Abstract
The effect of bone marrow microenvironment on the cell cycle of acute lymphocytic leukemia (ALL) and the underlying mechanism has not been elucidated. In this study, we found that in normal condition, bone marrow mesenchymal stromal cells (BM-MSCs) had no significant effect on the cell cycle and apoptosis of ALL; in the condition when the cell cycle of ALL was blocked by genotoxic agents, BM-MSCs could increase the S-phase cell ratio and decrease the G2/M phase ratio of ALL. Besides, BM-MSCs could protect ALL cells from drug-induced apoptosis. Then, we proved that BM-MSCs affect the cell cycle arrest effect of genotoxic agents on ALL cells via p21 down-regulation. Moreover, our results indicated that activation of Wnt/β-catenin and Erk pathways might be involved in the BM-MSC-induced down-regulation of p21 in ALL cells. Targeting microenvironment-related signaling pathway may therefore be a potential novel approach for ALL therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphocytic leukemia (ALL) as a clonal malignant disorder of lymphatic system is the most common cancer in children and often occurs in adults as well [1]. In recent years, the prognosis of ALL in children has much improved, thanks to the emerging chemotherapeutic approaches. Furthermore, although ALL patients in adults experience a complete remission of 78 to 93 %, recurrence is common with only 40 % exhibiting long-term disease-free survival. Among the recurrent patients, the 5-year survival rate is as low as 7 % [2]. Drug resistance is one of the main reasons for the low survival rate of recurrent ALL, and bone marrow microenvironment has been reported to enhance the drug resistance of ALL cells. The growth of leukemia cells disrupts the normal bone marrow microenvironment and creates an abnormal microenvironment, which reduces the number of normal CD34+ cells and prevents them from entering into the peripheral blood [3]. Genes that regulate cell interactions in the bone marrow were found highly expressed in pre-B ALL cells [4]. Bone marrow microenvironment is constituted by bone marrow mesenchymal stromal cells (BM-MSCs), and a number of molecular mechanisms, such as CXCR4/CXCL12 [5–9], VE-cadherin [10, 11], gap junction communication [12], Notch signaling [13], Wnt signaling [14], interleukins [7, 9, 15–17], growth factors [6, 18–20], asparagine [21, 22], and HIF-1a [23–25], had been identified to mediate the interaction between BM-MSCs and ALL cells. Currently, BM-MSCs are found to significantly enhance the drug resistance of ALL cells to various chemotherapy drugs, such as vincristine and cytarabine [4, 14], and became the focus of study.
Genotoxic agents such as etoposide (VP16) and idarubicin (IDA) cause DNA damage of ALL cells, inducing cell cycle arrest and cell apoptosis of ALL cells [26]. BM-MSC-mediated resistance of leukemia cells to those drugs can be explained by several different mechanisms including inhibition of apoptosis, change of cell cycle distribution, and promotion of proliferation. So far, many studies have proved that BM-MSCs could promote the viability of leukemia cells by inhibiting their apoptosis [4, 13, 14]. Growth and proliferation of all cell types require cell division especially for malignant cells. So cell cycle often becomes the target of some antitumor drugs. However, it is still controversial whether BM-MSCs promote or block the leukemia cell cycle, and the underlying mechanism is unclear. Gaundar et al. [6] and Yang et al. [14] suggested that BM-MSCs could promote the proliferation and cell cycle of ALL cells, while Zhu et al. [27] suggested that BM-MSCs could inhibit the proliferation of chronic myelogenous leukemia cells. Moreover, it is also unclear whether BM-MSCs affect the cell cycle arrest effect of genotoxic agents on ALL cells.
One important cell cycle inhibitor is p21, which blocks the interaction between cyclins and cyclin-dependent kinases (CDKs), inhibiting the CDK activity and blocking the G1/S and G2/M transitions [28]. Besides, a growing number of evidence shows the relationship between p21 and cell apoptosis [29, 30]. The expression of p21 is controlled by several different factors. At the transcription level, p53 as a transcription factor can promote the transcription of p21 gene [31], and c-myc inhibits the transcription of p21 gene via interacting with various transcription factors [32]. At the posttranscription level, p21 protein can be degraded through ubiquitin-proteasome pathway mediated by SCF-skp2 complex [33]. On the other hand, the MST signaling pathway mediates the phosphorylation of p21, and the p-p21 is unstable, which cannot accumulate in the cell [34]. The expression of p21 in ALL cells had been found down-regulated after cocultured with BM-MSCs [14]. Thus, we hypothesized that BM-MSCs regulate the cell cycle of ALL by down-regulating the p21 expression level in ALL cells.
