Abstract
Purpose
Vascularized pedicled bone-grafting from the cuboid to the talus provides low donor site morbidity and satisfactory outcomes in patients with early-stage talar avascular necrosis. We investigated the anatomy of the rotational vascularized pedicled bone graft from the cuboid.
Methods
15 embalmed cadaver specimens were perfused with red latex via the popliteal artery. The lateral malleolus was dissected. The course of the lateral tarsal artery and the vascular territory in the cuboid supplied by the lateral tarsal artery were observed. Vessel diameters were measured.
Results
The course of the lateral tarsal artery to the cuboid was consistent, and a vascularized pedicle of the lateral tarsal artery was present in all specimens. Mean diameter of the lateral tarsal artery was 1.40 ± 0.12 mm (range 1.67–1.25). Mean length of the vascularized pedicle was 67.15 ± 3.18 mm (range 62.43–74.36). The pedicle bone graft was long enough to reach the bony border of both the lateral and medial malleolus.
Conclusion
A vascularized pedicled cuboid bone graft based on the lateral tarsal artery has clinical utility for early-stage talar avascular necrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The talus has a limited blood supply as more than half its surface is covered with articular cartilage. Consequently, the talus is particularly susceptible to avascular necrosis, which can be challenging to treat [4, 6, 7, 11, 13]. In severe talar necrosis, the articular surface may collapse leading to ankle and subtalar joint incongruity, pain, and disability. Tibiotalar or tibiotalocalcaneal arthrodesis can be used as a salvage procedure for severe talar necrosis [1, 4]; however, arthrodesis may increase stress and lead to osteoarthritic degeneration in adjacent foot and ankle joints [2, 15].
Early talar avascular necrosis is best treated with procedures that induce revascularization. Although the blood supply of the talus is limited, there is scope for regeneration and reperfusion [8]. Previous studies have reported successful revascularization of the talus using auto-transplantation of a vascularized bone graft [19, 23]. After treatment, the function of the ankle was almost or completely normal in the majority of patients, and bone repair was excellent [23]. However, surgical revacularization using a vascularized bone graft is time consuming and associated with a high clinical risk. Some procedures, including those using a vascularized bone graft from the iliac crest [8] or medial femoral condyle [17], may damage the donor site. In contrast, vascularized pedicled bone-grafting from the cuboid uses a rotational graft, which avoids free tissue harvest and the need for a microvascular anastomosis that is at risk for disruption.
A previous study reported good results when using a rotational vascularized pedicle bone graft from the cuboid to treat patients with osteonecrosis involving < 60% of the talus [12]. However, discussion of the vascular anatomy of the rotational vascularized pedicled bone graft from the cuboid was limited. We hypothesize that a branch of the lateral tarsal artery supplies a consistent vascular territory in the cuboid, which is well vascularized and easy to harvest for bone-grafting procedures in the talus. The objective of this study was to investigate the anatomy of the rotational vascularized pedicled bone graft from the cuboid to support its clinical application in the treatment of talar avascular necrosis.
Methods
The experimental protocol for this study was approved by the Shenzhen medical and family plan commission. All methods were conducted in accordance with relevant guidelines and regulations of Ethics Committee. Written informed consent was provided by the legally authorized representatives/next of kin of the cadavers included in this study.
This study included 15 embalmed cadaver specimens perfused with red latex supplied via the popliteal artery. Specimens were obtained from the Anatomy Department of the Medical College at Shenzhen University, which granted permission for their use in this study. Prior to dissection, specimens were evaluated using computed tomography (CT) (GE HD 750, scanning layer thickness 2 mm, image resolution 512 × 512 matrix) to exclude specimens with pre-existing trauma, deformity, or bone neoplasms. Veins were not perfused in these specimens.
Dissections were performed under 4.0 loupe magnification and photographed. The origin of the lateral tarsal artery from the dorsalis pedis artery was examined. Vessel diameters were measured with a Digital Vernier Caliper (Lugong Industry, Shanghai, China), with a precision of 0.01 mm.
