Abstract
Background
This systematic review and meta-analysis aimed to evaluate the effectiveness of radioactive iodine (RAI) remnant ablation for thyroid cancer-related outcomes of patients with papillary thyroid microcarcinoma (PTMC).
Methods
A systematic literature search of PubMed, EMBASE OvidSP, and EBSCO was conducted. Studies were selected that provided multivariable analysis of the effectiveness of RAI ablation or provided specific data of a 10 years history of thyroid cancer-related outcomes in patients that presented with PTMC.
Results
Nineteen studies met the inclusion criteria. A multivariable analysis of the effectiveness of RAI ablation for any recurrence or thyroid cancer-related mortality in patients with PTMC was performed in several studies, among which only one study reported a positive result. Furthermore, for PTMC patients treated by total or near-total thyroidectomy (TT/NT), with or without RAI ablative therapy, the meta-analysis suggested that RAI ablation did not decrease the 10 years history of any tumor recurrence (relative risk [RR] 0.96; 95 % confidence interval [CI] 0.63–1.48; P = 0.87), locoregional recurrence (RR 1.15; 95 % CI 0.75–1.76; P = 0.51), distant metastases (RR 0.32; 95 % CI 0.08–1.32; P = 0.11) or thyroid cancer-related mortality (RR 0.76; 95 % CI 0.22–2.63; P = 0.66).
Conclusions
With regard to multivariable analyses, there was almost no positive treatment effect of RAI ablation noted for patients with PTMC. For PTMC patients already treated by TT/NT, incremental RAI ablation may not be beneficial at decreasing the 10 years recurrence of PTMC or incidence of thyroid cancer-related mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of papillary thyroid microcarcinoma (PTMC), defined by the World Health Organization (WTO) as papillary thyroid cancer (PTC) with greatest dimensions of 10 mm or less [1], has increased dramatically in recent years. [2]. Considerable debate has focused on the clinical significance of PTMC and whether this condition should be managed as aggressively as other thyroid malignancies [3]. The optimal therapeutic strategy, and especially the role of RAI ablation for PTMC remains controversial [4].
Radioactive iodine (RAI) ablation is often administered after total or near-total thyroidectomy (TT/NT), with the intention of ablating any residual thyroid tissue and potential microscopic residual tumor. It is also administered to facilitate long-term surveillance based on serum thyroglobulin (T g) measurement and diagnostic 131I total-body scanning radioiodine [5]. According to several guidelines [6, 7], it is recommended that RAI ablation is modulated on the basis of risk factors irrespective of the tumor size. There is little controversy over the value of RAI for higher risk thyroid cancer [8]. In addition, a systematic review and meta-analysis of the data have demonstrated that RAI ablation might benefit clinical management by decreasing the recurrence of well-differentiated thyroid cancer [9]. However, in a systematic review of a majority of patients presenting with very low-risk, low-risk, and other select cases of patients with moderate risk thyroid cancer; RAI therapy did not demonstrate any survival or disease-free survival benefit [10].
In regard to PTMC, some studies indicate that this condition has after all, the characteristics of a malignant tumor, and is often multifocal and prone to central lymph node metastasis, and thus requires aggressive surgery, and even post-operative RAI ablation [3, 11]. Moreover, some studies indicate that PTMC is mostly an inert carcinoma or has little or no impact on long-term outcome, wherein aggressive surgical treatment, RAI ablation or even any other treatment option is not recommended [12–14]. The many single-center studies described above could not accurately assess the impact of RAI treatment on the final outcome due to the small number of patients and event rates. Nevertheless, up to now, there is currently no special systematic review or meta-analysis of the effectiveness of RAI therapy for PTMC.
Thus, we aimed to carry out a systematic review and meta-analysis of the literature to determine whether RAI ablation decreases either the risk of recurrence or thyroid cancer-related mortality for patients that presented with PTMC, and that did not receive TT/NT surgery.
Materials and methods
The guidelines presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were used in this study [15, 16].
