Abstract
The mechanisms by which the bone marrow microenvironment regulates tumor cell survival are diverse. This study describes the novel observation that in addition to Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) cell lines, primary patient cells also express Hypoxia Inducible Factor-2α (HIF-2α) and Vascular Endothelial Cadherin (VE-cadherin), which are regulated by Abl kinase. Tumor expression of the classical endothelial protein, VE-cadherin, has been associated with aggressive phenotype and poor prognosis in other models, but has not been investigated in hematopoietic malignancies. Targeted knockdown of VE-cadherin rendered Ph+ ALL cells more susceptible to chemotherapy, even in the presence of bone marrow stromal cell (BMSC) derived survival cues. Pre-treatment of Ph+ ALL cells with ADH100191, a VE-cadherin antagonist, resulted in increased apoptosis during in vitro chemotherapy exposure. Consistent with a role for VE-cadherin in modulation of leukemia cell viability, lentiviral-mediated expression of VE-cadherin in Ph− ALL cells resulted in increased resistance to treatment-induced apoptosis. These observations suggest a novel role for VE-cadherin in modulation of chemoresistance in Ph+ ALL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia (ALL) patients are classified as “high risk” based on the presence of tumor cells harboring the 9;22 translocation (Philadelphia chromosome; Ph+). This translocation results in constitutively active Abl kinase, characteristic of cells resistant to standard chemotherapeutic regimens [1–5]. Although advances in treatment have increased the 5 year event free survival and cure rate, patients with Ph+ ALL continue to have a high incidence of bone marrow and central nervous system (CNS) relapse relative to Ph− ALL [6, 7]. While first line treatment with Imatinib Mesylate (Gleevec, IM), in combination with conventional treatments, was initially thought to be successful in Ph+ ALL, it has been demonstrated that specific mutations in the Abl kinase catalytic domain render this drug ineffective [8, 9]. In addition to mutations inherent to the tumor cell, the role of the microenvironment in modulating drug resistance has proven to be an important factor in the efficacy of treatment in many tumors, including primary and relapsed ALL [10]. Previous reports indicate the importance of the microenvironment in protection of tumor cell viability during disease initiation and progression, and during treatment [11–19].
We recently identified a unique population of Ph+ ALL cells that express Vascular Endothelial Cadherin (VE-cadherin) on their surface and respond to bone marrow stromal cells (BMSC) through stimulation of several anti-apoptotic pathways [20]. VE-cadherin expression is often discussed in the context of vasculogenic mimicry, a process by which tumor cells gain characteristics normally restricted to endothelial cells, allowing tumors to better utilize microenvironment cues contributing to increased survival [21]. VE-cadherin expression has also been shown to be an indicator of poor prognosis in melanoma and Ewings sarcoma [21, 22].
The Hypoxia Inducible Factor (HIF) family of proteins are transcriptional regulators of pro-angiogenic and survival proteins that have been shown to be important in tumor cell progression and aggressiveness [23]. HIF-2α can be regulated by Erk and Akt-induced phosphorylation cascades that either directly phosphorylate HIF-2α or are involved in its trans-activation [24–26]. Relevant to our model, HIF-2α has been shown to regulate the transcription of VE-cadherin in other systems [27]. Because HIF-2α can be active under normoxic conditions it is distinct from the more extensively studied, and more rigorously hypoxia-driven, HIF-1α. HIF-2α regulates transcriptional targets through binding to distinct promoters, as well as promoters overlapping with HIF-1α, and the ability of HIF-2α to respond to signals outside hypoxia driven regulation positions it to have both constitutive and hypoxia-induced functions in Ph+ ALL [28]. HIF-2α is up-regulated in some primary tumors including bladder, renal and high grade neuroblastomas and is associated with poor prognosis [23, 26, 29–31]. Therefore, the regulation of HIF-2α and VE-cadherin expression by bone marrow stromal cells (BMSC), and their role in hematopoietic tumor cell response to treatment, was investigated in the current study.
We demonstrate that, consistent with Ph+ ALL cell lines, primary Ph+ patient derived leukapheresis samples also express surface VE-cadherin. Primary ALL (Ph+ and Ph−) bone marrow core sections were positive for VE-cadherin expression by IHC. Exposure of Ph+/VE-cadherin+ ALL to IM resulted in diminished HIF-2α and VE-cadherin. Abl kinase activity, as well as expression of VE-cadherin and β-catenin, are maintained by BMSC contact during in vitro exposure to chemotherapy with lentiviral-mediated surface expression of VE-cadherin increasing chemoresistance. Disruption of VE-cadherin by siRNA or the antagonist ADH100191 (ADH) led to increased sensitivity of Ph+ ALL cells to chemotherapy, even in the presence of an adherent microenvironment, BMSC. Collectively, these observations suggest that BMSC may provide cues that converge on hematopoietic tumor cell VE-cadherin as one of the factors that modulate response to therapy.
