2O2 is a potential reactive oxygen species (ROS); however, in most cases, the peroxisomal localization of the APAO enzyme appears to protect the cell from its oxidative effects.
2.2 Spermine Oxidase
The SMOX gene encoding spermine oxidase is alternatively spliced, and multiple isoforms have been characterized in both human and mouse (Wang et al. 2001; Murray-Stewart et al. 2002, 2008; Cervelli et al. 2003). The catalytically active SMOX isoforms are FAD-dependent enzymes that directly oxidize spermine to yield spermidine, H2O2, and the aldehyde 3-aminopropanal. Importantly, these isoforms are found in significant amounts in both the cytoplasm and nucleus, resulting in the production of H2O2 as an ROS precursor in close proximity to DNA and chromatin while catalyzing the oxidation of the free-radical scavenger spermine (Murray-Stewart et al. 2008; Cervelli et al. 2004; Bianchi et al. 2005). SMOX activity therefore has the potential to significantly contribute to cellular oxidative damage and subsequent disease development. Additionally, the 3-aminopropanal produced through SMOX activity can be further metabolized to form acrolein, which also has toxic cellular effects and physiological implications.
3 Induction of Polyamine Catabolism
Regulation of SSAT occurs at nearly every level from transcription through protein stabilization, resulting in an enzyme that is highly inducible (Casero and Pegg 1993; Pegg 2008). Elevated levels of the natural polyamines themselves stimulate SSAT induction, and polyamine analogues have been extensively studied for their ability to highly induce SSAT activity in tumor cells as a chemotherapeutic strategy (Nowotarski et al. 2013; Casero and Marton 2007). Other stimuli for SSAT induction include certain proinflammatory cytokines, nonsteroidal antiinflammatory drugs (NSAIDs), hormones, stress, and common cytotoxic drugs such as cisplatin (Casero and Pegg 2009). As the acetylated polyamine substrates for oxidation by APAO are produced only through SSAT activity, APAO is in general constitutively expressed and rate limited by SSAT.
In contrast to APAO, oxidation of spermine via SMOX is inducible; however, most of its regulation appears to be at the level of transcription (Wang et al. 2005a). Similar to SSAT, SMOX can be induced by polyamine analogues and certain pro-inflammatory cytokines, leading to oxidative DNA damage. SMOX is also induced upon microbial pathogen infection, in states of chronic inflammation, and following tissue injury (Casero and Pegg 2009). Recent advances in the regulation and induction of the polyamine catabolic enzymes and their pathophysiological implications are discussed in the text that follows.
3.1 Epigenetic Regulation of Mammalian Polyamine Catabolism
Epigenetics refers to heritable alterations in gene expression that are not the result of changes in nucleotide sequence. Mechanisms that induce these changes include posttranslational modifications of histone proteins, such as acetylation, methylation, and phosphorylation, and the methylation of CpG dinucleotides. Few studies have focused on the direct epigenetic regulation of the polyamine catabolic enzymes, although the promoter regions of both SAT1 and SMOX genes contain identifiable CpG islands, suggesting the potential for transcriptional regulation through DNA methylation. The influence of methylation on SSAT expression was initially identified in lung cancer cell lines derived from female patients. The SAT1 gene is located on the X chromosome; therefore, the female cell lines expressed variable levels of basal SSAT expression and responsiveness to polyamine analogues that correlated with the expression of one or both alleles, and this expression was regulated by DNA methylation (Mank-Seymour et al. 1998). Recent studies in the prefrontal cortex have also correlated SAT1 promoter DNA CpG hypermethylation with decreased SSAT mRNA expression (Fiori and Turecki 2011). As DNA methylation is often increased in cancer, the downregulation of polyamine catabolic enzymes via DNA hypermethylation could provide a mechanism for maintaining the high levels of polyamines required for tumor proliferation.