Graft harvest
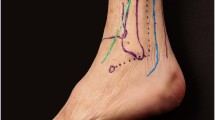
Graft harvest was performed as previously described by Nurley et al. [12]. First, the bony landmarks of the lateral malleolus and fifth metatarsal were marked. An incision was made 6–8 cm from the proximal lateral malleolus to the fifth metatarsal. The fascia of the inferior extensor retinaculum was incised at the lateral side of the extensor hallucis longus (EHL). The proximal part of the target artery could be find near the extensor hallucis brevis (EHB) and extensor digitorum brevis (EDB) fascia. The origin of the lateral tarsal artery was located near the level of talar neck where it branched off laterally from the dorsalis pedis artery. The lateral tarsal artery traveled beneath the EDB and EHB muscle bellies. After elevated the muscle belly, the branch to the cuboid could be found. Before entering the cuboid, the lateral tarsal artery produced branches that perforated the deep fascia (medially) and supplied the muscle bellies (laterally) and the sole of the foot (plantar side). The plantar branch of the lateral tarsal artery anastomosed with the lateral malleolar arteries.
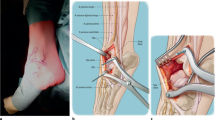
When harvesting a bone graft from the cuboid, the most important landmarks were the inferior borders of the EHB and EDB muscle bellies. After revealing the muscle bellies, the lateral tarsal branch was visualized by stretching the muscles toward the plantar surface. The lateral tarsal artery lay beneath these muscles, and its vascular territory and the cuboid were found in this plane. The vascular territory in the cuboid supplied by the lateral tarsal artery was dissected, and the tibiotalar joint was opened anteriorly. The vascularized pedicle was transferred to the talar bone (Fig. 1).
Anatomical measurements
Based on our observations, we divided the whole course of the lateral tarsal artery into three segments: Segment I, from origin to the muscle bellies of the EHB and EDB. In this segment, the artery gave off few branches and was easy to visualize; Segment II, this segment provided many branches, which supplied structures medially and laterally; Segment III, the transverse pedicle branch, which represented one of the two branches of the lateral tarsal artery at the dorsum of the proximal-medial aspect of the cuboid [3] (Fig. 2). The transverse pedicle branch coursed transversely lateral across the dorsal cortex of the cuboid and provided nutrient vessels to the cuboid.
The length of these three segments was measured and recorded. The number of nutrient branches to the cuboid was recorded. The distribution surface area of these vessels was calculated as length × width. The depth of the cuboid was measured on CT.
Data analysis
Statistical analysis was conducted using PASW18.0 (SPSS Inc. IBM Chicago, USA). Data are expressed as mean ± standard deviation (x ± SD).
Results
Across cadaveric specimens, there were variations in the origin of the lateral tarsal artery, which were classified into 3 types: Type I, the anterior lateral malleolar artery and the lateral tarsal artery originated from the trunk of the dorsalis pedis artery; the lateral tarsal artery had only one main trunk (40%, 6/15). Type II: the anterior lateral malleolar artery and the lateral tarsal artery originated from the trunk of the dorsalis pedis artery; the lateral tarsal artery divided into two main branches (46.7%, 7/15). Type III: the anterior lateral malleolar artery and the lateral tarsal artery originated from the same point on the dorsalis pedis artery (13.3%, 2/15) (Fig. 3).
A vascularized pedicle of the lateral tarsal artery was present in all 15 specimens. Almost all artery branches were accompanied by veins, as other authors reported [3, 10, 12, 14]. Mean diameter of the lateral tarsal artery was 1.40 ± 0.12 mm (range 1.67–1.25). The arterial pedicle included paired venae comitantes. Mean length of the vascularized pedicle was 67.15 ± 3.18 mm (range 62.43–74.36). The pedicled bone graft was long enough to reach the bony border of both the lateral and medial malleolus and possibly the distal fibula and tibia.
There were branches in two of the three segments of the lateral tarsal artery. In segment II, 1–2 branches distributed medially to the tarsal bone, and 2–3 branches distributed laterally to anastomose with the anterior lateral malleolar artery. In segment III (transverse pedicle branch), 2–3 branches distributed medially to the base of the cuneiform. The terminal branch of the transverse pedicle branch anastomosed with the anterior lateral malleolar artery to form a vascular network (Fig. 4).
The vessels in the vascular territory supplied by the transverse pedicle branch had no pattern. The length and width and distribution surface area of the vascular territory on the cuboid bone as well as the depth of the cuboid are summarized in Table 1; these should form the basis for the dimensions of the vascularized pedicled bone graft. The number of branches provided by the lateral tarsal artery is shown in Table 2.
Discussion
This study investigated the anatomy of the vascularized pedicled cuboid bone graft to support its clinical application in the treatment of talar avascular necrosis. Findings confirmed that a branch of the lateral tarsal artery supplies a consistent vascular territory in the cuboid, which is well vascularized and easy to harvest for bone-grafting procedures in the talus.
