Abstract
Purpose
The purpose of our study was to systematize the arterial supply of the talus and characterize the vessel damage occurring in the talus after total ankle replacement. Previous studies exist using vascular injection to visualize the topic [Giebel et al. (Surg Radiol Anat 19:231–235, 1997); Mulfinger and Trueta (J Bone Joint Surg Br 52:160–167, 1970); Peterson et al. (Acta Orthop Scand 46:1026–1034, 1975); Peterson and Goldie (Acta Orthop Scand 45:260–270, 1974)]. The vascularization of the talus has previously been described by various authors [Aquino et al. (J Foot Surg 25:188–193, 1986); Haliburton et al. (J Bone Joint Surg Am 40:1115–1120, 1958); Mulfinger and Trueta (1970); Peterson et al. (1975); Wildenauer (Z Orthop Ihre Grenzgeb 113:730, 1975)]. The plastination method provides excellent intraosseous view of the arterial system and offers a helpful method to demonstrate the influence of the Scandinavian Total Ankle Replacement (STAR) on the blood vessels.
Methods
In a first step, the nutritive foramina were analyzed on 20 macerated cadaver feet. After this, the articular surface was measured with a print using Optosil. The next step was the visualization of the vascularization of the talus using the plastination method. After vascular injection, a STAR was implanted in two specimens and a plastination was done.
Results
The highest amount and density of nutritive foramina were found in the sulcus tali. Using the imprint technique for the examined tali, we found a mean area covered by cartilage of 56.9 % in comparison to the total surface. The deep fin of STAR has the potential to eliminate important blood vessels of the talus.
Conclusion
The plastination methods were useful methods to analyze the arterial supply of the talus. In our study, the STAR showed a dominant influence on the vascularization of the talus. The fin appeared to be too long. A design modification with a short fin could provide the arterial supply, but should be tested biomechanically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
For decades, ankle fusion was the first choice in managing end-stage ankle osteoarthritis, whereas total ankle replacement was scarcely performed. Currently, ankle fusion may still be the standard procedure to manage end-stage ankle osteoarthritis for most patients and most surgeons. However, total ankle replacement seems to have evolved to be an available alternative. For selected patients with accumulating evidence of marked improvement such as impairments, quality of life, pain and function was shown [2, 4, 18, 29]. The current generation of total ankle replacement is uncemented and uses three components with a polyethylene meniscus designed prosthesis. A major problem in these implants is the high revision rate within the first 10 years. The 7-year survivorship of a total ankle replacement was shown to be 78 % (95 % CI: 71–85). The most common reasons for revision were aseptic loosening (39 %, n = 23) and instability (39 %, n = 23) [25].
Previous studies show a high correlation between osteoarthritis of the upper ankle joint and a prematured trauma [28, 30]. Particularly fractures of the talus neck are serious injuries with an influence on talar blood supply. The possibly developing osteoarthritis is caused by avascular necrosis, malunion or intraarticular fracture. With rising severity of fractures, rising influence on talar blood flow and consecutively a higher risk of avascular necrosis has been shown [19].
The knowledge of the arterial supply of the talus is necessary to understand as to which arteries could be destroyed during the implantation of a total ankle prosthesis. Different authors described the arterial supply of the talus using different techniques of visualization. Mulfinger and Trueta [20] described it by utilizing the Spalteholz technique. They classified the arteries into extraosseous and intraosseous vessels. In 1950 in his anatomical study, Wildenauer [31] reported on a periosteal rate providing the blood supply for the talus. His anatomic study describes the extraosseous blood supply on a high level of precision. He delineates an artery of the tarsal sinus and the tarsal canal, which is formed by the talar sulcus and the calcaneal sulcus [31]. According to the work of Dubreul-Chambardel, Wildenauer pointed out that the blood supply in the sinus tarsi comes through branches of the sinus tarsi artery and variable branches of the anterior and lateral malleolar artery. Wildenauer [31] identified an anastomosis of the sinus tarsi artery to a branch of the posterior tibial artery. He defines the difference between the part of the artery which comes from the dorsum pedis to the sinus tarsi as the “Arteria sinus tarsi” and a branch of the tibialis posterior, with origin 1 cm beneath the talocrural joint as “Arteria canalis tarsi”. Furthermore, the origin of the sinus tarsi artery was examined. As a potential origin the A. dorsalis pedis, the tarsal proximal lateral, the malleolaris anterior lateral and the ramus perforans peroneal arteries were named [1]. All authors agree that the three major arteries for blood supply of the talus are the posterior tibial artery, dorsalis pedis artery and the peroneal artery [11, 20, 23, 31].