SSAT expression is also indirectly regulated through epigenetic mechanisms. The SAT1 gene promoter contains a polyamine-responsive element (PRE) that enables transcriptional activation via the binding of NRF2 and polyamine-modulating factor 1 (PMF1) (Wang et al. 1998, 1999). In human non-small cell lung cancer cell lines that respond to polyamine analogue treatment with a large induction of SSAT, NRF2 is constitutively bound at the PRE by alterations of the KEAP1 protein, which normally sequesters it in the cytoplasm (Singh et al. 2006; Itoh et al. 1999). Recent studies have shown that NRF2-dependent transcriptional regulation of SSAT is influenced by the histone acetylation status of a specific microRNA, miR-200a, which targets the 3′-UTR of the KEAP1 mRNA. Treatment of polyamine analogue-resistant small cell lung cancer cells with a histone deacetylase (HDAC) inhibitor increased expression of miR-200a, which subsequently downregulated KEAP1 mRNA and protein and allowed the translocation of NRF2 to the nucleus. NRF2 occupancy was enriched at the PRE of the SSAT promoter, resulting in the sensitization of these phenotypically resistant cells to the antiproliferative effects of SSAT upregulation by the bis(ethyl)polyamine analogues (Murray-Stewart et al. 2013). These data suggest the use of HDAC inhibitors in combination with SSAT-inducing polyamine analogues as an effective chemotherapeutic strategy in patients harboring clinically aggressive small-cell lung cancers.
Last, PMF1, which responds to increased polyamines and their analogues through NRF2-dependent transcriptional activation of SAT1, is frequently hypermethylated and silenced in human bladder tumors (Aleman et al. 2008). The degree of PMF1 methylation and the corresponding loss of PMF1 expression is significantly correlated with increased tumor stage and grade and is prognostic of poor overall survival. Furthermore, PMF1 hypermethylation is detectable in urinary specimens and can accurately distinguish bladder cancer patients from controls; a subsequent study indicated PMF1 methylation status as a predictor for patient response to a common bladder tumor therapy (Alvarez-Mugica et al. 2013). These data indicate a role for PMF1-mediated induction of SSAT in blocking the progression of bladder cancer, perhaps by maintaining intracellular polyamine levels below those necessary for tumor proliferation. Unfortunately, the downstream effects of PMF1 silencing on polyamine catabolism have not been reported.
3.2 Regulation by Infectious/Inflammatory Agents
The upregulation of polyamine catabolism in response to microbial infection and inflammatory stimulus is becoming a common theme. Several bacterial pathogens have now been identified to induce SMOX expression in host cells, the ultimate outcome of which is oxidative DNA damage, apoptosis, and an increased potential for neoplastic transformation. These pathogens include Helicobacter pylori and the enterotoxigenic Bacteroides fragilis, both of which are described in greater detail in the sections that follow (Xu et al. 2004; Goodwin et al. 2011). Additionally, a constituent of gram-negative bacterial cell walls, endotoxin (LPS), induces the transcription of both SSAT and SMOX in the kidney (Zahedi et al. 2010), and expression of an HIV1-encoded protein induces SMOX in neuronal cells (Capone et al. 2013).
Polyamine catabolism contributes to inflammation through the production of ROS; however, it is also regulated as a result of inflammatory signals. Both SMOX and SSAT are activated by the inflammatory cytokine tumor necrosis factor (TNF)-α; SMOX can be similarly induced by interleukin-6 (Babbar and Casero 2006; Babbar et al. 2006b, 2007). Furthermore, oxidative stress itself, in the form of H2O2, has been shown to induce SAT1 transcription and alter intracellular polyamine concentrations (Chopra and Wallace 1998; Smirnova et al. 2012). Additionally, the alcohol metabolite acetaldehyde was recently shown to induce spermine oxidation (Uemura et al. 2013), ischemia–reperfusion injury induces both SSAT and SMOX catabolic pathways (Zahedi et al. 2009; Zahedi and Soleimani 2011), and exposure to carbon tetrachloride induces SSAT in hepatocytes (Zahedi et al. 2012). Each of these stimuli and their disease implications are discussed in the following sections.
4 Physiological Consequences of Increased Polyamine Catabolism
The higher polyamines, spermine in particular, play important physiological roles in protection from oxidative stress. Enhanced polyamine catabolism reduces this protection while concomitantly generating ROS and toxic aldehyde by-products. As a result, increased polyamine catabolism, resulting from the stimuli just mentioned, has been implicated in several pathophysiological conditions, including neurological and liver disease, stroke, kidney failure, and cancer.