Avascular necrosis of the talus can be classified according to Ficat [20] as Stage I: normal; Stage II: cystic and/or sclerotic lesions, normal talar contour, no subchondral fractures; Stage III: crescent sign, subchondral collapse; and Stage IV: joint-space narrowing, secondary tibial cysts, osteophytes, arthritic changes. Avascular necrosis that has progressed to Stage III or IV requires fusion because the articular surface cannot be preserved. It should be pointed out that, according to Ficat classification, pedicled bone revascularization can only be considered in regional necrosis of the talus without articular collapse.
The blood supply to the talus is complex, and includes: (1) the anterior tibial artery, which gives off the anterior lateral malleolar artery and the terminal branch and posterior recurrent branch of the lateral tarsal artery. The anterior lateral malleolar artery supplies the talus from the tarsal sinus artery. The terminal branch and the posterior recurrent branch of the lateral tarsal artery anastomose with the peroneal artery, from which the tarsal sinus artery originates. (2) The lateral tarsal artery, which branches off the dorsalis pedis artery and supplies the lateral half of the talar neck. (3) The medial tarsal branches of the dorsalis pedis artery, which supply the supermedial half of the talar neck [14]. (4) The deltoid branches of the posterior tibial artery, which supply the medial talus through the deltoid ligament. (5) The tarsal canal artery, which anastomoses with the tarsal sinus artery where the tarsal canal artery enters the sinus tarsi [5, 22]. As interruption of a single vessel does not cause avascular necrosis of the entire talar head, neck and body, Stage I or II avascular necrosis may be treated with vascularized bone grafts. Pedicled bone graft may provide additional blood supply; veinous reflux may also be achieved by both accompanied vein and cancellous bone in talus [13, 17, 22]. This allows the transfer of bone with viable osteoclasts and osteoblasts and a preserved circulation, which can replace deficient bone, revascularize necrotic bone, and accelerate healing [9, 17].
Previous reports have demonstrated the successful treatment of a partially necrotic talus with a vascularized bone graft from the iliac crest [8], and the management of three cases of Stage II and III talar osteonecrosis dissecans with free vascularized medial femoral condyle auto-transplantation [17]; however, these procedures were limited by substantial morbidity at the donor sites. Subsequently, the calcaneal branch of the posterior tibial artery was proposed as a vascular pedicle to treat medial osteochondral lesions of the talar dome. Pediculate transplantation did not require excessive enlargement of an incision because of the short distance from the donor site to the recipient site [18], but this technique could not be anatomically applied in 10% of the feet and is only relevant to osteochondral lesions of the talar dome [19]. A vascularized periosteal graft from the first metatarsal bone was used to treat Stage III osteonecrosis of the talus in an 11-year-old boy. The approach effectively induced bone revascularization and prevented further collapse of the talar dome. The diaphyseal dorsal periosteum of the first metatarsal was harvested, which required a longitudinal zig-zag dorsomedial incision of the foot, and the risk of occlusion of the vascular pedicle was high as the angle between the first metatarsal branch and the dorsalis pedis artery is relatively small [16].
There is a clinical need for a less-invasive and low-risk approach to revascularization of the talus. Evidence suggests that the lateral tarsal artery has clinical utility as an artery flap [10, 21]. In the present study, the lateral tarsal artery was easy to locate, and the position of the lateral tarsal artery and its branches in the lateral ankle was consistent across cadaveric specimens. In the longest (12 years) follow-up reports, Nunley et al. [12] reported a more than 80% (10–12) successful rate. Only 2 cases needed arthroplasty because of uncontrolled necrosis. The other 10 cases got revascularized and the ankles were preserved. Our anatomical observations confirmed that a vascularized cuboid pedicled bone graft based on the lateral tarsal artery has several advantages compared to the other vascularized bone grafts used for talar avascular necrosis. First, the lateral tarsal artery sends multiple periosteal branches to the cuboid. Second, our measurements confirmed that the lateral tarsal artery has an adequate length and vessel sizes for microvascular anastomoses to provide an excellent vascular pedicle. Third, the cuboid pedicled bone graft was long enough to reach the bony border of both the lateral and medial malleolus and possibly the distal fibula and tibia. Its supplying artery is located on the dorsal side; thus, the pedicle can rotate to either the medial or lateral side and is unlikely to be affected by ankle flexion or extension. Fourth, as potential donor and recipient sites can be confined to a single incision, surgery could be performed under regional anesthesia and avoid potential complications at a donor site in a previously uninvolved limb. Last, the terminal recurrent artery of the lateral tarsal artery sends branches posteriorly to the lateral anterior malleolar artery, which anastomose to create a vascular network. This may provide perforators to the skin and aid in wound healing (Fig. 5).