In the present study, we determined the effect of BM-MSCs on the cell cycle and apoptosis of ALL cells in the presence or absence of genotoxic agents. Our results confirmed that BM-MSCs affect the cell cycle arrest effect of genotoxic agents on ALL cells by down-regulation of p21 protein.
Materials And methods
Cell cultures
The Reh cell line was established in 1974 from the peripheral blood of a 15-year-old girl with acute lymphoblastic leukemia at relapse [35]. In this study, Reh cell line was provided by the Typical Cell Culture Collection Committee of the Chinese Academy of Sciences and cultured in Iscove’s Modified Dulbecco's Medium (IMDM) supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin-streptomycin at 37 °C and 5 % CO2 in a humidified incubator. Human BM-MSCs were isolated and cultured as previously described [36]. Briefly, bone marrow mononuclear cells were obtained from normal bone marrow donors by density gradient centrifugation and were cultured in DMEM with 10 % FBS and 1 % penicillin-streptomycin. The medium was replaced every 3 days, and the adherent cells were passaged when 80 % confluence was reached. Passages 2 to 8 BM-MSCs were used for the coculture experiments.
Characterization of BM-MSCs
BM-MSCs at passages 3–5 were analyzed for CD90-FITC, CD105-PE, CD73-APC, and CD45-FITC (eBioscience) expression using a FC 500 MCL Flow Cytometer (Beckman Coulter). For differentiation assays, BM-MSCs at passages 3–5 were cultured in either osteogenic or adipogenic differentiation medium (Cyagen). The osteogenic differentiation was revealed by the formation of calcium nodules stained with Alizarin Red (Cyagen), and the adipogenic differentiation was revealed by the formation of lipid droplets stained with Oil Red O (Cyagen).
Coculture of Reh cells with BM-MSCs
The coculture experiment was conducted as previously described [14]. Briefly, BM-MSCs were seeded onto the culture plates at a concentration of 4 × 104/well (12-well plate) or 105/well (6-well plate). After 1–2 days, Reh cells were cultured at a starting concentration of 4 × 105/well (12-well plate) or 106/well (6-well plate) with or without BM-MSC layer, VP16 (Sigma), IDA (Pfizer), or DMSO were added, with or without the SCF inhibitor MLN4924 (MedChemexpress). After coculture, the Reh cells were gently rinsed off and collected for next experiments, while adherent BM-MSCs could not be rinsed off.
Cell cycle analysis
Reh cells were cultured in serum-free IMDM for 24 h before coculture experiments. After coculture, Reh cells were carefully collected and washed once with cold phosphate-buffered saline (PBS), then fixed in 70 % ethanol overnight at −20 °C. The next day, ethanol was removed, and the samples were washed once with PBS and then stained with PI/RNase (Multisciences) at room temperature for 0.5–1 h. The samples were analyzed using a FC 500 MCL Flow Cytometer (Beckman Coulter).
Apoptosis analysis
Reh cells were carefully collected and washed once with cold phosphate-buffered saline (PBS), then stained with Annexin V-FITC and Prodium Iodide (PI) (both from Multisciences) at room temperature for 5 min. Flow cytometric analysis was performed using a FC 500 MCL Flow Cytometer (Beckman Coulter). Cells that were Annexin-V-positive and PI-negative were considered early apoptotic; Annexin-V-positive and PI-positive were considered apoptotic.
Western blot analysis
Reh cells were washed once with cold PBS and then lysed in lysis buffer containing 12 mM Tris (pH 6.8), 5 % glycerol, 0.4 % sodium dodecyl sulfate (SDS), and 5 % β-mercaptoethanol. Protein extracts were loaded onto a 10 % polyacrylamide gel containing SDS, electrophoresed, and transferred to a polyvinylidene difluoride membrane. The membrane was blocked in 5 % bovine serum albumin (BSA) at room temperature for 1 h, incubated with primary antibodies overnight, and then incubated with an IRDye secondary antibody (Licor) at room temperature for 1 h. Immunoreactive bands were visualized using an Odyssey infrared imaging system (Licor). β-actin was used to ensure equivalent loading of whole cell protein. Antibodies against p21, skp2, and Bax were from Epitomics (Burlingame, CA, USA), β-actin antibody was from MultiSciences Biotechnology (Hangzhou, China), and antibodies against Erk, phosphorylated-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (p-Erk), and β-catenin were from Cell Signaling Technology (Danvers, MA, USA).