In their work on talar neck fractures Aquino et al. described the anatomy of the extraosseous and intraosseous arteries according to the work of Mulfinger and Trueta. Following this, the origin of the artery of the tarsal canal is 1 cm proximal to the medial and lateral plantar arteries and the branches come from the posterior tibial artery and a network above the posterior tubercle. The entering points of the branches are the middle of the talus body and according to Wildenauer the artery terminates by anastomosing with the artery of the tarsal sinus. Five millimeter distal the origin of the artery canalis tarsi, the deltoid branch, which supplies the medial periosteal surface, arise and ends by anastomosing with branches from the dorsalis pedis artery over the neck of the talus. Branches of the dorsalis pedis artery run to the neck of the talus at the level of the ankle joint. The supply of the talar head comes from branches of the artery of the tarsal sinus. A plexus over the posterior tubercle area of the talus is the result of an association of branches of the peroneal artery and calcaneal branches of the posterior tibial artery [3, 20].
The intraosseous supply of the head arises from branches of the anterior tibial artery, from branches of the sinus tarsi anastomosis or direct branches of the lateral tarsal artery [20]. The body is supplied usually of four or five branches by the anastomotic artery in the tarsal canal [20].
Mulfinger et al. reported about extensive arterial anastomosis around the ankle between the medial and lateral malleolar extraosseous network, especially between the arteries entering the superior neck and those from the artery of the tarsal canal.
On 40 ft of adult cadavers Murakami examined the deep plantar arteries. He postulated that the lateral and medial tarsal arteries rise from the dorsalis pedis artery as it crosses the navicular bones, but the main stem descends to the proximal ends as the deep plantar branch to the first dorsal interosseous muscle [21].
The aim of our study was to systematize the arterial supply of the talus and characterize the vessel damage occurring in the talus after the implantation of the STAR (LINK Scandinavian Total Ankle Replacement; Waldemar Link GmbH & Co, Hamburg, Germany).
Methods and materials
Eight fresh-frozen lower extremities and 20 macerated tali were used in this study from body donors of the anatomical institute of a university hospital. The wet cadaveric (median age 70; range 69–71 years) and the macerate specimens (median age 73; range 51–95 years) were selected with occurrence of any fracture verified by conventional radiographs. The first step was the identification of the nutritive foramina of 20 tali. Therefore, drawings of the cranial, plantar, medial and lateral view were produced. Identical drawings were used for 20 macerated tali. After the individual drawing, using a transparent paper a distribution scheme could be prepared.
As a next step, the bony articular surface of the talus was analyzed by examining 20 tali. Macerate dry specimens served as material. An impression of the bare area and the area, which is covered by cartilage, was done by applying Optosil® (Heraeus Holding GmbH, Hanau, Germany). After preparing the impression, they were scanned and the surfaces were measured using the software Optimas® 6.5 (Media Cybernetics, Inc., Rockville, USA).
Afterward 6 ft of fresh-frozen cadavers were analyzed. The lumen of the posterior tibial artery was cannulated and irrigated with a common salt solution until it was clear. Subsequently, epoxide resin was injected into the artery. The epoxide resin was stained red and plumb minimum (grain size ≫99 % <0.0063 cm) was added. The grain size of plumb minimum slowed down the movement and only the arterial system was filled antegradely. Furthermore, the radiopacity of the plumb minimum enabled radiographic imaging of the arterial system.