Materials and Methods
Cells and Reagents
Ph+ SUP-B15, and Ph− REH, leukemic cell lines were obtained from the ATCC (CRL-1929 and CRL-8286, Manassas, VA) and have been verified by both RT-PCR and fluorescent in situ hybridization (FISH) for analysis of Bcr/Abl, and other, translocations. Primary de-identified Ph+ (IM resistant) and Ph− ALL leukophoresis and bone marrow core biopsies were evaluated by fluorescent in situ hybridization (FISH) for analysis of Bcr/Abl translocations. Maintenance and derivation of human BMSC has been previously described [11]. To establish long-term co-culture (LTCC) of BMSC and leukemic cells, SUP-B15 cells were seeded onto 70% confluent BMSC, and SUP-B15 were sub-cultured onto new BMSC weekly for more than 12 months. Long term media cultures (LTMC) include SUP-B15 cells cultured in media alone and were used in short term (24–120 h) co-culture experiments with adherent BMSC. The Bcr/Abl kinase inhibitor Imatinib Mesylate (IM) (LGM pharmaceuticals, Boca Raton, FL) was reconstituted in DMSO and used at 10 µM. Daunorubicin hydrochloride (DNR) (Sigma, St. Louis, MO) was diluted in Iscove’s DMEM (CellGRO/Mediatech, Inc. Herndon,VA) immediately prior to use at 0.01–0.1 mg/ml. The peptide antagonist of VE-cadherin, ADH100191, (ADH, Adherex Technologies Inc., Durham, NC) was used at a concentration of 1 mg/ml.
Knockdown of VE-cadherin and HIF-2α Expression by RNAi
SmartPool siRNA for VE-cadherin (CDH5) or HIF-2α (EPAS-1) was obtained from Dharmacon (Lafayette, CO) and Qiagen (Valencia, CA), respectively. Transient transfection of SUP-B15 cells, with sequence specific siRNA or scrambled control, was completed using oligofectamine (Invitrogen, Carlsbad, CA), 200 nM siRNA and 1.5 × 106 cells per reaction as previously described [20]. Cells were then treated with DNR, and collected at 72 h for evaluation of viability. Confirmation of knockdown was completed by flow cytometric analysis (FACS).
Confocal Microscopy, Flow Cytometry and Cell Viability
Anti-human antibodies specific for HIF-2α (flow cytometry), VE-cadherin (flow cytometry) and phosphorylated β-catenin (confocal microscopy) were purchased from Novus (Littleton,CO clone ep190b), R&D systems (Minneapolis, MN clone 123433) and Cell Signaling (Beverly, MA), respectively. In addition, a polyclonal antibody to VE-cadherin utilized for confocal microscopy was purchased from AXXORA LLC (San Diego, CA). Intracellular staining (for HIF-2α and phosphorylated β-catenin) were completed by fixing the cells in 10% formaldehyde for 30 min, followed by a 30 min ethanol permeabolization and a BSA block. For all staining, primary antibodies were used at 1 µg per sample and all experiments included matched isotype controls. Following incubation in secondary antibody (Alexa fluor 488 or 555), cells were analyzed using a FACScalibur flow cytometer (BD, Franklin Lakes, NJ) with 10,000 events collected. Remaining cells were observed by confocal microscopy. Confocal images were acquired on a Zeiss LSM510 confocal system attached to an AxioImager Z1 microscope using 40× and 63×/1.3 oil objective and the 405, 488, 543 and 633 nm lasers. DAPI was used as a nuclear stain. The images were acquired and processed using the Zeiss LSM510 software version 3.21 (Carl Zeiss, Thornwood, NY) [20]. To assess viability, cells were enumerated via trypan blue exclusion, or stained with Annexin V FITC (R&D systems, Minneapolis, MN) and analyzed by flow cytometry (FACS). Flow cytometric data were analyzed using Win Midi software.
Immunohistochemistry
Bone marrow core biopsy samples were formalin-fixed, lightly decalcified and paraffin-embedded with samples representative of Ph+ and Ph− patients at either primary or relapse stage of disease as indicated. Five micron sections were hand stained using ducal breast carcinoma as control tissue. In brief, the histologic sections were deparaffinized and antigen retrieval was performed by heating in a tris based buffer for 30 min. The primary antibody (mouse monoclonal anti-human VE-cadherin, clone BV6; Millipore Billerica, MA) was applied at a dilution of 1:10 for 24 h; negative controls used a 1:10 diluted mouse IgG2 matched isotype (BD Biosciences, San Jose CA). The slides were then incubated for 12 min with a conjugated secondary antibody and counterstained with hematoxylin. The slides were then analyzed using routine light microscopy and all images shown are 400×.
Reverse Transcriptase (RT-PCR)
Total RNA was isolated from leukemic cells using the Micro-to-Midi Total RNA Isolation kit (Invitrogen). Primers for HIF-2α (EPAS1, PPH02551B), VE-cadherin (CDH5, PPH00668E) and actin, as a housekeeping gene control, were purchased from Superarray (Fredrick, MD). PCR conditions were in the linear range of amplification. Images were cropped to show only data relevant to this paper. However, all comparison data were run on the same gel.
Constitutive Expression of VE-cadherin
Cloning of human VE-cadherin (CDH5) into pLenti6.2-DEST/V5 (Invitrogen), and generation of the pLenti6.2-DEST/V5 empty vector control and lentiviral particles has been described [20]. To generate lentiviral particles the pLenti6.2-Dest/V5 vectors were transfected into 293FT packaging cells and viral particles were collected, filtered, and concentrated as described by Burns et. al. [32]. Lentiviral stocks were titered with HT1080 (ATCC CCL-121) using the blasticidin-resistance colony assay. To generate REH cell lines stably expressing empty vector (REHvect), or VE-cadherin (REHCDH5), cells were infected with lentiviral supernatants with an MOI of 1. Clones stably expressing the genes of interest were selected by blasticidin (3 µg/mL) and expression was confirmed by quantitative real time RT-PCR, flow cytometry and western blot.
Western Blot Analysis
Anti-GAPDH (1:10,000) and anti-VE-cadherin (1:200) antibodies were purchased from Research Diagnostics Inc., (Flanders, NJ). Anti-β-catenin (1:200) and anti-phosphorylated CrkL (1:1,000) antibodies were purchased from Santa Cruz (Santa Cruz, CA) and Cell Signaling Technology, Inc. (Danvers, MA), respectively. Leukemic cells were lysed in complete cell lysis buffer, protein concentrations were determined and westerns were performed as described by Wang et al. [20]. Signal was visualized with enhanced chemiluminescence reagents (Amersham, Pharmacia Biotech, Piscataway, NJ). Images were cropped to show only data relevant to this paper. However, all comparison data were run on the same gel.