This experimental cadaveric study demonstrated that a vascularized cuboid pedicled bone graft based on the lateral tarsal artery is easy to harvest; however, attentions must be paid when performing this procedure in the clinical setting. First, the EDB and EHB muscles must be elevated to facilitate dissection, which may cause postoperative pain and weakness leading to loss of toe flexion for a short time. Second, the bony graft was from the cuboid. According to our measurements of the cuboid on CT, the size of the harvested bone should be limited to 2–3 cm (length) × 2 cm (width) × < 1 cm (depth) to include cancellous bone but avoid cuboid fracture or damage to the joint surface. These dimensions may vary between patients because the size of the cuboid may differ. Also, the limit of graft size limits the application of pedicle cuboid revascularization. If the osteonecrosis is large, it will not be suitable for this procedure. Nurley et al. [10, 21] suggest only if patients with osteonecrosis involving < 60% of the talus on the basis of MRI scans met the criterion. Third, pedicle cuboid graft does not contain cartilage, if necrosis involved cartilage, or any kind of articulation collapse, the procedure could not be used for these situations.
This study was associated with several limitations. First, ink-based perfusion of the fresh-frozen specimens was not performed; therefore, the distribution of the perforating vessels of the cuboid could not be described. Second, we did not analyze the number of nutrient foramen on the surface of the cuboid, which is a novel method used to evaluate perforators supplying the bone. Third, we did not review the medical histories of the donors that provided the cadaveric specimens. These medical histories may have included diabetes, nicotine use, or other peripheral vascular disease, which could have altered the efficacy of vessel perfusion and identification. Last, this study included a small sample size of cadaveric specimens.
A vascularized pedicle cuboid bone graft based on the lateral tarsal artery can be harvested for use in early-stage talar avascular necrosis. Vascular anatomy was consistent and bony graft harvest was simple and straightforward in all cadaveric specimens.
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computed tomography
- EHL:
-
Extensor hallucis longus
- EHB:
-
Extensor hallucis brevis
- EDB:
-
Extensor digitorum brevis
References
Dhillon MS, Rana B, Panda I, Patel S, Kumar P (2018) Management options in avascular necrosis of talus. Indian J Orthop 52:284–296. https://doi.org/10.4103/ortho.IJOrtho_608_17
Fuchs S, Sandmann C, Skwara A, Chylarecki C (2003) Quality of life 20 years after arthrodesis of the ankle. A study of adjacent joints. J Bone Joint Surg Br 85:994–998. https://doi.org/10.1302/0301-620x.85b7.13984
Gilbert BJ, Horst F, Nunley JA (2004) Potential donor rotational bone grafts using vascular territories in the foot and ankle. J Bone Joint Surg Am 86:1857–1873. https://doi.org/10.2106/00004623-200409000-00002
Gross CESR, Frank JM, Easley ME, Holmes GB (2016) Treatment of osteonecrosis of the talus. Jbjs Rev 4:e2–e2
Haynes JA, Gosselin M, Cusworth B, McCormick J, Johnson J, Klein S (2017) The arterial anatomy of the deltoid ligament: a cadaveric study. Foot Ankle Int 38:785–790. https://doi.org/10.1177/1071100717702464
He JQ, Ma XL, Zhang X, Xin JY, Li N (2016) Three-dimensional computer-assisted modeling of talus morphology in Chinese patients. Orthop Surg 8:383–392. https://doi.org/10.1111/os.12258
Horst F, Gilbert BJ, Nunley JA (2004) Avascular necrosis of the talus: current treatment options. Foot Ankle Clin 9:757–773. https://doi.org/10.1016/j.fcl.2004.08.001
Hussl H, Sailer R, Daniaux H, Pechlaner S (1989) Revascularization of a partially necrotic talus with a vascularized bone graft from the iliac crest. Arch Orthop Trauma Surg 108:27–29. https://doi.org/10.1007/bf00934153
Jawad MU, Haleem AA, Scully SP (2012) In brief: Ficat classification: avascular necrosis of the femoral head. Clin Orthop Relat Res 470:2636–2639. https://doi.org/10.1007/s11999-012-2416-2