4.1 ROS Generation and Oxidative DNA Damage
4.1.1 Inflammation-Associated Hyperproliferative Conditions
Approximately 20 % of all human cancers can be causally linked to chronic inflammation, particularly through infection with human pathogens (Zur Hausen 2009). Representatives of these pathogens are also inducers of SMOX, and elevated levels of SMOX have been observed in several inflammation-associated human conditions that are risk factors for the development of epithelial cancers. These observations suggest a role for spermine oxidation in the initiation of tumorigenesis. In addition to generating oxidative stress capable of DNA damage in the epithelial cell, several systems have now also demonstrated inductions of the polyamine oxidases in infiltrating inflammatory cells as a potential means for immune response evasion.
An active area of investigation regarding infection and inflammation focuses on Helicobacter pylori, a gram-negative bacterium that infects the stomach mucosa and causes inflammation in the form of chronic gastritis and peptic ulcers (Hardbower et al. 2013). Although eliciting acute and chronic immune and inflammatory responses, H. pylori evades the antimicrobial mechanisms of the immune response and often persists for the life of the host (Gobert et al. 2001; Bussiere et al. 2005). Approximately 50 % of the world’s population is infected with H. pylori, which is considered a class I carcinogen and is believed to be the causal agent of 95 % of gastric cancers (Malfertheiner et al. 2005).
Gastric epithelial cells respond to infection with H. pylori through an induction of SMOX mRNA and activity. The generation of H2O2 that results from SMOX induction by H. pylori has been causally linked to DNA damage and apoptosis in the gastric mucosae of humans and mice (Xu et al. 2004). Importantly, a subpopulation of gastric epithelial cells in which H. pylori infection has induced SMOX activity and high amounts of DNA damage remains resistant to apoptosis, therefore increasing the likelihood that these cells will undergo malignant transformation (Chaturvedi et al. 2011). SMOX-mediated H2O2 production in response to H. pylori is also induced in the host macrophages responding to the infection, resulting in macrophage apoptosis and contributing to immune evasion and bacterial persistence (Chaturvedi et al. 2004, 2013). Recent studies with affected patient samples have further implicated the critical role played by SMOX in the etiology of H. pylori-induced gastric cancer (Chaturvedi et al. 2014b, 2014c).
Chronic inflammation and the associated oxidative damage is also a risk factor for the development of colorectal neoplasia. Induction of SMOX has been demonstrated in a mouse model of infection with the enterotoxigenic bacterium B. fragilis (ETBF), which results in ulcerative colitis, acute diarrheal disease, inflammatory bowel disease, and ultimately causes colon cancer. This SMOX induction has been implicated as the source of ROS-induced DNA damage in ETBF-infected colonic epithelium, and treatment with the SMOX inhibitor MDL72, 527 decreased ETBF-induced DNA damage, colonic inflammation, proliferation, and tumorigenesis (Goodwin et al. 2011). In patient samples of ulcerative colitis, SMOX protein expression in infiltrating mononuclear cells was found to correlate with the severity of inflammatory disease scoring, consistent with a role for SMOX in colitis pathogenesis (Hong et al. 2010).
Chronic inflammation in the form of prostatitis is believed to contribute to the development of prostate cancer. In prostate tissue samples from patients with prostate disease spanning the spectrum from inflammation to prostate adenocarcinoma, SMOX protein expression was increased in comparison with individuals without disease (Goodwin et al. 2008). When examining patient-matched samples, SMOX expression was increased in prostatic intraepithelial neoplasia (PIN) and prostate cancer samples relative to benign prostatic epithelium from the same individual, with the greatest increase observed in PIN lesions, which are recognized as precursors to prostate carcinoma. Consistent with the inflammation-associated conditions already described, these data suggest a role for SMOX in precursor lesion development and carcinogenic initiation events. Furthermore, patients who developed prostate disease demonstrated significantly higher SMOX expression even in the nondiseased areas of prostatic epithelium when compared to those without disease, suggesting increased SMOX expression is an important component early in the disease process.
Pneumocystis infection is also associated with upregulated polyamine catabolism. Pneumocystis opportunistically infects the lungs of immunocompromised patients and results in a decrease in alveolar macrophages. This apoptosis appears to be the result of H2O2 production by the macrophages through increased APAO activity subsequent to the induction of polyamine biosynthesis (Liao et al. 2009).