Statistics
Where appropriate, data were analyzed using the Students-t test or ANOVA with statistical significance of p ≤ .001 denoted by “#”, p ≤ .01 by “*”, and p ≤ .05 by “+”. Annexin experiments were not merged into one data set, but rather representative data from a single experiment are presented that are indicative of the consistent trend. As such, no error bars are shown. While absolute values between experiments may differ based on slightly different tumor cell viabilities at the start of the experiment, the magnitude and trend of the response is consistent.
Results
VE-cadherin is Expressed in Primary, Patient Derived ALL Cells
Several studies have documented the unexpected presence of VE-cadherin in aggressive types of tumors [20, 22, 33–35]. Wang et al. showed that VE-cadherin was present in a panel of Philadelphia chromosome positive (Ph+) cell lines that express a stem cell like phenotype [20]. To further investigate our previous findings, primary patient derived Ph+ and Ph− ALL cells, obtained from leukapheresis, were assessed for the presence or absence of VE-cadherin and HIF-2α, a known regulator of VE-cadherin. Transcripts for VE-cadherin and HIF-2α were detected in the Ph+ patient leukapheresis cells while patient cells lacking the Ph translocation had minimal levels of both VE-cadherin and HIF-2α mRNA (Fig. 1a). Consistent with the gene expression profiles, Fig. 1b shows that VE-cadherin and HIF-2α proteins were expressed on the Ph+ patient cells (black line) but were negligible on the Ph− patient cells (gray line). Additionally, 11/12 patient bone marrow core biopsies were positive for VE-cadherin, with four representative samples shown (Fig. 1c and Table 1). Although IHC does not allow determination of the precise localization of VE-cadherin, the results are consistent with intracellular flow cytometry staining of Ph− and Ph+ ALL following paraformaldehyde fixation of cell lines in which expression of VE-cadherin was detected intracellularly (unpublished data) while only being consistently detected on the surface of Ph+ tumor lines and primary cells. These data demonstrate that the expression patterns and localization of VE-cadherin and HIF-2α in Ph+ primary patient cells correlate with representative cell lines and suggest that SUP-B15 cells are a relevant model of Ph+ ALL.
Patient derived ALL cells express VE-cadherin and HIF-2α. a RNA was isolated from 5 × 106 patient derived cells and RT-PCR was performed for HIF-2α, VE-cadherin or actin. b 1 × 106 patient derived Ph+ (black line) and Ph− (gray line) cells were obtained from leukapheresis and stained to detect HIF-2α (intracellular), VE-cadherin (surface) , or matched isotype control and analyzed by FACS. c Ph+ and Ph− primary patient bone marrow core biopsies were stained for VE-cadherin or matched isotype control. 11/12 samples were positive for VE-cadherin and four representative samples are shown. All samples were positive for CD19, CD10, CD22, HLADR and TDT
BMSC Maintain Expression of Ph+ ALL VE-cadherin During Treatment
It has been previously reported that VE-cadherin protein levels are decreased by treatment with Imatinib Mesylate (IM) without significantly altering cell viability in the absence of additional stress, such as chemotherapy [20, 36]. However, the mechanism that underlies this decrease, and the contribution of bone marrow stromal cells (BMSC) to blunting this decrease, are unknown. To determine if the decrease in VE-cadherin occurs at the transcriptional level, and if BMSC can offset reduced expression, SUP-B15 cells were co-cultured with BMSC in the presence of IM, or DMSO control, for 24 h. The relative abundance of VE-cadherin transcripts were decreased by treatment with IM, but co-culture with BMSC blunted the reduction (Fig. 2a, left panel). In contrast, the HIF-2α transcript was not affected by IM exposure (Fig. 2a, right panel).