Jia Y, Xu J, Kang Q, Zhang C, Chai Y (2016) Reverse-flow lateral tarsal island flap for covering the great toe donor site of wraparound flap. Ann Plast Surg 77:445–449. https://doi.org/10.1097/sap.0000000000000612
Leduc S, Clare MP, Laflamme GY, Walling AK (2008) Posttraumatic avascular necrosis of the talus. Foot Ankle Clin 13:753–765. https://doi.org/10.1016/j.fcl.2008.09.004
Nunley JA, Hamid KS (2017) Vascularized pedicle bone-grafting from the cuboid for talar osteonecrosis: results of a novel salvage procedure. J Bone Joint Surg Am 99:848–854. https://doi.org/10.2106/jbjs.16.00841
Parr WC, Chatterjee HJ, Soligo C (2011) Inter- and intra-specific scaling of articular surface areas in the hominoid talus. J Anat 218:386–401. https://doi.org/10.1111/j.1469-7580.2011.01347.x
Prasarn ML, Miller AN, Dyke JP, Helfet DL, Lorich DG (2010) Arterial anatomy of the talus: a cadaver and gadolinium-enhanced MRI study. Foot Ankle Int 31:987–993. https://doi.org/10.3113/fai.2010.0987
Schweitzer KMLJ, Davis H (2018) Replacing the fusion: conversion of an ankle arthrodesis to a total ankle arthroplasty. Tech Foot Ankle Surg 17:1
Soldado F, Barrera-Ochoa S, Fontecha CG, Haddad S, Barastegui D, Barber I, Rego P (2013) Vascularized periosteal graft from the first metatarsal bone: a new technique to prevent collapse of osteonecrosis of the talus in children. A case report. Microsurgery 33:56–59. https://doi.org/10.1002/micr.22045
Struckmann VF, Harhaus L, Simon R, Woelfl C, von Recum J, Thiele J, Kneser U, Kremer T (2017) Surgical revascularization-an innovative approach to the treatment of talar osteonecrosis dissecans stages ii and iii. J Foot Ankle Surg 56:176–181. https://doi.org/10.1053/j.jfas.2016.02.012
Tanaka Y, Omokawa S, Fujii T, Kumai T, Sugimoto K, Takakura Y (2006) Vascularized bone graft from the medial calcaneus for treatment of large osteochondral lesions of the medial talus. Foot Ankle Int 27:1143–1147. https://doi.org/10.1177/107110070602701222
Tanaka Y, Omokawa S, Ryu J, Clovis N, Takakura Y (2005) Anatomical consideration of vascularized bone graft transfer from the medial calcaneus to the talus. Clin Anat 18:115–120. https://doi.org/10.1002/ca.20065
Vani PC, Arthi G, Jessy JP, Rani N, Jhajhria SK (2019) Vascular foramina of talus: an anatomical study with reference to surgical dissection. Surg Radiol Anat. https://doi.org/10.1007/s00276-019-02394-6
Wang C, Wang Q, Wang Z, Li G, Yang D (2015) Lateral tarsal artery flap: an option for hypopharyngeal reconstruction in patients with hypopharyngeal carcinomas after surgery. Int J Clin Exp Med 8:4855–4861
Weber M, Bellwald D, Wingenfeld C, Hempfing A, Leunig M (2004) The avascular talus: revascularization in an animal model. Foot Ankle Int 25:151–158. https://doi.org/10.1177/107110070402500308
Zhang Y, Liu Y, Jiang Y (1998) Treatment of avascular necrosis of talus with vascularized bone graft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 12:285–287
Acknowledgements
Not applicable
Funding
This study was supported by a grant from Researching and Developing Founding of People’s Hospital of Peking University (RDH2018-02), and Founding of Shenzhen Health and Family Planning Commission (SZXJ2018085). Founding of Shenzhen Science and Technology Project (JCYJ20180228175315535).
Author information
Authors and Affiliations
Contributions
LB and XZ designed the study and performed most of the investigation and wrote the manuscript; SL did data analysis. LB, YP, and XX did micro-anatomy and measurements. All of the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Written informed consent for participation in the study was obtained from the Legally authorized representatives/next of kin.
Consent for publication
Not applicable.
Ethical approval
The experimental protocol for this study was approved by the Shenzhen medical and family plan commission. All methods were conducted in accordance with relevant guidelines and regulations of Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bai, L., Peng, Yb., Liu, Sb. et al. Anatomical basis of a pedicled cuboid bone graft based on the lateral tarsal artery for talar avascular necrosis. Surg Radiol Anat 43, 1703–1709 (2021). https://doi.org/10.1007/s00276-021-02789-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-021-02789-4