4.1.2 Ischemia–Reperfusion and Toxin-Induced Injury
Renal ischemia–reperfusion has been shown to induce polyamine catabolism through SSAT and SMOX in both the kidney and liver, resulting in oxidative stress and apoptosis that do not occur in SSAT-deficient animals (Zahedi et al. 2009; Zahedi and Soleimani 2011). Furthermore, endotoxin, or LPS, is a major cause of sepsis-related acute kidney injury, and injection of mice with LPS resulted in inductions of renal SSAT and SMOX. Pharmacological inhibition of polyamine oxidation or ablation of SSAT decreased renal cell damage, implicating the catalysis of polyamines in the mediation of endotoxin-induced acute kidney injury (Zahedi et al. 2010). In similar studies, it was demonstrated that exposing mice to carbon tetrachloride resulted in a large induction of hepatocyte SSAT activity that was associated with liver damage, and this damage was prevented by pharmacological inhibition of polyamine oxidation or SSAT ablation (Zahedi et al. 2012).
4.1.3 HIV-Related Dementia
Chronic oxidative stress occurs in brain tissues of HIV-infected patients and is associated with the development of human immunodeficiency virus (HIV)-associated dementia. Recently, the HIV-1 Tat gene was shown to induce SMOX activity, resulting in ROS generation and a reduction in intracellular spermine content in neuroblastoma cells. These studies provided evidence that SMOX-derived H2O2 induces oxidative stress that plays a role in neuronal cell death and the etiology of dementia associated with HIV infection (Capone et al. 2013).
4.2 Acrolein Generation
In addition to generating ROS, polyamine oxidation results in toxic aldehydes that have been implicated in neurological conditions and diseases (Pegg 2013). The aldehyde product of SMOX activity, 3-amidopropanal, can be spontaneously metabolized to acrolein, a major toxicity factor that is being actively investigated as a factor in several pathologies.
In this regard, multiple studies have implicated a role for polyamine oxidation-associated acrolein production in neuronal damage. APAO and SMOX proteins and acrolein adducts can be measured in the plasma of patients and hold potential as biomarkers for the detection of stroke (Igarashi and Kashiwagi 2011a, b; Tomitori et al. 2005). Acrolein conjugates, when combined with amyloid-β ratios, also provide an accurate indication for Alzheimer’s disease, even in patients with only mild cognitive impairment (Waragai et al. 2012).
Increases in free and protein-conjugated acrolein have also been detected in various renal pathologies, including renal failure, and the metabolism of alcohol in the liver has been shown to induce spermine oxidation resulting in increased detection of acrolein (Sakata et al. 2003a, b; Uemura et al. 2013). This increase in hepatocellular SMOX suggests a mechanism for the liver toxicity and decreased regenerative abilities associated with chronic alcohol intake.
4.3 Reduction of Intracellular Polyamine Pools
The studies described here focus on the cellular effects of toxic by-products generated through elevated polyamine catabolism; however, the resulting reduction in intracellular polyamine concentrations must also be considered, as their depletion can significantly exacerbate the toxic outcome. Spermine and spermidine function to protect DNA from oxidative damage, and spermine has been shown to act directly as a free-radical scavenger (Ha et al. 1998a, b; Khan et al. 1992a, b; Rider et al. 2007; Nilsson et al. 2000). Therefore, elevated polyamine catabolism, particularly through nuclear SMOX induction, not only generates ROS but also functionally reduces the antioxidant levels of the cell.
Similarly, polyamines have been recognized as mediators of immune function by negatively regulating the production of certain inflammatory cytokines, including TNF-α and interleukin (IL)-1β, and nitric oxide (Zhang et al. 2000; Perez-Cano et al. 2003; Paul and Kang 2013) Therefore, the transcriptional activation of SSAT and SMOX by pro-inflammatory cytokines, such as TNF-α, decreases the abundance of spermine and spermidine that would normally repress further production of TNF-α, thus having the potential to exacerbate the chronic inflammatory response.