Bone marrow stromal cells maintain Ph+ ALL expression of VE-cadherin during Imatinib treatment. a SUP-B15 cells were cultured in media alone or co-cultured with BMSC and subsequently treated with 10 µM Imatinib Mesylate (IM). RT-PCR was performed for VE-cadherin, HIF-2α and actin. b SUP-B15 cells were exposed to DMSO as the control solvent, or to 10 uM IM, for 24 h. HIF-2α and VE-cadherin proteins were evaluated by FACS. c SUP-B15 cells were cultured in either media alone, in contact with BMSC, or in contact with BMSC and Imatinib for 24 h. Tumor cells were subsequently fixed with 4% PFA, permeabilized with 0.5% Triton-X 100 and stained with anti-VE-cadherin antibody or a matched isotype control for evaluation by confocal microscopy (images shown are 63× with no zoom) or following the standard protocol described in materials and methods for flow cytometric analysis. d SUP-B15 cells were cultured in media alone, or in the presence of BMSC for 24 h, and subsequently treated with 0.1 mg/ml DNR overnight. Cells were collected, lysed and western blots were completed to analyze Bcr/abl activity as well as VE-cadherin and β-catenin expression. GAPDH was used as a loading control. Loading of gels is as follows: Lane 1 is media alone, Lane 2 is DNR treatment, Lane 3 is co-culture with BMSC and Lane 4 is co-culture with BMSC and DNR treatment
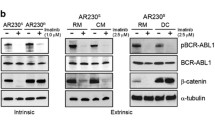
The mechanisms by which HIF-2α, an upstream regulator of VE-cadherin, is regulated in Ph+ ALL, potentially through Abl kinase activity, has not yet been elucidated. Data from our laboratory shows that the activity of Abl kinase can be increased, and maintained during cellular stress, by bone marrow stromal cells (BMSC) and can physically interact with VE-cadherin and β-catenin [20]. Interestingly, Akt and Erk, both established regulators of HIF-2α, can be regulated by both Abl kinase and the stromal cell niche [12, 37–39]. Therefore, we investigated if HIF-2α and VE-cadherin were regulated by Abl kinase activity in Ph+ ALL cells. SUP-B15 cells were treated with Imatinib with HIF-2α and VE-cadherin protein expression analyzed by FACS (Fig. 2b). Inhibition of Abl kinase resulted in diminished levels of HIF-2α and VE-cadherin protein without significantly altering cell viability when no additional stress was applied to the cells, suggesting a link between Abl kinase activity and regulation of the levels of VE-cadherin and HIF-2α. The specific mechanism between Abl activity and HIF-2α remain to be determined in studies that are beyond the scope of this manuscript and may have relevance in the setting of physiological levels of Abl kinase as well in tumors that do not harbor the Bcr/Abl translocation. We subsequently evaluated the ability of BMSC contact to modulate Imatinib-induced down regulation of VE-cadherin at the protein level, and found that consistent with the observation at the mRNA level, Imatinib induced a modest down regulation in VE-cadherin protein that was partially offset by the presence of BMSC during treatment as demonstrated by flow cytometric analysis and confocal microscopy (Fig. 2c; top and bottom panels respectively). Due to the observations that Abl kinase activity can be increased by BMSC, and that VE-cadherin expression can be maintained by BMSC during IM treatment, we investigated the ability of BMSC to maintain VE-cadherin, phosphorylated CrkL (as an indicator of Abl kinase activity) and β-catenin (a pro-survival factor) during chemotherapy. Figure 2d shows that during treatment with Daunorubicin (DNR) the levels of all three proteins are reduced in leukemia cells in the absence of BMSC cues. However, BMSC co-culture increases the levels of all three proteins and maintains the increase in the presence of chemotherapy (Fig. 2d). These data suggest that HIF-2α and VE-cadherin protein expression is regulated by Abl kinase activity, with BMSC contact maintaining the expression of Abl kinase, VE-cadherin and β-catenin during treatment.
The Bone Marrow Microenvironment Regulates HIF-2α
We have shown that leukemic cell Bcr/Abl kinase activity, as well as β-catenin and VE-cadherin, can be maintained by bone marrow stromal cells (BMSC), even in the presence of chemotherapy (Fig. 2) and we have previously shown a physical interaction between Bcr/Abl, β-catenin, VE-cadherin [20]. The current study, and previous publication, demonstrates that in addition to a physical interaction, a signaling cascade may occur between Bcr/Abl and subsequent stabilization of HIF-2α, VE-cadherin and β-catenin (Fig. 2d) [20, 40]. However, the modulation of HIF-2α by BMSC has not been investigated. Therefore, we determined the influence of BMSC on HIF-2α expression. LTMC and LTCC SUP-B15 cells were cultured in media alone or with BMSC. HIF-2α message and protein expression were analyzed by RT-PCR and FACS, respectively. Data presented in Fig. 3a show that in both media alone and during co-culture with BMSC, the message levels of HIF-2α remain constant. However, HIF-2α protein is increased by co-culture with BMSC, regardless of whether ALL cells were originally from LTMC or LTCC conditions (Fig. 3b). Consistent with this observation, VE-cadherin levels increase with BMSC contact as well (Fig. 2c) [20]. Additionally, when the baseline HIF-2α expression in LTMC and LTCC cells was compared, by placing SUP-B15 cells from LTMC or LTCC in media for 24 (Fig. 3c), or 120 h (data not shown), the LTCC derived Ph+ ALL cells had higher levels of HIF-2α. This was intriguing as the LTCC cells also have increased resistance to treatment compared to the LTMC cells, and have higher levels of VE-cadherin at baseline [20] (data not shown). To further substantiate the functional significance of BMSC increasing HIF-2α protein levels, Oct-4, a stem cell marker and direct transcriptional target of HIF-2α, was evaluated and shown to increase in the presence of BMSC (Fig. 3d).Taken together, these data suggest that BMSC increase HIF-2α protein, as well as targets of HIF-α, Oct-4 and VE-cadherin, in our model.
Bone marrow stromal cells modulate HIF-2α. a & b LTMC and LTCC SUP-B15 cells were cultured in media alone or in the presence of BMSC. HIF-2α mRNA and protein were evaluated by RT-PCR and FACS, respectively. c LTMC and LTCC cells were placed in media for 24 h and their baseline expression of HIF-2α was evaluated by FACS. d SUP-B15 cells were cultured in media alone or in the presence of BMSC and evaluated for expression of Oct-4
Modulation of VE-cadherin Reduces Chemotherapy-induced Apoptosis
To further investigate the roles of HIF-2α and VE-cadherin in regulation of apoptosis, SUP-B15 cells were treated with siRNA specific to HIF-2α, VE-cadherin, or scrambled control sequence. After 72 h, efficiency of knockdown was determined by FACS (Fig. 4a). The labels above the histogram represent the specific siRNA utilized for that experiment compared to the scrambled (Scr) control while the labels on the X axis correspond to the staining detected for either HIF-2α or VE-cadherin in those cells. HIF-2α has been shown to directly regulate the transcription of VE-cadherin and as expected, when HIF-2α was down-regulated, VE-cadherin decreased as well (Fig. 4a top panel). Surprisingly, when VE-cadherin was down-regulated, slightly less HIF-2α protein was detected (Fig. 4a bottom panel). There are potentially intermediate regulators in this pathway, along with the possibility that HIF-2α is acting directly as a transcription factor for VE-cadherin gene expression, as documented in studies from other laboratories [27]. Following down-regulation of HIF-2α or VE-cadherin, SUP-B15 cells were treated with Imatinib Mesylate (IM), Daunorubicin (DNR) or a combination of IM/DNR and viability was evaluated. Cells in which either VE-cadherin or HIF-2α were down-regulated were more susceptible to chemotherapy-induced death than their controls, and VE-cadherin down-regulation rendered the cells susceptible, even when co-cultured with BMSC (Fig. 4b and c).