Quantitative Reverse Transcription PCR (qRT-PCR)
Total cellular RNA was extracted using Trizol reagent (Takara, Liaoning, China). Reverse transcription was performed using a PrimeScript RT Master Mix (Takara). Quantitative PCR was performed using an iTaq Universal SYBR Green Supermix (Bio-rad) with a CFX96 Touch Real-Time PCR Detection system (Bio-rad). Primer sets used for these analyses are listed in Table 1.
Statistical analysis
The data were presented as mean ± SEM and analyzed by SAS 9.1 software using Student’s t test. Differences were considered statistically significant at p < 0.05.
Results
Characterization of BM-MSCs
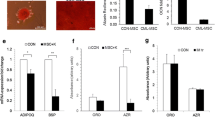
BM-MSCs at passage 1 presented fibroblast-like, spindle-shaped morphology (Fig. 1a). The adipogenic and osteogenic differentiation potentials of BM-MSCs were verified by Oil Red O and Alizarin Red staining, respectively (Fig. 1b, c). Strong expression of CD90, CD105, and CD73 was also detected in BM-MSCs by flow cytometry, while the expression of CD45 was negative, confirming that these BM-MSCs were from a nonhematopoietic origin (Fig. 1d).
Characterization of BM-MSCs. a BM-MSCs were grown in culture at passage 1 without differentiation, magnification × 100. b Adipogenic differentiation capacity of BM-MSCs. The differentiation into adipocytes was revealed by the formation of lipid droplets stained with Oil Red O, magnification × 100. c Osteogenic differentiation of BM-MSCs. The osteogenic differentiation was revealed by the formation of calcium nodules stained with Alizarin Red, magnification × 100. d Flow cytometric analysis of expression of surface antigens in BM-MSCs (solid lines). Solid histograms represent unstained controls. Data shown were from one representative experiment of three
BM-MSCs protect ALL cells from drug-induced apoptosis
Reh cells were cultured with or without BM-MSCs in the presence or absence of genotoxic agents for 72 h, and the apoptosis and early apoptosis rate were measured using Annexin V/PI method (Fig. 2). In the absence of drugs, there was no significant difference of apoptosis rate or early apoptosis rate between Reh cells cultured alone or with BM-MSCs. In the presence of VP16, the early apoptosis rate of Reh cultured with BM-MSCs was lower than that of Reh cultured alone (11.81 ± 2.52 and 21.04 ± 0.74 %, respectively, p < 0.05), and there was no significant difference of apoptosis rate between Reh cells cultured alone or with BM-MSCs. In the presence of IDA, the early apoptosis rate of Reh cultured with BM-MSCs was lower than that of Reh cultured alone (5.49 ± 0.90 and 37.00 ± 5.32 %, respectively, p < 0.05), and the apoptosis rate of Reh cultured with BM-MSCs was also lower than that of Reh cultured alone (66.09 ± 5.89 and 79.93 ± 3.15 %, respectively, p < 0.05). These results suggest that BM-MSCs can protect ALL cells from drug-induced apoptosis.