5 Polyamine Catabolism as a Therapeutic Target
5.1 Chemotherapeutic Strategies
In the cancer setting, much research has focused on inducing polyamine catabolism in established tumors with the goal of reducing the natural polyamines required for tumor proliferation while selectively inducing tumor cell apoptosis through the generation of ROS (Nowotarski et al. 2013; Battaglia et al. 2013). Several classes of polyamine analogues have now been characterized to accomplish this goal in vitro; however, their clinical utility as single agents has been limited. Combining these analogues with other agents targeting the tumor cell has become a promising chemotherapeutic option. For example, cotreatment of small cell lung tumor cells with a bis(ethyl) polyamine analogue and the HDAC inhibitor MS-275 produced a synergistic induction of SSAT activity that was associated with growth inhibition (Murray-Stewart et al. 2013). Furthermore, these analogues have been observed to work in concert with other common cytotoxic agents, including 5-fluorouracil and platinum-based compounds, to induce polyamine catabolism (Hector et al. 2004, 2008; Pledgie-Tracy et al. 2010).
5.2 Chemopreventive Strategies
SSAT induction is observed in response to treatment with NSAIDs, and this induction contributes to the antiproliferative activity of NSAIDs in regard to the development of colorectal carcinoma (Babbar et al. 2003, 2006a). Recently, a phase III clinical trial examined the chemopreventive potential of combining treatment with a specific NSAID (sulindac) with an inhibitor of polyamine biosynthesis (difluoromethylornithine) (Meyskens et al. 2008). Patients in this study who received the combination therapy demonstrated dramatic reductions in the occurrence of colon polyps and adenomas, verifying the utility of targeting polyamine metabolism in conjunction with inflammation.
In light of the growing evidence supporting a role for polyamine oxidation in the etiologies of several human conditions and diseases, including cancer initiation, attention must also be given to attenuating this response as a means for chemoprevention. As SMOX activity has been demonstrated in the cell nucleus, its inhibition in the presence of cancer-predisposing, SMOX-inducing factors such as H. pylori infection appears to provide protection from the oxidative DNA damage that leads to carcinogenesis. It is likely that this inhibition of polyamine catabolism prevents the generation of ROS while maintaining the intracellular spermine pools capable of free-radical scavenging. Although effective inhibitors for SMOX exist, including MDL72527, they are nonspecific and also inhibit APAO. The identification of specific inhibitors of the polyamine oxidases will provide both experimental and therapeutic benefit.
6 Summary
In conclusion, although consisting of only three enzymes, intracellular mammalian polyamine catabolism is a dynamic process that is capable of responding to a multitude of environmental stimuli. Depending on the context, these interactions can have positive or negative cellular effects: polyamine catabolism can protect cells from the toxic effects of excessive polyamine accumulation and limit uncontrolled proliferation in instances of upregulated polyamine biosynthesis. Yet, excessive polyamine catabolism can cause oxidative stress and toxin generation with the potential to increase carcinogenic events, limit the regeneration or viability of essential cells, or diminish the innate immune response to infection. Thus, both targeted increases in polyamine catabolism and targeted inhibition of polyamine catabolism have the potential for therapeutic benefit, depending on the precise context of the changes occurring. Additional studies are necessary to fully understand and exploit these dynamic pathways for maximum therapeutic advantage.