Modulation of VE-cadherin results in altered chemosensitivity. a SUP-B15 cells were transiently transfected with scrambled, HIF-2α, or VE-cadherin siRNA and conformation of down-regulation was determined using FACS. b & c siRNA transfected cells were subsequently cultured in media alone or in the presence of BMSC and exposed to 10 uM IM or DMSO and 0.01 mg/ml DNR. Cell viability was determined by trypan blue exclusion and Annexin-V-FITC
To confirm the potential role of surface VE-cadherin in modulation of therapeutic response, Ph−/surface VE-cadherin- REH cells were transduced with a lentiviral vector containing full length VE-cadherin (REHCDH5) or empty vector control (REHVect). Western blot and FACs analysis confirmed expression of VE-cadherin (Fig. 5a, data not shown). REHCDH5 and REHVect expressing cells were challenged with DNR and viability was evaluated. Ph− REH cells expressing surface VE-cadherin show a modest, but statistically significant, increase in survival during chemotherapy compared to their VE-cadherin negative vector control (Fig. 5b).
Expression of VE-cadherin decreases leukemic cell sensitivity to chemotherapy. a Ph−/surface VE-cadherin- REH cells were transduced with virus containing empty vector control (REHVect) or wild type CDH5 (REHCDH5). Western blot was completed to demonstrate expression of VE-cadherin. b REHVect and REHCDH5 cells were challenged with 0.6 mg/ml DNR for 48 h and viability was evaluated by trypan blue exclusion
Disruption of VE-cadherin Signaling as a Therapeutic Target
To address the role of VE-cadherin as a potential therapeutic target in Ph+ ALL, ADH100191 (ADH), a specific peptide inhibitor directed against the surface cell adhesion recognition sequence of the VE-cadherin extracellular domain, was utilized. SUP-B15 cells were pre-treated with 1 mg/ml ADH for 6 h prior to treatment with Daunorubicin (DNR). ADH pretreated Ph+/surface VE-cadherin+ leukemic cells were more susceptible to DNR as shown by trypan blue exclusion and Annexin-V-FITC staining (Fig. 6a). This effect was consistent and was partially sustained in the presence of BMSC (Fig. 6b). In experiments where all cells shown were treated with chemotherapy, ADH/IM, in combination with DNR, increased the sensitivity of the Ph+ cells in media alone with the increased sensitivity sustained in the presence of BMSC (Fig. 6c). In contrast, Ph−/surface VE-cadherin- REH cells pre-treated with ADH100191 (ADH) prior to Daunorubicin (DNR) had no increase in response to chemotherapy, demonstrating the specificity of the ADH for surface VE-cadherin positive cells poised to respond to a signaling antagonist (Fig. 6d). Due to the specificity of the VE-cadherin antagonist ADH100191 (ADH) for surface VE-cadherin, and the ability of VE-cadherin to be endocytosed from the membrane, we evaluated the expression of surface VE-cadherin in the presence of ADH100191 (Fig. 6e).
Disruption of VE-cadherin signaling using the VE-cadherin antagonist ADH increases Ph+ ALL sensitivity to apoptosis. a & b SUP-B15 were cultured in media alone or co-cultured with BMSC for 24 h. The cells were then pre-treated with 1 mg/ml ADH, or media control, for 6 h and subsequently challenged with chemotherapy (0.1 mg/ml Daunorubicin (DNR)/24 h). Viability was determined by trypan blue exclusion and Annexin-V-FITC. c SUP-B15 cells we cultured in media alone or in the presence of BMSC for 24 h, pre-treated with either media/DMSO, IM, ADH or a combination of IM & ADH for 6 h prior to treatment with DNR. Cell viability was then determined by trypan blue analysis. Statistical significance is shown comparing both the SUP-B15 on BMSC treated with DNR to the IM/DNR, ADH/DNR or IM/ADH/DNR groups as well as the comparison between the IM/DNR or ADH/DNR to IM/ADH/DNR. d Ph− REH cells were pre-treated with 1 mg/ml or 2 mg/ml ADH for 6 h and subsequently treated with 0.6 mg/ml DNR. Viability was determined by Annexin-V-FITC. e SUP-B15 cells were treated with media alone or with 1 mg/ml ADH for 24 h and surface stained for VE-cadherin
Our laboratory has previously shown physical interaction between Bcr/Abl, VE-cadherin and β-catenin in Ph+ cells, a stabilization of β-catenin in cells expressing all three proteins, and the ability of BMSC to increase their expression and maintain expression during treatment (Fig. 2c) [20]. We have additionally determined that Ph+ cell lines have higher baseline levels of β-catenin compared to Ph− cell lines (unpublished data). Therefore, we sought to determine if the mechanism by which VE-cadherin signaling influences response to cytotoxic agents was potentially β-catenin mediated. Treatment of SUP-B15 cells with ADH100191 (ADH) for 6 h showed an increase in the amount of Ser(33,37)/Thr(41) phosphorylated β-catenin, characteristic of that targeted for degradation, compared to untreated control cells (Fig. 7a). Additionally, after treatment with the VE-cadherin antagonist ADH for 24 h there was a decrease in total β-catenin protein detected (Fig. 7b).