BM-MSCs protect ALL cells from drug-induced apoptosis. Reh cells were cultured with or without BM-MSCs in the presence or absence of genotoxic agents for 72 h, then the apoptosis rate (b) and early apoptosis rate (c) were measured using Annexin V/PI method. a Representative result of flow cytometric analysis. Experiments were repeated three times, Asterisks denote significance at p < .05, N.S. not statistically significant
BM-MSCs affect the cell-cycle arrest effect of genotoxic agents on ALL cells
In this study, we cultured leukemia cell line Reh with or without BM-MSCs in the presence of 250 nM VP16 or 25 nM IDA, or without drug treatment for 24, 48, and 72 h. VP16 induced G2/M phase arrest of the Reh cell cycle (Fig. 3a), and IDA induced S-phase arrest of the Reh cell cycle (Fig. 3b). In the absence of drugs, BM-MSCs had no significant effect on the cell cycle of Reh cells. In the presence of VP16 or IDA, BM-MSCs increased the S-phase ratio of Reh cells and decrease the G2/M phase ratio of Reh cells (Fig. 3a, b). Then, we chose the time points that BM-MSCs had the most significant effect on Reh cell cycle to perform three replicate experiments (48 h for IDA treatment and 72 h for VP16 treatment). Under the treatment of VP16 (Fig. 3c), the S-phase ratio of Reh cultured with BM-MSCs was higher than that of Reh cultured alone (10.893 ± 1.077 and 6.842 ± 0.652 %, respectively, p < 0.05); the G2/M phase ratio of Reh cultured with BM-MSCs was lower than that of Reh cultured alone (25.812 ± 1.720 and 38.818 ± 3.508 %, respectively, p < 0.05). Under the treatment of IDA (Fig. 3d), the S-phase ratio of Reh cultured with BM-MSCs was higher than that of Reh cultured alone (58.720 ± 5.765 and 45.519 ± 7.886 %, respectively, p < 0.05); the G2/M phase ratio of Reh cultured with BM-MSCs was lower than that of Reh cultured alone (1.888 ± 0.809 and 6.449 ± 0.325 %, respectively, p < 0.05). The results suggest that when genotoxic agents inhibit the cell cycle of ALL passing the G1/S or G2/M checkpoint, BM-MSCs can promote the cell cycle of ALL passing these cell cycle checkpoints, thus affecting the cell cycle arrest effect of genotoxic agents on ALL cells.
Effect of BM-MSCs on ALL cell cycle in the presence or absence of genotoxic agents. a Cell cycle analysis of Reh cells cultured with or without BM-MSCs in the presence or absence of 250 nM VP16 for 24, 48, and 72 h. b Cell cycle analysis of Reh cells cultured with or without BM-MSCs in the presence or absence of 25 nM IDA for 24, 48, and 72 h. c Cell cycle distribution of Reh cells cultured with or without BM-MSCs in the presence or absence of 250 nM VP16 for 72 h. Asterisks denote significance at p < .05. d Cell cycle distribution of Reh cells cultured with or without BM-MSCs in the presence or absence of 25 nM IDA for 48 h. Asterisks denote significance at p < .05
BM-MSCs down-regulate the p21 expression and activate the Erk and Wnt signaling pathways in ALL cells
We cultured the Reh cells with or without BM-MSCs in the presence or absence of genotoxic agents and analyzed the protein expression of p21 and S-phase kinase protein 2 (skp2) in Reh cells. In the absence of genotoxic agents, the expression of p21 was low in Reh cells. Treatment of VP16 or IDA induced the expression of p21, while BM-MSCs down-regulate the p21 expression in Reh cells. Besides, there was no significant difference of skp2 expression between Reh cultured alone and Reh cultured with BM-MSCs (Fig. 4a). The results of qRT-PCR showed that, under the drug treatment, the messenger RNA (mRNA) expression level of p21 was also lower in Reh cells cultured with BM-MSCs than in Reh cells cultured alone (Fig. 4b, c). We also detected the expression of key proteins in the Erk and Wnt signaling pathways. In the presence of VP16 or IDA, the expression of p-Erk and β-catenin was higher in Reh cells cultured with BM-MSCs than in Reh cells cultured alone (Fig. 4d). These results suggest that BM-MSCs might down-regulate the p21 expression in ALL cells via certain pathways such as Wnt and Erk pathways, thus promoting the G1/S and G2/M transitions of ALL cell cycle.