Search strategy
Two trained investigators (i.e., G.H. and W.Z.) independently conducted a search of the following databases: PubMed, EMBASE, OvidSP, and EBSCO for English-language studies that were published between January 1966 and June 2015. The search was conducted using Medical Subject Headings (MeSH) or keywords, and when appropriate as search terms. Search terms were Boolean search criteria and included “papillary thyroid microcarcinoma”, “papillary microcarcinoma”, “thyroid microcarcinoma” OR “PTMC” and “RAI remnant ablation”, “RAI”, “iodine radioisotopes” OR “iodine radioisotopes”. The reference lists of the articles that we retrieved and previous reviews were manually searched to identify and aggregate suitable studies. The last search was performed on June 4, 2015. Detailed search methods are included in Supplementary Table 1.
Eligibility criteria
Two trained reviewers (i.e., G.H. and W.Z.) independently screened all of the retrieved abstracts and titles to determine articles that were “potentially” and deemed “relevant” references. The same two reviewers then independently reviewed the full articles, using the following inclusion criteria: (1) studies that provided multivariable analysis for the benefit of RAI ablation for any recurrence or thyroid cancer-related mortality in patients with PTMC; (2) studies that provided specific data of the 10 years outcome of recurrence or thyroid cancer-related death in patients that presented with PTMC treated by TT/NT, with or without RAI ablation therapy. Studies were excluded if (1) they were single case reports, regular review or systematic review articles; (2) a median or mean follow-up period less than 5 years; (3) a cohort size smaller than 50 patients; (4) studies that lacked a control or non-exposed group; (5) studies that included PTMC that were associated with medullary, or only high-risk or very low-risk PTMC. Disagreements were resolved by consensus discussion. When studies of overlapping groups of patients were identified, only the most recent studies were retained, with the notable exception of earlier studies presenting analyses that were not repeated in the most recent study.
Quality assessment
Two reviewers (i.e., G.H. and L.S.) independently conducted the quality assessment of studies with the Newcastle-Ottawa Scale (NOS) [17, 18]. The NOS was developed to assess the quality of case–control and cohort studies, containing three parameters of quality that included: (1) selection; (2) comparability; and (3) exposure/outcome assessment. Studies that achieved five or more points were considered to be of high quality. Any discrepancies between reviewers were addressed by a joint reevaluation of the original article. In addition, the publication bias was assessed using the funnel plot [19, 20].
Data abstraction
Two trained investigators (i.e., W.Y. and H.W.) independently abstracted the data from the finally included articles. First author’s name, publication year of the article, demographics, pathological characteristics of PTMC, surgical procedure and RAI ablation were extracted from each study. Any multivariable analysis for effectiveness of RAI therapy for tumor recurrence or thyroid cancer-related mortality was tabulated, respectively. Whenever possible, the numbers of patients with any recurrence, locoregional recurrence, distant metastases, and death from thyroid cancer at 10 years following TT/NT treatment with or without RAI ablation were extracted. If the numbers of events were missing, data were extrapolated from graphs, tabulated proportions of events or by subgroup analyses. Any disagreements were discussed to reach a consensus agreement. Studies that provide multivariable analyses or meta-analysis for effectiveness of RAI ablation for 10 years thyroid cancer-related outcomes are included in Supplementary Table 2.
Data analysis
Data from multivariable analyses that were adjusted for prognostic factors were tabulated as presented in the primary studies. An alpha value of P < 0.05 was defined statistically significant for multivariate analyses. Meta-analyses were performed for the 10 years outcomes of any recurrence, locoregional recurrence, distant metastases and thyroid cancer-specific mortality, respectively. The X 2 test and I 2 statistic were performed to assess the statistical heterogeneity of the RAI effect for each treatment outcome. Values of P > 0.10 and I 2 < 50 % suggested that the observed heterogeneity might be accepted, and thus meta-analysis was performed. The relative risk (RR), with a 95 % confidence interval (CI), was used to estimate the RAI effect for 10 years thyroid cancer-related outcomes. All of the statistical analyses were conducted using the RevMan version 5.2 statistical software package (Cochrane Collaboration, RevMan software, Oxford, UK).