Three to four millimeter slices in the three anatomical planes were done. The slices were degreased, dehydrated and fixed in epoxide resin.
In two other right lower extremities of unfixed cadaver specimens (69 and 71 years, female gender) the influence of the STAR was investigated.
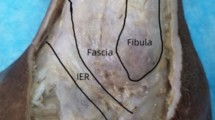
After flushing the arterial system of the two lower extremities with a common salt solution the specimen was fixed by a 1 % formalin solution over the superficial femoral artery. Afterward the same procedure described above was performed using epoxide resin. After the epoxide was finally cured the implantation of the STAR was performed using the original equipment by a competent and experienced orthopedic foot surgeon. An anterior approach was used. After a 25 cm long skin incision centered over the ankle lateral to the anterior tibial tendon, the incision was deepened until the ankle joint was reached. After identification of the superficial branch of the peroneal nerve, the deep peroneal nerve and artery were identified and retracted. Osteophytes were removed until a good visualization of the articular surface was achieved. The handling of instruments and implantation of the prosthesis followed the surgical technique guidelines, and included resection of the distal tibial joint surface and the cranial talar surface.
Finally the plastination was fabricated and the preparation was cut into 2.6 and 3 mm slices by a diamant precision mill.
Results and discussion
The blood supply of the talus is ensured by branches of peroneal artery, the dorsal pedis and posterior tibial artery. These findings appear to be consistent with the findings of Mulfinger or Wildenauer [20, 31]. The highest amount and density of nutritive foramina are found in the sulcus tali between the facies articularis calcanea medialis and the facies articularis calcanea posterior. The gap between them is just 1–2 mm (Fig. 1). In some of the examined feet nutritive foramina were found in the passage of the sinus tali to the talus neck (Fig. 2). They originated from the dorsalis pedis artery or from the posterior tibial artery. In the medial view, some of the foramina nutricia were observed to lie directly beneath the articular surface and at the processus posterior tali (Fig. 3). Nutritive foramina also proved to be located in the dorsal area of the neck of the talus (upper left quarter). The involvement arteries are branches of the dorsal pedis artery and the posterior tibial artery (Fig. 4). In 1904 Sewell described the anatomy of the talus in his studies of more than 1,000 dried specimens. He found vascular foramina on the superior, lateral and inferior surfaces of the neck and on the medial surface of the body with a maximum on the part of the inferior surface of the neck [24]. However, in contrast to his description, our findings are more precise and point out that the area between the facies articularis calcanea medialis and lateralis needs special attention.
In contrast to the most skeletal bones vast majority of the talus is coated with cartilage. With the method of an imprint of the bare area it was possible to examine this. The results of these measures are shown in Table 1. For the examined tali a mean area of the area covered with cartilage in comparison to the total surface of 56.9 %. The results are similar with the findings of Dörenberg [7], who examined the anatomical articular surface. He examined the cartilage surfaces of six ankle joints using a triple dyeing techniques and a pressure of 1,600 N in dorsal extension, in a 0 degree position either in plantar flexion. The largest weight bearing area was found in the 0° position, namely 56.4 ± 4.9 % and the smallest in dorsal extension with 47.1 ± 3.6 %. This shows that the access for the blood supply is very limited.
The plastination method used in our study proved to be an excellent method to map the intraosseous vascular network of the talus. The plastination revealed a vascular network supplying the talus which is filled by the three lower leg arteries. Considering the distribution of the nutritive foramina, the vessels of the lower surface of the talus had the major share of blood supply toward the corpus tali. The dorsal collum tali receive blood of the branches of the anterior tibial and of the dorsalis pedis artery. Intraosseous anastomoses are described by Gelberman and Mortensen [9] between the sinus tarsi and the dorsal collum tali. In the 1930s radiology had developed the possible to show that the tarsal bones are supplied by a dorsal arterial net of periosteal vessels as well as branches of the plantar arteries [33]. In our study, as in the study of Giebel et al. [10], such anastomoses were not found. A part of the branch of the deltoideus artery penetrates deep into the body of the talus and not far from exertions of the tarsal canal artery (Fig. 5). It could be possible that because of the saw cut the anastomosis is not visible any more. Haliburton [11] showed in a plastination study of the intraosseous vessels of the talus that the head is supplied by vessels entering from the superior surface of the neck, the body by vessels which enter antero-inferiorly, and the neck of the talus by vessels which enter through the medial surface below the articular facet.