References
Aleman A, Cebrian V, Alvarez M, Lopez V, Orenes E, Lopez-Serra L, Algaba F, Bellmunt J, Lopez-Beltran A, Gonzalez-Peramato P, Cordon-Cardo C, Garcia J, del Muro JG, Esteller M, Sanchez-Carbayo M (2008) Identification of PMF1 methylation in association with bladder cancer progression. Clin Cancer Res 14:8236–8243
Alvarez-Mugica M, Fernandez-Gomez JM, Cebrian V, Fresno F, Escaf S, Sanchez-Carbayo M (2013) Polyamine-modulated factor-1 methylation predicts Bacillus Calmette-Guerin response in patients with high-grade non-muscle-invasive bladder carcinoma. Eur Urol 63:364–370
Babbar N, Casero RA Jr (2006) Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res 66:11125–11130
Babbar N, Ignatenko NA, Casero RA Jr, Gerner EW (2003) Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem 278:47762–47775
Babbar N, Gerner EW, Casero RA Jr (2006a) Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J 394:317–324
Babbar N, Hacker A, Huang Y, Casero RA Jr (2006b) Tumor necrosis factor alpha induces spermidine/spermine N1-acetyltransferase through nuclear factor kappaB in non-small cell lung cancer cells. J Biol Chem 281:24182–24192
Babbar N, Murray-Stewart T, Casero RA Jr (2007) Inflammation and polyamine catabolism: the good, the bad and the ugly. Biochem Soc Trans 35:300–304
Battaglia V, Destefano Shields C, Murray-Stewart T, Casero RA Jr (2013) Polyamine catabolism in carcinogenesis: potential targets for chemotherapy and chemoprevention. Amino Acids 46:511–519. doi:10.1007/s00726-013-1529-6
Bianchi M, Amendola R, Federico R, Polticelli F, Mariottini P (2005) Two short protein domains are responsible for the nuclear localization of the mouse spermine oxidase mu isoform. FEBS J 272:3052–3059
Bussiere FI, Chaturvedi R, Cheng Y, Gobert AP, Asim M, Blumberg DR, Xu H, Kim PY, Hacker A, Casero RA Jr, Wilson KT (2005) Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem 280:2409–2412
Capone C, Cervelli M, Angelucci E, Colasanti M, Macone A, Mariottini P, Persichini T (2013) A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radic Biol Med 63:99–107
Casero RA Jr, Marton LJ (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6(5):373–390
Casero RA Jr, Pegg AE (1993) Spermidine/spermine N1-acetyltransferase: the turning point in polyamine metabolism. FASEB J 7:653–661
Casero RA, Pegg AE (2009) Polyamine catabolism and disease. Biochem J 421:323–338
Cervelli M, Polticelli F, Federico R, Mariottini P (2003) Heterologous expression and characterization of mouse spermine oxidase. J Biol Chem 278:5271–5276
Cervelli M, Bellini A, Bianchi M, Marcocci L, Nocera S, Polticelli F, Federico R, Amendola R, Mariottini P (2004) Mouse spermine oxidase gene splice variants. Nuclear subcellular localization of a novel active isoform. Eur J Biochem 271:760–770
Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA Jr, Wilson KT (2004) Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem 279:40161–40173
Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA Jr, Correa P, Gobert AP, Polk DB, Peek RM Jr, Wilson KT (2011) Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141:1696–1708
Chaturvedi R, Asim M, Barry DP, Frye JW, Casero RA Jr, Wilson KT (2014a) Spermine oxidase is a regulator of macrophage host response to Helicobacter pylori: enhancement of antimicrobial nitric oxide generation by depletion of spermine. Amino Acids 46:531–542
Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC, Delgado AG, Hill S, Casero RA, Jr., Bravo LE, Dominguez RL, Correa P, Polk DB, Washington MK, Rose KL, Schey KL, Morgan DR, Peek RM, Jr, Wilson KT (2014b) Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology 146:1736–1751
Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM, Delgado AG, Schneider BG, Israel DA, Romero-Gallo J, Nagy TA, Morgan DR, Murray-Stewart T, Bravo LE, Peek RM, Jr, Fox JG, Woster PM, Casero RA, Jr, Correa P, Wilson KT. (2014c) Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene
Chopra S, Wallace HM (1998) Induction of spermidine/spermine N1-acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem Pharmacol 55:1119–1123
Fiori LM, Turecki G (2011) Epigenetic regulation of spermidine/spermine N1-acetyltransferase (SAT1) in suicide. J Psychiatr Res 45:1229–1235
Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT (2001) Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci USA 98:13844–13849
Goodwin AC, Jadallah S, Toubaji A, Lecksell K, Hicks JL, Kowalski J, Bova GS, De Marzo AM, Netto GJ, Casero RA Jr (2008) Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 68(7):766–772.
Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA Jr (2011) Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci USA 108:15354–15359
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr (1998a) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95:11140–11145
Ha HC, Yager JD, Woster PA, Casero RA Jr (1998b) Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem Biophys Res Commun 244:298–303
Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT (2013) Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes 4:475–481
Hector S, Porter CW, Kramer DL, Clark K, Prey J, Kisiel N, Diegelman P, Chen Y, Pendyala L (2004) Polyamine catabolism in platinum drug action: interactions between oxaliplatin and the polyamine analogue N1, N11-diethylnorspermine at the level of spermidine/spermine N1-acetyltransferase. Mol Cancer Ther 3:813–822
Hector S, Tummala R, Kisiel ND, Diegelman P, Vujcic S, Clark K, Fakih M, Kramer DL, Porter CW, Pendyala L (2008) Polyamine catabolism in colorectal cancer cells following treatment with oxaliplatin, 5-fluorouracil and N1, N11 diethylnorspermine. Cancer Chemother Pharmacol 62:517–527
Holtta E (1977) Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry 16:91–100
Hong SK, Chaturvedi R, Piazuelo MB, Coburn LA, Williams CS, Delgado AG, Casero RA Jr, Schwartz DA, Wilson KT (2010) Increased expression and cellular localization of spermine oxidase in ulcerative colitis and relationship to disease activity. Inflamm Bowel Dis 16:1557–1566
Igarashi K, Kashiwagi K (2011a) Protein-conjugated acrolein as a biochemical marker of brain infarction. Mol Nutr Food Res 55:1332–1341
Igarashi K, Kashiwagi K (2011b) Use of polyamine metabolites as markers for stroke and renal failure. Methods Mol Biol 720:395–408
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86
Khan AU, Di Mascio P, Medeiros MH, Wilson T (1992a) Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc Natl Acad Sci USA 89:11428–11430
Khan AU, Mei YH, Wilson T (1992b) A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci USA 89:11426–11427
Liao CP, Lasbury ME, Wang SH, Zhang C, Durant PJ, Murakami Y, Matsufuji S, Lee CH (2009) Pneumocystis mediates overexpression of antizyme inhibitor resulting in increased polyamine levels and apoptosis in alveolar macrophages. J Biol Chem 284:8174–8184
Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Megraud F, Xiao SD, Sugano K, Nyren O (2005) Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol 100:2100–2115
Mank-Seymour AR, Murray TR, Berkey KA, Xiao L, Kern S, Casero RA Jr (1998) Two active copies of the X-linked gene spermidine/spermine N1-acetyltransferase (SSAT) in a female lung cancer cell line are associated with an increase in sensitivity to an antitumor polyamine analogue. Clin Cancer Res 4:2003–2008
Matsui I, Wiegand L, Pegg AE (1981) Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem 256:2454–2459
Meyskens FL Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW (2008) Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 1:32–38
Murray-Stewart T, Wang Y, Devereux W, Casero RA Jr (2002) Cloning and characterization of multiple human polyamine oxidase splice variants that code for isoenzymes with different biochemical characteristics. Biochem J 368:673–677
Murray-Stewart T, Wang Y, Goodwin A, Hacker A, Meeker A, Casero RA Jr (2008) Nuclear localization of human spermine oxidase isoforms: possible implications in drug response and disease etiology. FEBS J 275:2795–2806
Murray-Stewart T, Hanigan CL, Woster PM, Marton LJ, Casero RA Jr (2013) Histone deacetylase inhibition overcomes drug resistance through a miRNA-dependent mechanism. Mol Cancer Ther 12:2088–2099
Nilsson J, Gritli-Linde A, Heby O (2000) Skin fibroblasts from spermine synthase-deficient hemizygous gyro male (Gy/Y) mice overproduce spermidine and exhibit increased resistance to oxidative stress but decreased resistance to UV irradiation. Biochem J 352:381–387
Nowotarski SL, Woster PM, Casero RA Jr (2013) Polyamines and cancer: implications for chemotherapy and chemoprevention. Expert Rev Mol Med 15:e3
Park MH, Nishimura K, Zanelli CF, Valentini SR (2010) Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38:491–500
Paul S, Kang SC (2013) Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res 62:681–688
Pegg AE (1988) Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res 48:759–774
Pegg AE (2008) Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab 294:E995–E1010
Pegg AE (2009) Mammalian polyamine metabolism and function. IUBMB Life 61:880–894
Pegg AE (2013) Toxicity of polyamines and their metabolic products. Chem Res Toxicol 26:1782–1800