Results
Results of the search
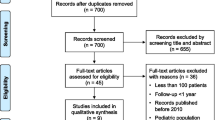
The search strategy above yielded no randomized controlled trials. We obtained 1101 abstracts and titles through electronic searches, and of these, one full-text article was identified and retrieved through manual hand searches. After reviewing the titles, abstracts, and full text, 19 studies met the inclusion/exclusion criteria for this review [21–39] (Fig. 1).
Patient demographic characteristics, tumor pathological findings and details of TT/NT surgery with or without RAI ablation of patients in the included studies are shown in Table 1. The dose of RAI that was administered was unavailable in seven studies [25, 27, 28, 31, 33–35] and ranged from 30 to 150 mCi in the remaining included studies. The use of thyroid hormone suppressive therapy was clearly reported in the majority of studies, with the exception of seven other studies [22, 26, 27, 31, 32, 34, 35]. The mean or median follow-up period in the 19 selected studies ranged from 5.1 to 17.2 years.
Assessment of methodologic quality
The results of quality assessment according to the NOS are included in Supplementary Table 3. In total, the detailed search strategies yielded 19 cohort studies, and 100 percent of the studies were assessed as high quality: nine studies had a NOS score of seven, eight studies had a NOS score of eight, and two studies had a NOS score of nine.
Furthermore, funnel plots for 10 years any tumor recurrence, and locoregional recurrence included in the meta-analysis suggested little publication bias, respectively. Funnel plots for 10 years distant metastases and thyroid cancer-related mortality did not suggest any publication bias (Supplementary Fig. 1).
Summary of multivariable analysis for effectiveness of radioiodine for thyroid cancer-related outcomes
Multivariable analysis of any thyroid cancer-related recurrence is shown in Table 2. In addition, eight of the studies included in the review were subjected to a multivariable analysis of the effectiveness of RAI ablation for any tumor recurrence [21–24, 26, 31, 33, 39]. Post-operative RAI ablation decreased the adjusted risk of any thyroid cancer-related recurrence to just one small study (i.e., 130 patients; P = 0.005) [22]. This positive study had any thyroid cancer-related recurrence rates of 10.8 % with a median follow-up period over 10 years. A positive treatment effect of RAI ablation was not noted in the other six remaining largest studies [21, 23, 26, 31, 33, 39] as well as in one other small study [24]. In addition, another study reported a statistically significant benefit of RAI ablation in decreasing locoregional recurrence (P = 0.01, RR = 0.2, 95 % CI 0.07–0.7) [37].
Multivariable analysis of thyroid cancer-related mortality is shown in Table 3. Four of the included studies in the review performed a multivariable analysis of the benefits of RAI ablation in terms of the outcomes of cause-specific mortality [22, 31, 33, 37]. These four studies examined 130–892 patients, with a median follow-up period that ranged from 8.4 to 17.2 years, with an overall thyroid cancer-related mortality rate that ranged from 0.2 to 3.1 %. None of these studies reported a mortality advantage for the ablated patients, after adjusting for prognostic factors and cointerventions.
Summary of the meta-analysis for effectiveness of radioiodine for 10 years thyroid cancer-related outcomes
Meta-analysis of a 10 years any tumor recurrence is shown in Fig. 2. The numbers of patients with a 10 years any recurrence after TT/NT and treated with or without RAI ablation could be extracted from ten of the studies that were included in the review. Upon pooling the 10 years data for any tumor recurrence of 2295 patients, there was no evidence of any marked statistical heterogeneity (P = 0.10, I 2 = 39 %), thus the results were pooled. The pooled estimate suggested that there was no significant benefit of RAI ablation on any tumor recurrence (P = 0.87). The pooled 10 years any recurrence rates in the selected studies were determined as follows: 49 of 1536 (3.19 %) in the TT/NT- and RIA-treated patients, and 36 of 759 (4.74 %) in the TT/NT-treated patients (relative risk [RR] = 0.96, 95 % confidence interval [CI] 0.63–1.48).