The presented study shows in accordance to previous studies that the blood supply of the talus tends to be of minor quality [10, 11, 22, 23, 27, 31] (Figs. 6, 7, 8, 9). Our plastination slices show that the three big lower legs arteries build up the vessel network of the talus. The dorsal collum tali get the most supply of branches of the tibialis anterior artery just before it becomes the dorsal pedis artery. These findings magnified the results of previous studies [1, 20, 31]. The findings in our slices are similar to the diagrammatic drawing of Gelberman et al. or of Mulfinger et al. [9, 20].
With this study it is not possible to give a statement to the influence of the surgical approach on the blood supply of the talus. The anterior approach is the most popular approach and must be chosen to implant this prosthesis. According to the knowledge which branches of the three lower limb arteries are important it could be possible to destroy important small vessels of the dorsalis pedis artery in the first steps of the operation.
The results of ankle arthroplasty have generally been disappointing compared to other forms of arthroplasty, because of the unique anatomical structure with several articulating joint surfaces and complex ligamentous attachments [6]. The goal of a total replacement is the recovery to a normal ankle joint motion with a stable situation. The Scandinavian Total Ankle Replacement device (LINK STAR; Waldemar Link GmbH & Co, Hamburg, Germany) is a three-component, porous-coated prosthesis designed to be implanted with an uncemented technique. There are few studies which report clinical results of the STAR [2, 8, 13, 16, 25, 29, 32]. In a population-based study of 515 cases from the Finnish Arthroplasty Register Skyttä et al. examined the results of 217 STAR and 298 AES primary total ankle replacement. During a period of 9 years, 59 revisions were reported and the most common reasons for revision were aseptic loosening (39 %, n = 23) and instability (39 %, n = 23). Furthermore Skyttä et al. [25] found that only 83 % of the implanted prostheses are revision-free after 5 years of follow-up. Fevang et al. [8] reported of a revision rate of up to 76 %. His findings were underlined by Henricson et al. [14], who found an overall survival rate of the implants of 69 % in 10 years. This is in contrast to the study of Carlsson; he presented a 12-year follow-up of 51 single-coated STAR endoprostheses which revealed that 15 ankle joints had undergone fusion. A 5-year follow-up of 58 double-coated STAR endoprostheses showed a clinical survival rate of 98 % with a radiographic survival rate of 94 % [5]. The implantation of a total ankle replacement is a surgically demanding procedure and a long learning curve is well known [2, 5].
One of the biggest goals of a successful implantation of an artificial joint is the osteointegration of the implant. Several studies showed that the osteointegration of the talar component with the talus is difficult [15, 26]. Contrary to this context and the knowledge about the specific blood supply of the talus, our study shows the influence of the fin of the STAR in a plastination model. In addition to this, the slices show the effective contact surface, which is usually not visible because of the cap-shaped design (Figs. 10 and 11).