BM-MSCs down-regulate the p21 expression and activate the Erk and Wnt signaling pathways in ALL cells. a Western blot analysis of p21 and skp2 expressions in Reh cells cultured with or without BM-MSCs in the presence or absence of genotoxic agents. b, c qRT-PCR analysis of p21 mRNA expression in Reh cells cultured with or without BM-MSCs under treatment of VP16 or IDA. Asterisks denote significance at p < .05. d Western blot analysis of p-Erk, Erk, and β-catenin expression in Reh cells cultured with or without BM-MSCs in the presence of 250 nM VP16 or 25 nM IDA
BM-MSC-induced p21 down-regulation promotes the G1/S and G2/M transitions of ALL cell cycle
To confirm the role of p21 down-regulation in the BM-MSC-mediated cell cycle promotion of ALL, we utilized MLN4924, a specific small molecular inhibitor of SCF complex, to induce high expression of p21 in Reh cells, and to see whether this would eliminate the cell cycle promotion effect of BM-MSCs on Reh cells. Using different concentration of MLN4924 to treat Reh cells, we found that MLN4924 treatment could significantly up-regulate the p21 expression of Reh cells at every concentration and down-regulate the skp2 expression at high concentrations (1000 and 3000 nM), besides, the expression of apoptosis-associated protein Bax was not affected (Fig. 5a). Then, we used MLN4924 to induce high expression of p21 in both Reh cultured alone and cultured with BM-MSCs, thus eliminating the down-regulation of p21 caused by BM-MSC coculture (Fig. 5b, c). Cell cycle analysis showed that MLN4924 eliminated the cell cycle promotion effect of BM-MSCs on Reh cells under the treatment of genotoxic agents (Fig. 5d–g). In addition, apoptosis analysis showed that MLN4924 did not affect the antiapoptosis effect of BM-MSCs on Reh cells (Fig. 6a–d). Through these experiments, we confirmed that BM-MSC-induced p21 down-regulation in ALL cells promote the G1/S and G2/M transitions of ALL cell cycle but does not contribute to the antiapoptosis effect of BM-MSCs on ALL cells.
MLN4924-induced p21 high expression eliminates the cell cycle promotion effect of BM-MSCs on Reh cells. a Western blot analysis of p21, skp2, and bax expression in Reh cells treated by different concentration of MLN4924. b Under the treatment of 25 nM IDA, western blot analysis of p21 and skp2 expressions in Reh cells cultured with or without BM-MSCs in the presence of different concentrations of MLN4924 (0, 300, 700 nM). c Under the treatment of 250 nM VP16, western blot analysis of p21 and skp2 expressions in Reh cells cultured with or without BM-MSCs in the presence of different concentrations of MLN4924 (0, 100, 300 nM). d, e Under the treatment of 250 nM VP16 (d) or 25 nM IDA (e), the S-phase cell ratio of Reh cells cultured with or without BM-MSCs in the presence of different concentrations of MLN4924 (0, 100, 300 nM). f, g Under the treatment of 250 nM VP16 (f) or 25 nM IDA (g), the G2/M phase cell ratio of Reh cells cultured with or without BM-MSCs in the presence of different concentrations of MLN4924 (0, 100, 300 nM). Asterisks denote significance at p < .05, N.S. not statistically significant
Discussion
It has been shown that BM-MSCs promote the viability of leukemia cells by inhibiting their apoptosis, while the effect of BM-MSCs on leukemia cell cycle is still unclear. In the present study, we proved that in the absence of chemotherapeutic drugs, BM-MSCs have no significant effect on the cell cycle and apoptosis of ALL cells. Genotoxic agents like VP16 and IDA can block the cell cycle of ALL in different phases and induce apoptosis of ALL cells at the same time, while BM-MSCs affect the cell cycle arrest effect of genotoxic agents on ALL cells and also protect ALL cells from drug-induced apoptosis. The BM-MSC-induced p21 down-regulation in ALL cells promotes the G1/S and G2/M transitions of ALL cell cycle, which has not been reported previously, but does not contribute to the antiapoptosis effect of BM-MSCs on ALL cells.
Both VP16 and IDA can inhibit the function of topoisomerase II, inducing DNA double-strand breakage, thus activating DNA-damage-related signaling pathway and inducing the cell cycle arrest [26]. Our results show that both VP16 and IDA induce the expression of p21 in Reh cells. VP16 induces G2/M phase arrest of the Reh cells, which is due to the VP16-induced DNA damage inhibiting the cell cycle passing the DNA damage checkpoint; IDA induces S-phase arrest of the Reh cell-cycle, and the possible cause is the blockage of DNA synthesis during the S-phase. The present study found that, through down-regulating the p21 expression, BM-MSCs promote the G1/S and G2/M transitions of ALL cell cycle in the presence of genotoxic agents, which increase the S-phase cell ratio and decrease the G2/M phase cell ratio of ALL cells. Since the BM-MSC-induced change of cell cycle distribution will affect the killing effect of genotoxic agents, we propose that eliminating the effect of BM-MSCs on ALL cell cycle might enhance the chemotherapy efficacy on ALL cells.