A Forest plot that details the 10 years history of any tumor recurrence. TT total thyroidectomy, NT near-total thyroidectomy, RAI radioiodine ablation. Note: In Hay 2008 [31], the data were extrapolated from the survival curve for node-positive PTMC
Meta-analysis of the 10 years locoregional recurrence is shown in Fig. 3, and the 10 years distant metastases are shown in Fig. 4. Further pooled analyses were performed by examining the effect of radioiodine on the 10 years locoregional recurrence or distant metastases in 11 different studies that were included in the review. Statistical heterogeneities were not significant by either test (P = 0.15, I 2 = 31 %, P = 0.63, I 2 = 0 %, respectively). For the outcome of locoregional recurrence in 2509 patients, the event rate in the TT/NT- and RIA-treated patients was 66 of 1703 (3.87 %) and in TT/NT-treated patients it was 35 of 806 (4.34 %), such that the pooled RR for locoregional recurrence was 1.15 (95 % CI 0.75–1.76) in ablated patients (Fig. 3). For the outcome of distant metastases in 2711 patients, the rate of distant metastases was 5 of 1925 (0.25 %) in TT/NT- and RIA-treated patients as compared with 4 of 786 (0.50 %) in TT/NT-treated patients. Moreover, there was no significant benefit of radioiodine ablation on distant metastases between either group (RR = 0.32; 95 % CI 0.08–1.32; P = 0.11; Fig. 4).
A Forest plot that details the 10 years history of locoregional recurrence of a tumor. TT total thyroidectomy, NT near-total thyroidectomy, RAI radioiodine ablation. Note: In Hay 2008 [31], the data were extrapolated from survival curve for node-positive PTMC
A Forest plot that details the 10 years history of distant metastases. TT total thyroidectomy, NT near-total thyroidectomy, RAI radioiodine ablation. Note: In Hay 2008 [31], the data were extrapolated from survival curve for node-positive PTMC
Meta-analysis of the 10 years thyroid cancer-related mortality is shown in Fig. 5. The numbers of patients with a 10 years thyroid cancer-related mortality after TT/NT with or without RAI remnant ablation could be extracted in 17 of the included studies. The 10 years thyroid cancer-related mortality for PTMC was calculated as 0–3.77 % [35] for TT/NT- plus RIA-treated patients, and from 0 to 5.17 % [22] in TT/NT-treated patients. Significant statistical heterogeneity of the treatment effect was not observed (P = 0.57, and I 2 = 0 %), and thus pooling was performed. The pooled 10 years thyroid cancer-related mortalities in the studies were 4 of 2516 (0.16 %) in the TT/NT- plus RIA-treated patients and 5 of 1502 (0.47 %) in the TT/NT alone treated patients (RR = 0.76, 95 % CI 0.22–2.63; P = 0.66).
Discussion
Our systematic review of the literature revealed that any tumor recurrence was adjusted to a variable degree for prognostic factors or cointerventions in eight cohort studies, among which only one study appeared to have a positive result. Four cohort studies examined the thyroid cancer-related mortality; however, none of them appeared to have a positive result. Demographics and tumor pathological characteristics were adjusted in the majority of the multivariable models. Furthermore, for patients already treated by TT/NT, the pooled analysis suggested that any tumor recurrence, locoregional recurrence, distant metastases, and thyroid cancer-related mortality at 10 years did not decrease with incremental RAI ablation.
It is a remarkable fact that the prevalence of regional nodal metastases in PTMC is known to be high [40, 41]. All studies, with the exception of one article [26] in our review, described a central neck node dissection being performed, which was combined with a lateral cervical node dissection in patients with intraoperative suspect lymph node. In addition, the rates of lymph node metastases ranged from 11 to 43 % in all studies, respectively. Since it was not possible to extract the prognostic data of the PTMC patients with lymphatic metastases, the meta-analysis of this subgroup of patients could not be conducted.
However, our meta-analysis suggested inferentially that in TT/NT therapy with lymph nodes resected, the vast majority of these lymphatic metastases did not progress to clinical recurrence without RAI treatment.