The achievement of an optimal contact between the surfaces of the talus and the prosthesis is very difficult and could be shown in the plastination (Fig. 8). The topography of the blood supply of the talus is very important for the design of the prosthesis and also regarding the choice of the operative approach. A deep fin could possibly destroy supplying arteries as shown in Fig. 9. It is possible that during the implantation branches of the anterior tibial artery could be destroyed through the surgical approach. In addition by the resection of the articular surface of the talus, the blood supply could be seriously disrupted. With a deeply implanted fin of the cap of the STAR, a destruction of ascending arteries of the tarsal canal seems to be likely; clinically the danger of damage to the microvascular circulation must not be underestimated. Kohonen et al. examined 123 patients who had undergone TAA with the AES implant by computed tomography, concerning the detection of periprosthetic osteolysis after implantation. The study showed at least one large (>10 mm) osteolytic lesion on radiographs in 34.9 % at a minimum follow-up of 14 months. He pointed out that the first major osteolytic lesion on radiographs was noticed as early as 14 months after operation by follow-up investigations at 6 weeks, 2 or 3 months and 1 year after the implantation and between 1 and 2 years [17]. It must be assumed that the osteolytic lesion is either caused by the necrosis or by wear debris. Hawkins [12] mentioned that the aseptic necrosis of the talus is usually secondary to a fracture of the talar neck or dome with interruption of its intraosseous blood supply.
Our study had some limitations. First, we did not prepare an MRI of the ankle before implantation, so it is not possible to give a statement on the vascular situation before implantation, but with the method of plastination we were able to examine the interruption of small vessels as shown above. Furthermore, we were unable to obtain detailed information about the stress of the joint in life time or if there were upper ankle traumata, which could have caused osteolysis.
Conclusion
In conclusion the total ankle replacement poses a particular challenge, because of the unusual distribution of the bare area to the cartilage-covered areas and the poor blood supply of the talus. We were able to demonstrate that the highest amount and density of foramina nutricia were found in the sulcus tali between the facies articularis calcanea medialis and the facies articularis calcanea posterior. The plastination after the implantation of STAR shows the negative influence of the deep fin on the arterial supply. The question rises whether a shorter fin could fulfill the biomechanical requirements and could possibly reduce the number of revisions due to aseptic loosening.
References
Adachi B (1928) Das Arteriensystem der Japaner. Anatomie der Japaner, vol 1. Kaiserlich-japanische Universitaet zu Kyoto, Kyoto
Anderson T, Montgomery F, Carlsson A (2003) Uncemented STAR total ankle prostheses. Three to eight-year follow-up of fifty-one consecutive ankles. J Bone Joint Surg Am 85(7):1321–1329
Aquino MD, Aquino L, Aquino JM (1986) Talar neck fractures: a review of vascular supply and classification. J Foot Surg 25(3):188–193
Bonnin M, Judet T, Colombier JA, Buscayret F, Graveleau N, Piriou P (2004) Midterm results of the Salto total ankle prosthesis. Clin Orthop Relat Res 424:6–18
Carlsson A (2006) Single-and double-coated star total ankle replacements: a clinical and radiographic follow-up study of 109 cases. Orthopaede 35(5):527–532
Dettwyler M, Stacoff A, Kramers-de-Quervain, Stüssi E (2004) Modelling of the ankle joint complex. Reflections with regards to ankle prostheses. J Foot Ankle Surg 10(3):109–119
Dorenberg KO (1983) Contact surfaces and anatomical joint surfaces of the upper ankle joint––methods for the determination of facet size and case representation. Morphol Med 3(2):97–108