Next, elimination of the cell cycle promotion effect of BM-MSCs on Reh cells through MLN4924-induced high expression of p21 in Reh cells confirms that BM-MSCs promote the G1/S and G2/M transitions of ALL cell-cycle via p21 down-regulation. Notably, MLN4924 specifically inhibits the neddylation of Cullin1 via blocking Nedd8-activating enzyme and subsequently inhibits the degradation of p21 protein by suppressing the function of SCF-skp2 ubiquitin ligase complex, leading to the accumulation of p21 protein [37]. Up-regulating p21 expression can overcome drug resistance in different types of tumor. For example, Chen et al. [38] up-regulated the p21 expression in ovarian cancer cells using epigallocatechin gallate and cruciferous vegetables, and enhanced the cisplatin-induced apoptosis and G2/M phase arrest. Vijayaraghavalu et al. [39] activated the expression of p21 in breast cancer cells using epigenetic drugs, which caused G2/M phase arrest and overcame the drug resistance. Our results showed for the first time that up-regulation of p21 expression in ALL cells eliminates the effect of BM-MSCs on ALL cell cycle, which suggests that the combined approach of small molecular inhibitor modulating p21 protein expression in leukemia cells with novel cell cycle specific chemotherapeutic drugs may enhance the chemotherapy efficacy.
The Wnt signaling pathway and Erk signaling pathway have been proved to mediate the microenvironment protection of ALL [14, 40], and both these two pathways can up-regulate the c-myc expression [41, 42], while c-myc is the transcription inhibitor of p21 [32]. Our results show that BM-MSCs up-regulate the expression of p-Erk and β-catenin in Reh cells, thus, we predict that BM-MSCs might down-regulate the p21 expression in ALL cells via activating the Wnt and Erk signaling pathways. More work is needed to determine the cross-talk between these pathways in leukemia microenvironment.
SCF-skp2 complex mediates the ubiquitin-proteasome pathway, which plays the major role in the posttranscriptional degradation of p21. SCF-skp2 complex contains skp1, cullin1, Rbx1, and skp2. Skp2 can bind specifically to p21, guiding the degradation of p21 [33]. Cullin1 is the scaffold protein of SCF-skp2 complex, which also plays an important role in the function of SCF-skp2 complex, and the activation of SCF-skp2 complex requires neddylation of cullin1. More work is needed to see whether BM-MSCs would regulate protein level in ALL cells via activating the ubiquitin-proteasome pathway.
Our results also show that BM-MSCs can reduce the apoptosis rate of ALL cells induced by genotoxic agents, which is consistent with the previous studies [4, 13, 14]. Since a growing number of evidence shows that p21 is not only a cell cycle inhibitor but also has a relationship with cell apoptosis [29, 30], we examined whether the BM-MSC-induced p21 down-regulation in ALL cells also contributed to the antiapoptosis effect of BM-MSCs on ALL cells. However, the results indicate that the MLN4924-induced high expression of p21 in ALL cells does not affect the antiapoptosis effect of BM-MSCs on ALL cells. Our explanation for these results is that the effect of BM-MSCs on the cell cycle of ALL cells promotes the proliferation rather than reduces the apoptosis of ALL cells, and the antiapoptosis effect of BM-MSCs on ALL cells might be through affecting apoptosis signaling pathway rather than down-regulation of p21. The relation between cell cycle and apoptosis is complicated depending on different cell types, which requires further research.
A number of studies have shown the occurrence of immunophenotypic changes in BM-MSCs from ALL patients [43, 44], which suggested that microenvironmental conditions might play a pivotal role in facilitating BM-MSC function. More work is needed to determine the effect of leukemia microenvironment on BM-MSC properties.
In conclusion, the present study shows for the first time that BM-MSCs affecting the cell cycle arrest effect of genotoxic agents on ALL cells are mediated by down-regulation of p21 protein. Activation of Wnt/β-catenin and Erk pathways might be involved in the BM-MSC-induced down-regulation of p21 in ALL cells. Targeting microenvironment-related signaling pathway may therefore be a potential novel therapeutic strategy for the treatment of ALL.