Inhibition of VE-cadherin signaling leads to targeted degradation of β-catenin. a SUP-B15 cells were treated with 1 mg/ml ADH or media control for 4 h and subsequently stained for phospho-β-catenin (Ser33/37 and Thr41) or matched isotype control. Cells were analyzed by confocal microscopy (images shown are 40× with zoom). b SUP- B15 cells were treated with 1 mg/ml ADH or media control for 24 h and analyzed by western blot to observe changes in total β-catenin. Samples shown were run on the same gel, in outer lanes, and therefore the image was cut to show only bands relevant to the current study
Discussion
In the current study we characterize the impact of VE-cadherin on the sensitivity of Philadelphia chromosome positive (Ph+) ALL cells to Daunorubicin (DNR) and Imatinib Mesylate (IM) in vitro. While VE-cadherin surface expression in this unique and difficult to treat, sub-type of ALL, and its expression in a panel of patient samples, was somewhat surprising initially, it is not the first description of its expression in non-endothelial cells. Previous studies have shown the expression of VE-cadherin in fetal liver cells with expression subsequently lost in adult stem cells and not present on the surface of B-lineage cells [41, 42]. Recent studies have also demonstrated that VE-cadherin is induced during epithelial-to-mesenchymal transition (EMT) in mammary tumor cells [35], contributes to vascular mimicry in highly aggressive melanoma [43, 44] is required for successful endovascular invasion and normal placentation in cytotrophoblast stem cells [45], is involved during trans-endothelial migration of metastatic cancer cells and is up-regulated in neighboring vasculature during tumor induced angiogenesis [46–49], and is a critical regulator of TGF-β signaling in endothelial cells [50]. Therefore, it is tempting to speculate that the surface expression of VE-cadherin in Ph+ ALL cells would position these tumors to better interact with signals from the microenvironment that promote tumor cell survival, including TGF-β. Interestingly, unpublished data from our laboratory observed that BMSC knock down of TGF-β diminishes the ability of bone marrow stromal cells (BMSC) to protect B lineage leukemic cells from treatment.
Implications of VE-cadherin as a mediator of aggressive phenotype underscore our interest in understanding its regulation by the microenvironment in which ALL cells thrive. One upstream factor of interest, based on its stabilization by microenvironment-derived cues, and its influence on VE-cadherin expression, is HIF-2α which can respond to low oxygen levels but also regulates VE-cadherin independent of hypoxia [27]. Therefore, HIF-2α supported VE-cadherin expression would allow for homotypic tumor interactions as well as interactions with endothelial cells in bone marrow niches or other anatomical locations that include VE-cadherin positive cells. The expression of high levels of both HIF-2α and VE-cadherin in primary ALL cells is consistent with the literature describing the role of these two proteins in other aggressive tumor models (Fig. 1) [23, 26, 33–35, 46, 51]. Although one caveat of staining formalin fixed bone marrow core biopsy samples is the lack of specific cellular protein localization determination, it is intriguing to detect expression of VE-cadherin on the majority (11/12) of samples and on the surface of Ph+ leukapheresis samples (Fig. 1). While the interpretation of the IHC staining is limited, it supports the assertion that VE-cadherin protein expression is observed in primary B lineage ALL, with the potential that it is stabilized and regulated uniquely in the context of high Abl kinase activity characteristic of Ph+ ALL. Accumulation of high levels of VE-cadherin may result in stabilization of β-catenin and transcription of downstream targets as we have previously published via binding to the TCF/LEF consensus sequence [20] while also being presented on the cell surface where it may respond to inhibition via signaling antagonists such as ADH.
Data presented in the current study also show that forced surface expression of VE-cadherin in Ph− cells significantly increased their viability during treatment (Fig. 5). In our model, bone marrow stromal cells (BMSC) modulated and maintained HIF-2α, Oct-4 and VE-cadherin in Ph+ ALL cells (Figs. 2, 3) [20]. Our data suggests that the signaling pathway relies on active Abl kinase influencing HIF-2α, VE-cadherin and subsequently β-catenin (Figs. 2 and 7) with potential intermediate effector proteins still to be determined. Importantly, the targeted inhibition of either HIF-2α or VE-cadherin, during chemotherapy exposure in vitro, significantly diminished cell viability even in the presence of BMSCs (Figs. 4 and 6) suggesting relevance in the context of the marrow microenvironment in which leukemic cells are often refractory to therapy, at relapse in particular. Somewhat speculative in nature, but worth consideration, is the possibility that BMSC cues influence tumor resistance to therapy, in part, by maintaining proteins associated with a tumor stem cell phenotype that is coincident with resistance. HIF-2α has been documented to increase expression of at least two factors relevant to the stem like phenotype of the Ph+ ALL cells in this study; Oct-4 (Oct-3/4, Pou5F1) [52] and VE-cadherin [27] under hypoxic and normoxic conditions, respectively. Of note, evaluations of anatomical regions within the marrow that support hematopoietic stem cells were localized predominantly to areas of low oxygen perfusion [53]. Recent literature suggests that over expression of HIF family members, and particularly HIF-2α, promoted differentiation of BMSC to the endothelial lineage [28]. The hypoxic nature of the stem cell niche in the marrow emphasizes the need to consider the influence of hypoxia on the expression and activity of factors, including the hypoxia inducible factors (HIF-1α and HIF-2α), that can regulate genes that sustain tumor cell survival, proliferation, and self-renewal.