Our systematic review and meta-analysis were subject to some methodologic limitations too. The systematic review yielded totally retrospective cohort studies. Patients were often not stratified into low-risk, intermediate-risk, and high-risk groups in the statistical analysis, and patient demographics and tumor characteristics were inconsistent from study to the next. Furthermore, the multivariate analyses were limited by the inconsistency of independent variables in the model among studies, the lack of adjustment for the cointervention of thyroid hormone suppressive therapy, and particularly, under-powering of studies due to the small sample sizes or event rates. Moreover, with regard to the meta-analysis, about half of the studies lacked a complete 10 years dataset due to the follow-up period being of insufficient time or because subjects were lost to follow-up during the 10 years period after their initial treatment. Although the 20 years data could be abstracted in five studies [21, 22, 26, 28, 31], significant statistical heterogeneity was present when pooling was performed. Our meta-analysis was also limited by the fact that some patient data had to be extrapolated from graphs, tabulated proportions of events, subgroup analyses or it had to be calculated from information derived directly from selected articles, possibly resulting in some human error.
In sum, our systematic review and meta-analysis supports the finding of several previous studies [23, 31, 39] that RAI ablation does not result in superior outcomes for PTMC.
Recurrences were localized mostly to the locoregional sites, and rarely to distant sites, and thus the post-operative follow-up period by clinical examination, ultrasound of the neck, assessment of serum T g levels and T g antibodies, and TSH suppressive therapy without RAI ablation, might already represent an adequate management strategy for PTMC patients treated by TT/NT.
Given the methodologic limitations of the meta-analysis of retrospective data, a long-term, prospective, randomized controlled trial with a larger sample size of selected patients may definitively resolve this issue. In addition, in this unprecedented gene-molecular era, the novel gene-molecular-based management strategies might also be used to select RAI-required patients with PTMC in the near future [42, 43].
References
Hedinger C, Williams E, Sobin L (1988) Histological typing of thyroid tumours. World Health Organisation histological classification of tumors, 2nd edn. Springer-Verlag, Berlin, pp 9–10
Davies L, Welch HG (2014) Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322
Arora N, Turbendian HK, Kato MA et al (2009) Papillary thyroid carcinoma and microcarcinoma: is there a need to distinguish the two? Thyroid 19:473–477
Wartofsky L (2012) Management of papillary microcarcinoma: primum non nocere? J Clin Endocrinol Metab 97:1169–1172
Haymart MR, Banerjee M, Stewart AK et al (2011) Use of radioactive iodine for thyroid cancer. JAMA 06:721–728
Cooper DS, Doherty GM, Haugen BR et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Pacini F, Castagna MG, Brilli L et al (2012) Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:vii110–vii119
Jung TS, Kim TY, Kim KW et al (2007) Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J 54:265–274
Sawka AM, Thephamongkhol K, Brouwers MA et al (2004) Systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab 89:3668–3676
Sacks W, Fung CH, Chang JT et al (2010) The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid 20:1235–1245
Cappelli C, Castellano M, Braga M et al (2007) Aggressiveness and outcome of papillary thyroid carcinoma (PT C) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol 95:555–560
Ito Y, Miyauchi A, Inoue H et al (2010) An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34:28–35. doi:10.1007/s00268-009-0303-0
Sugitani I, Toda K, Yamada K et al (2010) Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34:1222–1231. doi:10.1007/s00268-009-0359-x
Lee J, Park JH, Lee CR et al (2013) Long-term outcomes of total thyroidectomy versus thyroid lobectomy for papillary thyroid microcarcinoma: comparative analysis after propensity score matching. Thyroid 23:1408–1415
Moher D, Liberati A, Tetzlaff J et al (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34
Castillo JJ, Dalia S, Pascual SK (2010) Association between red blood cell transfusions and development of non-Hodgkin lymphoma: a meta-analysis of observational studies. Blood 116:2897–2907