Reference
Pui CH, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371(9617):1030–1043
Fielding AK, Richards SM, Chopra R et al (2007) Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 109(3):944–950
Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA (2008) Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322(5909):1861–1865
Tesfai Y, Ford J, Carter KW et al (2012) Interactions between acute lymphoblastic leukemia and bone marrow stromal cells influence response to therapy. Leuk Res 36(3):299–306
Parameswaran R, Yu M, Lyu MA et al (2012) Treatment of acute lymphoblastic leukemia with an rGel/BLyS fusion toxin. Leukemia 26(8):1786–1796
Gaundar SS, Bradstock KF, Bendall LJ (2009) p38MAPK inhibitors attenuate cytokine production by bone marrow stromal cells and reduce stroma-mediated proliferation of acute lymphoblastic leukemia cells. Cell Cycle 8(18):2975–2983
Scupoli MT, Donadelli M, Cioffi F et al (2008) Bone marrow stromal cells and the upregulation of interleukin-8 production in human T-cell acute lymphoblastic leukemia through the CXCL12/CXCR4 axis and the NF-kappaB and JNK/AP-1 pathways. Haematologica 93(4):524–532
Juarez J, Dela Pena A, Baraz R et al (2007) CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia 21(6):1249–1257
Juarez J, Baraz R, Gaundar S, Bradstock K, Bendall L (2007) Interaction of interleukin-7 and interleukin-3 with the CXCL12-induced proliferation of B-cell progenitor acute lymphoblastic leukemia. Haematologica 92(4):450–459
O'Leary H, Akers SM, Piktel D et al (2010) VE-cadherin regulates Philadelphia chromosome positive acute lymphoblastic leukemia sensitivity to apoptosis. Cancer Microenviron 3(1):67–81
Wang L, O'Leary H, Fortney J, Gibson LF (2007) Ph+/VE-cadherin + identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood 110(9):3334–3344
Zhang X, Liu Y, Si YJ et al (2012) Effect of Cx43 gene-modified leukemic bone marrow stromal cells on the regulation of Jurkat cell line in vitro. Leuk Res 36(2):198–204
Nwabo Kamdje AH, Mosna F, Bifari F et al (2011) Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood 118(2):380–389
Yang Y, Mallampati S, Sun B et al (2013) Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett 333:9–17
Silva A, Laranjeira AB, Martins LR et al (2011) IL-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res 71(14):4780–4789
de Vasconcellos JF, Laranjeira AB, Zanchin NI et al (2011) Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr Blood Cancer 56(4):568–577
Batista A, Barata JT, Raderschall E et al (2011) Targeting of active mTOR inhibits primary leukemia T cells and synergizes with cytotoxic drugs and signaling inhibitors. Exp Hematol 39(4):457–472 e453
Dosen-Dahl G, Munthe E, Nygren MK, Stubberud H, Hystad ME, Rian E (2008) Bone marrow stroma cells regulate TIEG1 expression in acute lymphoblastic leukemia cells: role of TGFbeta/BMP-6 and TIEG1 in chemotherapy escape. Int J Cancer 123(12):2759–2766
Markovic A, MacKenzie KL, Lock RB (2012) Induction of vascular endothelial growth factor secretion by childhood acute lymphoblastic leukemia cells via the FLT-3 signaling pathway. Mol Cancer Ther 11(1):183–193
Fragoso R, Pereira T, Wu Y, Zhu Z, Cabecadas J, Dias S (2006) VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood 107(4):1608–1616
Fung KL, Liang RH, Chan GC (2010) Vincristine but not imatinib could suppress mesenchymal niche's support to lymphoid leukemic cells. Leuk Lymphoma 51(3):515–522
Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D (2007) Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117(4):1049–1057
Frolova O, Samudio I, Benito JM et al (2012) Regulation of HIF-1alpha signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther 13(10):858–870
Dong-Feng Z, Ting L, Cheng C et al (2012) Silencing HIF-1alpha reduces the adhesion and secretion functions of acute leukemia hBMSCs. Braz J Med Biol Res 45(10):906–912
Benito J, Shi Y, Szymanska B et al (2011) Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One 6(8):e23108
Hande KR (1998) Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34(10):1514–1521
Zhu Y, Sun Z, Han Q et al (2009) Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 23(5):925–933