HIF target specificity has been shown to originate through its N-terminal trans-activation domain and distinct target genes have been identified for these two factors, with little functional redundancy even though they have the potential to bind comparable DNA motifs [54]. The co-factors that contribute to their specificity of binding and gene regulation appear to be cell, tissue, and “circumstance” specific, and investigation of the details are underway to better understand these important factors in regulation of growth, survival, and differentiation. Relevant to our model is the unique role of HIF-2α in support of VE-cadherin expression, and therefore its potential importance as a transcription factor that may regulate therapeutic response. Further investigation will determine if BMSC derived cues impact on binding partners of HIF-2α that may influence its effect on ALL cells beyond its role as a transcription factor for VE-cadherin.
Exciting reports have recently emerged describing the use of diverse agents, including the microtubule destabilizing agent CA4P, to selectively disrupt VE-cadherin in endothelial cells [55, 56]. CA4P was shown to increase tumor cell apoptosis without negatively affecting smooth muscle cells or the normal vasculature, highlighting its promise as a therapeutic agent. Our recent observations suggest that, in general, agents designed to disrupt vasculature and tumor angiogenesis, particularly those that target VE-cadherin, may have previously unappreciated benefit through their direct effects on hematopoietic tumor cells while not damaging normal cells or the stem cell pool [41, 42]. Our utilization of the VE-cadherin antagonist, ADH100191 (ADH), demonstrates the importance of VE-cadherin in Ph+ ALL cells, with the VE-cadherin antagonist having no effect on Ph− ALL cells (Fig. 6d) while decreasing the viability of Ph+ cells during chemotherapy (Figs. 6 and 7). The fact that hematopoietic tumor cells lacking surface VE-cadherin were spared any effect by ADH100191 underlies speculation that normal hematopoietic cells might also be unaffected by this agent making it a potentially useful chemo-sensitization therapy. It also suggests that while the majority of both Ph+ and Ph− primary cells expressed VE-cadherin protein when a “total” protein IHC approach was utilized, the efficacy of VE-cadherin antagonists may be logically limited to those tumors in which the VE-cadherin is presented on the cell surface. The modulation of β-catenin in response to inhibition of VE-cadherin in our model of lymphoid leukemia (Fig. 7) is consistent with a recent report documenting that β-catenin is essential for survival of Ph+ myeloid leukemic stem cells [57, 58]. Although the changes in viability when VE-cadherin signaling is inhibited are quantitatively modest, they remain relevant in the context of the significant proliferative potential of a small residual tumor load in the setting of aggressive leukemia which may subsequently contribute to relapse of disease.
Potentially, signals from the bone marrow microenvironment culminate on targets that are also impacted by the hypoxic nature of protective niches. Sustained expression of surface VE-cadherin in Ph+ ALL cells, supported by signals inherent to the tumor such as high Abl kinase activity and through microenvironment cues that influence transcriptional regulators including HIF-2α and β-catenin, provide us a model in which we can begin to investigate the complexity of survival signals in the marrow milieu. From these models we can optimize novel therapeutic strategies designed to target residual tumor cells that are refractory to standard therapy by understanding the critical targets on which survival signals converge. Pathways that we have traditionally considered in the context of a solid tumor cell survival and support of vasculogenesis and angiogenesis warrant further consideration in the biology of hematological malignancies as well.
References
Carroll WL, Bhojwani D, Min DJ et al (2003) Pediatric acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 102–131
Pieters R, Carroll WL (2008) Biology and treatment of acute lymphoblastic leukemia. Pediatr Clin North Am 55:1–20, ix
Hoelzer D (1993) Acute lymphoblastic leukemia—progress in children, less in adults. N Engl J Med 329:1343–1344
Faderl S, Kantarjian HM, Thomas DA et al (2000) Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma 36:263–273
Radich JP (2001) Philadelphia chromosome-positive acute lymphocytic leukemia. Hematol Oncol Clin North Am 15:21–36
Bailey LC, Lange BJ, Rheingold SR, Bunin NJ (2008) Bone-marrow relapse in paediatric acute lymphoblastic leukaemia. Lancet Oncol 9:873–883
Pui CH, Howard SC (2008) Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol 9:257–268
Li S, Li D (2007) Stem cell and kinase activity-independent pathway in resistance of leukaemia to BCR-ABL kinase inhibitors. J Cell Mol Med 11:1251–1262
Jones D, Thomas D, Yin CC et al (2008) Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer 113:985–994
Meads MB, Hazlehurst LA, Dalton WS (2008) The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 14:2519–2526
Mudry RE, Fortney JE, York T, Hall BM, Gibson LF (2000) Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood 96:1926–1932
Wang L, Fortney JE, Gibson LF (2004) Stromal cell protection of B-lineage acute lymphoblastic leukemic cells during chemotherapy requires active Akt. Leuk Res 28:733–742
Hall BM, Fortney JE, Taylor L et al (2004) Stromal cells expressing elevated VCAM-1 enhance survival of B lineage tumor cells. Cancer Lett 207:229–239
Sethi T, Rintoul RC, Moore SM et al (1999) Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med 5:662–668
Hazlehurst LA, Damiano JS, Buyuksal I, Pledger WJ, Dalton WS (2000) Adhesion to fibronectin via beta1 integrins regulates p27kip1 levels and contributes to cell adhesion mediated drug resistance (CAM-DR). Oncogene 19:4319–4327
Aoudjit F, Vuori K (2001) Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 20:4995–5004
Shain KH, Yarde DN, Meads MB et al (2009) Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res 69:1009–1015
Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D (2007) Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117:1049–1057
Nishigaki H, Ito C, Manabe A et al (1997) Prevalence and growth characteristics of malignant stem cells in B-lineage acute lymphoblastic leukemia. Blood 89:3735–3744
Wang L, O’Leary H, Fortney J, Gibson LF (2007) Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood 110:3334–3344
Hendrix MJ, Seftor EA, Hess AR, Seftor RE (2003) Molecular plasticity of human melanoma cells. Oncogene 22:3070–3075
van der Schaft DW, Hillen F, Pauwels P et al (2005) Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res 65:11520–11528
Maynard MA, Ohh M (2007) The role of hypoxia-inducible factors in cancer. Cell Mol Life Sci 64:2170–2180
Akeno N, Robins J, Zhang M, Czyzyk-Krzeska MF, Clemens TL (2002) Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2alpha. Endocrinology 143:420–425
Conrad PW, Freeman TL, Beitner-Johnson D, Millhorn DE (1999) EPAS1 trans-activation during hypoxia requires p42/p44 MAPK. J Biol Chem 274:33709–33713
Qing G, Simon MC (2009) Hypoxia inducible factor-2alpha: a critical mediator of aggressive tumor phenotypes. Curr Opin Genet Dev 19:60–66
Le Bras A, Lionneton F, Mattot V et al (2007) HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene 26:7480–7489
Ben-Shoshan J, Schwartz S, Luboshits G et al (2008) Constitutive expression of HIF-1alpha and HIF-2alpha in bone marrow stromal cells differentially promotes their proangiogenic properties. Stem Cells 26:2634–2643
Jones A, Fujiyama C, Blanche C et al (2001) Relation of vascular endothelial growth factor production to expression and regulation of hypoxia-inducible factor-1 alpha and hypoxia-inducible factor-2 alpha in human bladder tumors and cell lines. Clin Cancer Res 7:1263–1272
Holmquist-Mengelbier L, Fredlund E, Lofstedt T et al (2006) Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10:413–423
Raval RR, Lau KW, Tran MG et al (2005) Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 25:5675–5686
Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK (1993) Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 90:8033–8037
Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE (2001) Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 98:8018–8023
Smith ME, Brown JI, Fisher C (1998) Epithelioid sarcoma: presence of vascular-endothelial cadherin and lack of epithelial cadherin. Histopathology 33:425–431
Labelle M, Schnittler HJ, Aust DE et al (2008) Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor beta signaling. Cancer Res 68:1388–1397
Vrekoussis T, Stathopoulos EN, De Giorgi U et al (2006) Modulation of vascular endothelium by imatinib: a study on the EA.hy 926 endothelial cell line. J Chemother 18:56–65
Atfi A, Abecassis L, Bourgeade MF (2005) Bcr-Abl activates the AKT/Fox O3 signalling pathway to restrict transforming growth factor-beta-mediated cytostatic signals. EMBO Rep 6:985–991
Cortez D, Reuther G, Pendergast AM (1997) The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene 15:2333–2342
Tabe Y, Jin L, Tsutsumi-Ishii Y et al (2007) Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res 67:684–694
Coluccia AM, Vacca A, Dunach M et al (2007) Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J 26:1456–1466
Kim I, Yilmaz OH, Morrison SJ (2005) CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood 106:903–905
Koga H, Sugiyama S, Kugiyama K et al (2005) Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 45:1622–1630
Hendrix MJ, Seftor EA, Hess AR, Seftor RE (2003) Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3:411–421
Seftor EA, Meltzer PS, Schatteman GC et al (2002) Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol 44:17–27
Zhou Y, Fisher SJ, Janatpour M et al (1997) Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 99:2139–2151
Parker BS, Argani P, Cook BP et al (2004) Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res 64:7857–7866
Shih SC, Robinson GS, Perruzzi CA et al (2002) Molecular profiling of angiogenesis markers. Am J Pathol 161:35–41
Weis S, Cui J, Barnes L, Cheresh D (2004) Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 167:223–229
Voura EB, Sandig M, Siu CH (1998) Cell–cell interactions during transendothelial migration of tumor cells. Microsc Res Tech 43:265–275
Rudini N, Felici A, Giampietro C et al (2008) VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J 27:993–1004
Lofstedt T, Fredlund E, Holmquist-Mengelbier L et al (2007) Hypoxia inducible factor-2alpha in cancer. Cell Cycle 6:919–926
Covello KL, Kehler J, Yu H et al (2006) HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 20:557–570
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD (2007) Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 104:5431–5436
Hu CJ, Sataur A, Wang L, Chen H, Simon MC (2007) The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell 18:4528–4542
Vincent L, Kermani P, Young LM et al (2005) Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest 115:2992–3006
Petit I, Karajannis MA, Vincent L et al (2008) The microtubule-targeting agent CA4P regresses leukemic xenografts by disrupting interaction with vascular cells and mitochondrial-dependent cell death. Blood 111:1951–1961
Hu Y, Chen Y, Douglas L, Li S (2009) Beta-catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia 23:109–116
Abrahamsson AE, Geron I, Gotlib J et al (2009) Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA 106:3925–3929
Acknowledgements
This work was supported, in part, by NIH RO1HL056888 (LFG), NIH RO1CA134573 (LFG), and NIH P20 RR016440 (LFG). Image acquisition and data analysis were completed in the WVU Microscope Imaging Facility. Flow cytometry experiments were performed in the West Virginia University Flow Cytometry Core Facility, which is supported in part by NIH grants P20 RR106440 and RR020866. Immunohistochemical analysis was performed by the Pathology Core Facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Leary, H., Akers, S.M., Piktel, D. et al. VE-cadherin Regulates Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Sensitivity to Apoptosis. Cancer Microenvironment 3, 67–81 (2010). https://doi.org/10.1007/s12307-010-0035-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-010-0035-6