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Langan D, Higgins JPT, Gregory W et al (2012) Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta-analysis. J Clin Epidemiol 65:511–519
Lau J, Ioannidis JPA, Terrin N et al (2006) The case of the misleading funnel plot. BMJ 333:597–600
Pedrazzini L, Baroli A, Marzoli L et al (2013) Cancer recurrence in papillary thyroid microcarcinoma: a multivariate analysis on 231 patients with a 12-year follow-up. Minerva Endocrinol 38:269–279
Mihailovic J, Stefanovic L, Stankovic R (2013) Influence of initial treatment on the survival and recurrence in patients with differentiated thyroid microcarcinoma. Clin Nucl Med 38:332–338
Kim HJ, Kim NK, Choi JH et al (2013) Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol 78:614–620
Riss JC, Peyrottes I, Chamorey E et al (2012) Prognostic impact of tumour multifocality in thyroid papillary microcarcinoma based on a series of 160 cases. Eur Ann Otorhinolaryngol Head Neck Dis 129:175–178
Gershinsky M, Barnett-Griness O, Stein N et al (2012) Total versus hemithyroidectomy for microscopic papillary thyroid cancer. J Endocrinol Invest 35:464–468
Neuhold N, Schultheis A, Hermann M et al (2011) Incidental papillary microcarcinoma of the thyroid—further evidence of a very low malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol 18:3430–3436
Moon HJ, Kim EK, Chung WY et al (2011) Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor?[J]. Ann Surg Oncol 18:1916–1923
Mercante G, Frasoldati A, Pedroni C et al (2009) Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 19:707–716
Pisanu A, Reccia I, Nardello O et al (2009) Risk factors for nodal metastasis and recurrence among patients with papillary thyroid microcarcinoma: differences in clinical relevance between nonincidental and incidental tumors. World J Surg 33:460–468. doi:10.1007/s00268-008-9870-8
Kim TY, Hong SJ, Kim JM et al (2008) Prognostic parameters for recurrence of papillary thyroid microcarcinoma. BMC Cancer 8:296–307
Hay ID, Hutchinson ME, Gonzalez-Losada T et al (2008) Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery 144:980–988
Gülben K, Berberoğlu U, Çelen O et al (2008) Incidental papillary microcarcinoma of the thyroid—factors affecting lymph node metastasis. Langenbecks Arch Surg 393:25–29
Pelizzo MR, Boschin IM, Toniato A et al (2006) Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol 32:1144–1148
Cheema Y, Olson S, Elson D et al (2006) What is the biology and optimal treatment for papillary microcarcinoma of the thyroid? J Surg Res 134:160–162
Lo CY, Chan WF, Lang BHH et al (2006) Papillary microcarcinoma: is there any difference between clinically overt and occult tumors. World J Surg 30:759–766. doi:10.1007/s00268-005-0363-8
Roti E, Rossi R, Trasforini G et al (2006) Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab 91:2171–2178
Chow SM, Law SCK, Chan JKC et al (2003) Papillary microcarcinoma of the thyroid—prognostic significance of lymph node metastasis and multifocality. Cancer 98:31–40
Appetecchia M, Scarcello G, Pucci E et al (2002) Outcome after treatment of papillary thyroid microcarcinoma. J Exp Clin Cancer Res 21:159–164
Baudin E, Travagli JP, Ropers J et al (1998) Microcarcinoma of the thyroid gland. Cancer 83:553–559
Wada N, Duh QY, Sugino K et al (2003) Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 237:399–407
Zhang L, Wei WJ, Ji QH et al (2012) Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 97:1250–1257
Xing M, Haugen BR, Schlumberger M (2013) Progress in molecular-based management of differentiated thyroid cancer. Lancet 381:1058–1069
Nucera C, Pontecorvi A (2012) Clinical outcome, role of BRAFV600E, and molecular pathways in papillary thyroid microcarcinoma: is it an indolent cancer or an early stage of papillary thyroid cancer? Front Endocrinol 3:33
Acknowledgments
The authors acknowledge the helpful methodological advice of Dr. Xiaochun Chou (Shanghai Jiao Tong University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that there are no conflicts, either perceived or real, with respect to this article.
Additional information
Guangfu Hu and Wei Zhu have contributed equally to this work and co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, G., Zhu, W., Yang, W. et al. The Effectiveness of Radioactive Iodine Remnant Ablation for Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-analysis. World J Surg 40, 100–109 (2016). https://doi.org/10.1007/s00268-015-3346-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3346-4