Perez-Cano FJ, Franch A, Castellote C, Castell M (2003) Immunomodulatory action of spermine and spermidine on NR8383 macrophage line in various culture conditions. Cell Immunol 226:86–94
Pledgie-Tracy A, Billam M, Hacker A, Sobolewski MD, Woster PM, Zhang Z, Casero RA, Davidson NE (2010) The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother Pharmacol 65:1067–1081
Rider JE, Hacker A, Mackintosh CA, Pegg AE, Woster PM, Casero RA Jr (2007) Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 33:231–240
Saini P, Eyler DE, Green R, Dever TE (2009) Hypusine-containing protein eIF5A promotes translation elongation. Nature (Lond) 459:118–121
Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K (2003a) Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans 31:371–374
Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K (2003b) Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun 305:143–149
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S (2006) Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3:e420
Smirnova OA, Isaguliants MG, Hyvonen MT, Keinanen TA, Tunitskaya VL, Vepsalainen J, Alhonen L, Kochetkov SN, Ivanov AV (2012) Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie 94:1876–1883
Tabor CW, Rosenthal SM (1956) Pharmacology of spermine and spermidine; some effects on animals and bacteria. J Pharmacol Exp Ther 116:139–155
Tomitori H, Usui T, Saeki N, Ueda S, Kase H, Nishimura K, Kashiwagi K, Igarashi K (2005) Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke J Cerebr Circ 36:2609–2613
Uemura T, Tanaka Y, Higashi K, Miyamori D, Takasaka T, Nagano T, Toida T, Yoshimoto K, Igarashi K, Ikegaya H (2013) Acetaldehyde-induced cytotoxicity involves induction of spermine oxidase at the transcriptional level. Toxicology 310:1–7
Wang Y, Casero RA Jr (2006) Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J Biochem 139:17–25
Wang Y, Xiao L, Thiagalingam A, Nelkin BD, Casero RA Jr (1998) The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J Biol Chem 273:34623–34630
Wang Y, Devereux W, Stewart TM, Casero RA Jr (1999) Cloning and characterization of human polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermine N(1)-acetyltransferase gene. J Biol Chem 274:22095–22101
Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA Jr (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 61:5370–5373
Wang Y, Hacker A, Murray-Stewart T, Fleischer JG, Woster PM, Casero RA Jr (2005a) Induction of human spermine oxidase SMO(PAOh1) is regulated at the levels of new mRNA synthesis, mRNA stabilization and newly synthesized protein. Biochem J 386:543–547
Wang Y, Hacker A, Murray-Stewart T, Frydman B, Valasinas A, Fraser AV, Woster PM, Casero RA Jr (2005b) Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother Pharmacol 56:83–90
Waragai M, Yoshida M, Mizoi M, Saiki R, Kashiwagi K, Takagi K, Arai H, Tashiro J, Hashimoto M, Iwai N, Uemura K, Igarashi K (2012) Increased protein-conjugated acrolein and amyloid-beta40/42 ratio in plasma of patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 32:33–41
Wu T, Yankovskaya V, McIntire WS (2003) Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J Biol Chem 278: 20514–20525
Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA Jr, Wilson KT (2004) Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res 64:8521–8525
Zahedi K, Soleimani M (2011) Spermidine/spermine-N(1)-acetyltransferase in kidney ischemia reperfusion injury. Methods Mol Biol 720:379–394
Zahedi K, Lentsch AB, Okaya T, Barone S, Sakai N, Witte DP, Arend LJ, Alhonen L, Jell J, Janne J, Porter CW, Soleimani M (2009) Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 296:899–909
Zahedi K, Barone S, Kramer DL, Amlal H, Alhonen L, Janne J, Porter CW, Soleimani M (2010) The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol 299:164–174
Zahedi K, Barone SL, Xu J, Steinbergs N, Schuster R, Lentsch AB, Amlal H, Wang J, Casero RA Jr, Soleimani M (2012) Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am J Physiol Gastrointest Liver Physiol 303:546–560
Zhang M, Wang H, Tracey KJ (2000) Regulation of macrophage activation and inflammation by spermine: a new chapter in an old story. Crit Care Med 28(4 suppl):60–66
Zur Hausen H (2009) The search for infectious causes of human cancers: where and why. Virology 392:1–10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Murray-Stewart, T., Casero, R.A. (2015). Mammalian Polyamine Catabolism. In: Kusano, T., Suzuki, H. (eds) Polyamines. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55212-3_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55212-3_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55211-6
Online ISBN: 978-4-431-55212-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)