Warfel NA, El-Deiry WS (2013) p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol 25(1):52–58
Shiu TY, Huang SM, Shih YL, Chu HC, Chang WK, Hsieh TY (2013) Hepatitis C virus core protein down-regulates p21(Waf1/Cip1) and inhibits curcumin-induced apoptosis through microRNA-345 targeting in human hepatoma cells. PLoS One 8(4):e61089
Ahmad N, Feyes DK, Agarwal R, Mukhtar H (1998) Photodynamic therapy results in induction of WAF1/CIP1/P21 leading to cell cycle arrest and apoptosis. Proc Natl Acad Sci U S A 95(12):6977–6982
el-Deiry WS, Tokino T, Velculescu VE et al (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75(4):817–825
Mitchell KO, El-Deiry WS (1999) Overexpression of c-Myc inhibits p21WAF1/CIP1 expression and induces S-phase entry in 12-O-tetradecanoylphorbol-13-acetate (TPA)-sensitive human cancer cells. Cell Growth Differ 10(4):223–230
Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278(28):25752–25757
Cornils H, Kohler RS, Hergovich A, Hemmings BA (2011) Human NDR kinases control G(1)/S cell cycle transition by directly regulating p21 stability. Mol Cell Biol 31(7):1382–1395
Drexler HG, Quentmeier H, Dirks WG, Uphoff CC, MacLeod RA (2002) DNA profiling and cytogenetic analysis of cell line WSU-CLL reveal cross-contamination with cell line REH (pre B-ALL). Leukemia 16(9):1868–1870
Zhao YM, Li JY, Lan JP et al (2008) Cell cycle dependent telomere regulation by telomerase in human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 369(4):1114–1119
Tanaka T, Nakatani T, Kamitani T (2012) Inhibition of NEDD8-conjugation pathway by novel molecules: potential approaches to anticancer therapy. Mol Oncol 6(3):267–275
Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO (2013) Enhancement of cisplatin-mediated apoptosis in ovarian cancer cells through potentiating G2/M arrest and p21 upregulation by combinatorial epigallocatechin gallate and sulforaphane. J Oncol 2013:872957
Vijayaraghavalu S, Dermawan JK, Cheriyath V, Labhasetwar V (2013) Highly synergistic effect of sequential treatment with epigenetic and anticancer drugs to overcome drug resistance in breast cancer cells is mediated via activation of p21 gene expression leading to G2/M cycle arrest. Mol Pharm 10(1):337–352
Wu KN, Zhao YM, He Y et al (2014) Rapamycin interacts synergistically with idarubicin to induce T-leukemia cell apoptosis in vitro and in a mesenchymal stem cell simulated drug-resistant microenvironment via Akt/mammalian target of rapamycin and extracellular signal-related kinase signaling pathways. Leuk Lymphoma 55(3):668–676
Li Y, Gao Q, Yin G, Ding X, Hao J (2012) WNT/beta-catenin-signaling pathway stimulates the proliferation of cultured adult human Sertoli cells via upregulation of C-myc expression. Reprod Sci 19(11):1232–1240
Pintus G, Tadolini B, Posadino AM et al (2002) Inhibition of the MEK/ERK signaling pathway by the novel antimetastatic agent NAMI-A down regulates c-myc gene expression and endothelial cell proliferation. Eur J Biochem 269(23):5861–5870
Lanza F, Campioni D, Moretti S et al (2007) Aberrant expression of HLA-DR antigen by bone marrow-derived mesenchymal stromal cells from patients affected by acute lymphoproliferative disorders. Leukemia 21(2):378–381
Campioni D, Lanza F, Moretti S, Ferrari L, Cuneo A et al (2008) Loss of Thy-1 (CD90) antigen expression on mesenchymal stromal cells from hematologic malignancies is induced by in vitro angiogenic stimuli and is associated with peculiar functional and phenotypic characteristics. Cytotherapy 10(1):69–82
Acknowledgments
This work was supported by grants from National High-tech R&D Program of China (863 Program, grant No. N20120221) and Zhejiang Provincial Natural Science Foundation of China (grant No. Y2110152).
Conflicts of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, K., Hu, Y. et al. Bone marrow mesenchymal stromal cells affect the cell cycle arrest effect of genotoxic agents on acute lymphocytic leukemia cells via p21 down-regulation. Ann Hematol 93, 1499–1508 (2014). https://doi.org/10.1007/s00277-014-2069-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2069-1