Fevang BT, Lie SA, Havelin LI, Brun JG, Skredderstuen A, Furnes O (2007) 257 ankle arthroplasties performed in Norway between 1994 and 2005. Acta Orthop 78(5):575–583
Gelberman RH, Mortensen WW (1983) The arterial anatomy of the talus. Foot Ankle Int 4(2):64–72
Giebel GD, Meyer C, Koebke J, Giebel G (1997) The arterial supply of the ankle joint and its importance for the operative fracture treatment. Surg Radiol Anat 19(4):231–235
Haliburton RA, Sullivan CR, Kelly PJ, Peterson LF (1958) The extraosseous and intraosseous blood supply of the talus. J Bone Joint Surg Am 40(5):1115–1120
Hawkins LG (1970) Fractures of the neck of the talus. J Bone Joint Surg Am 52(5):991–1002
Henricson A, Skoog A, Carlsson A (2007) The Swedish Ankle Arthroplasty Register: an analysis of 531 arthroplasties between 1993 and 2005. Acta Orthop 78(5):569–574
Henricson A, Nilsson JA, Carlsson A (2011) 10-year survival of total ankle arthroplasties: a report on 780 cases from the Swedish Ankle Register. Acta Orthop 82(6):655–659
Knecht SI, Estin M, Callaghan JJ, Zimmerman MB, Alliman KJ, Alvine FG, Saltzman CL (2004) The Agility total ankle arthroplasty. Seven to sixteen-year follow-up. J Bone Joint Surg Am 86((6):1161–1171
Kofoed H (2004) Scandinavian Total Ankle Replacement (STAR). Clin Orthop Relat Res 424:73–79
Kohonen I, Koivu H, Pudas T, Tiusanen H, Vahlberg T, Mattila K (2013) Does computed tomography add information on radiographic analysis in detecting periprosthetic osteolysis after total ankle arthroplasty? Foot Ankle Int 34(2):180–188
Kopp FJ, Patel MM, Deland JT, O’Malley MJ (2006) Total ankle arthroplasty with the Agility prosthesis: clinical and radiographic evaluation. Foot Ankle Int 27(2):97–103
Metzger MJ, Levin JS, Clancy JT (1999) Talar neck fractures and rates of avascular necrosis. J Foot Ankle Surg 38(2):154–162
Mulfinger GL, Trueta J (1970) The blood supply of the talus. J Bone Joint Surg Br 52(1):160–167
Murakami T (1971) On the position and course of the deep plantar arteries, with special reference to the so-called plantar metatarsal arteries. Okajimas Folia Anat Jpn 48(5):295–322
Peterson L, Goldie IF (1975) The arterial supply of the talus. A study on the relationship to experimental talar fractures. Acta Orthop Scand 46(6):1026–1034
Peterson L, Goldie I, Lindell D (1974) The arterial supply of the talus. Acta Orthop Scand 45(2):260–270
Sewell RB (1904) A study of the Astragalus. J Anat Physiol 38(Pt 3):233–247
Skytta ET, Koivu H, Eskelinen A, Ikavalko M, Paavolainen P, Remes V (2010) Total ankle replacement: a population-based study of 515 cases from the Finnish Arthroplasty Register. Acta Orthop 81(1):114–118
Spirt AA, Assal M, Hansen ST Jr (2004) Complications and failure after total ankle arthroplasty. J Bone Joint Surg Am 86(6):1172–1178
Tanaka Y, Omokawa S, Ryu J, Clovis N, Takakura Y (2005) Anatomical consideration of vascularized bone graft transfer from the medial calcaneus to the talus. Clin Anat 18(2):115–120
Thomas RH, Daniels TR (2003) Ankle arthritis. J Bone Joint Surg Am 85(5):923–936
Valderrabano V, Pagenstert G, Horisberger M, Knupp M, Hintermann B (2006) Sports and recreation activity of ankle arthritis patients before and after total ankle replacement. Am J Sports Med 34(6):993–999
Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B (2009) Etiology of ankle osteoarthritis. Clin Orthop Relat Res 467(7):1800–1806
Wildenauer E (1975) Proceedings: discussion on the blood supply of the talus. Z Orthop Ihre Grenzgeb 113(4):730
Wood PL, Karski MT, Watmough P (2010) Total ankle replacement: the results of 100 mobility total ankle replacements. J Bone Joint Surg Br 92(7):958–962
Zchakaja MJ (1932) Blutversorugung der Knochen des Fußes (Ossa pedis). Fortschr Röntgenstr 45:160–176
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oppermann, J., Franzen, J., Spies, C. et al. The microvascular anatomy of the talus: a plastination study on the influence of total ankle replacement. Surg Radiol Anat 36, 487–494 (2014). https://doi.org/10.1007/s00276-013-1219-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-013-1219-9