Abstract
Background
In patients with Bismuth type I and II hilar cholangiocarcinoma (HCCA), bile duct resection alone has been the conventional approach. However, many authors have reported that concomitant liver resection improved surgical outcomes.
Methods
Between January 2000 and January 2012, 52 patients underwent surgical resection for a Bismuth type I and II HCCA (type I: n = 22; type II: n = 30). Patients were classified into two groups: concomitant liver resection (n = 26) and bile duct resection alone (n = 26).
Results
Bile duct resection alone was performed in 26 patients. Concomitant liver resection was performed in 26 patients (right side hepatectomy [n = 13]; left-side hepatectomy [n = 6]; volume-preserving liver resection [n = 7]). All liver resections included a caudate lobectomy. Patient and tumor characteristics did not differ between the two groups. Although concomitant liver resection required longer operating time (P < 0.001), it had a similar postoperative complication rate (P = 0.764), high curability (P = 0.010), and low local recurrence rate (P = 0.006). Concomitant liver resection showed better overall survival (P = 0.047).
Conclusions
Concomitant liver resection should be considered in patients with Bismuth type I and II HCCA.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since its first review by Klatskin in 1965, hilar cholangiocarcinoma (HCCA) remains one of most difficult malignant tumors in which a curative resection can be obtained [1]. To achieve long-term survival, surgical resection and complete removal of malignant tissue has been recognized as the treatment of choice for HCCA [2–6]. Historically, in the early period of the use of this approach, local resection of the hilar area and hepaticojejunostomy was the standard approach in patients with HCCA because of the high mortality and morbidity of liver resection [7, 8]. However, high marginal recurrence and low curability rates have been reported. At present, development of preoperative management and surgical technique has facilitated a more aggressive resection approach. Preoperative bile drainage reduces postoperative morbidity and mortality [9]. Preoperative portal vein embolization increases the safety of the concomitant liver resection [10]. Combined vascular resection and reconstruction have expanded the surgical indications [11–13].
Nowadays, concomitant liver resection is accepted as a standard procedure in patients with HCCA [8, 14–19]. Nevertheless, the optimal procedure for Bismuth type I and II HCCA remains controversial. Several authors have reported that bile duct resection without liver resection can be applied in selected patients with Bismuth type I and II HCCA [20–22]. Other investigators have recommended concomitant liver resection because of the low curability rate of bile duct resection alone [14, 15, 17]. In these studies, the survival rate in patients with no residual tumor (R0 resection) was higher after concomitant liver resection than after bile duct resection alone. In the present study, we identified surgical outcomes of the bile duct resection alone and the concomitant liver resection in patients with Bismuth type I and II HCCA.
Methods
Patients
From January 2000 through January 2012, a total of 174 patients with HCCA underwent surgical resection at Yonsei University Health System, Seoul, Korea. According to the Bismuth classification criteria [23], 24 patients were classified as Bismuth type I and 30 patients were classified as Bismuth type II. A total of 52 patients with Bismuth type I and II HCCA were enrolled in this study, excluding two patients who underwent palliative resection. The patients were divided into two groups according to the resection scale: patients who received concomitant liver resection (group A) and patients who received bile duct resection alone (group B).
Preoperative evaluation
All patients underwent preoperative magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP) to determine the extent of disease along the intrahepatic ducts. Regional lymph nodes and distant metastases were imaged by computed tomography (CT) or magnetic resonance imaging (MRI). The majority of patients (39 of 52 cases) were jaundiced on admission; biliary decompression was performed using endoscopic retrograde biliary drainage (ERBD) and/or percutaneous transhepatic biliary drainage (PTBD). When jaundice could not be controlled following biliary drainage, additional biliary drainage was added to resolve jaundice.
Surgical strategy
At first, bile duct resection alone was performed in patients with T1 or T2 stage and/or Bismuth type I HCCA on preoperative imaging studies; concomitant liver resection was performed in patients with advanced T stage and/or Bismuth type II HCCA on preoperative imaging studies, but surgical strategy was changed in the late 2000s because of the high recurrence rate. Right hepatectomy with caudate lobectomy has become the standard procedure for the treatment of Bismuth type I or II HCCA. Left hepatectomy with caudate lobectomy or segmentectomy 4 with caudate lobectomy was performed in patients who were expected to have a small remnant liver.
Surgical procedure
All surgical procedures included resection of lymph nodes adjacent to the retropancreatic area and proper hepatic artery. Lymph nodes and connective tissues around the hepatoduodenal ligament from the suprapancreatic portion to the liver hilum were dissected in an en-bloc fashion. Lymph nodes of hepatoduodenal ligament, common hepatic artery, retopancreatic region and celiac trunk were routinely collected and sent to the pathologist. Para-aortic lymph nodes were dissected when lymph node enlargement was observed in preoperative imaging. The distal bile duct was transected at the upper border of the pancreatic head. The distal stump of the distal bile duct was ligated to prevent bile spillage. In the bile duct resection alone group, after the hepatic artery and portal vein were skeletonized (Fig. 1c), the extrahepatic bile duct was fully exposed and the proximal site was transected. In the concomitant liver resection group, liver parenchymal transection was performed using the Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab, Bloomfield, CT). To obtain a wide resection margin, the left bile duct was transected near the origin of the umbilical portion during a right side hepatectomy with a caudate lobectomy (Fig. 1a). During left side hepatectomy with caudate lobectomy, the right proximal bile duct was transected at the bifurcation site of the right anterior and right posterior bile duct (Fig. 1b). A volume-preserving liver resection was performed in a similar manner (Fig. 1d). The entire bile duct resection margin was examined by frozen-section histologic assessment during surgery. Further bile duct resection was performed when tumor invasion was reported from the analysis of the frozen section. The surgical concepts of the no touch technique and en-bloc resection were followed during the procedure. All liver resections included caudate lobectomy. Liver resection, including caudate lobectomy, was not performed in patients in the bile duct resection alone group.
Surgical procedure of concomitant liver resection. The left side of the proximal bile duct resection margin was clearly obtained near the origin point of the umbilical portion during right hepatectomy with caudate lobectomy (a). The right side of the proximal bile duct was resected at the right anterior and the right posterior bile duct bifurcation site (b). Right and left hepatic arteries and portal vein were skeletonized during segmentectomy 1, 4 (c). Bilateral proximal bile duct resection margin was clearly obtained during segmentectomy 1, 4 (d)
Perioperative and follow-up data
During surgery, operative time, intraoperative blood loss (calculated by the volume of suction and the weight of gauze or tape), and intraoperative transfusion requirements were recorded. Complications were documented according to the Clavien-Dindo classification [24]. Postoperative mortality was defined as death within 30 days. Patients with microscopic residual tumor (R1 resection) received adjuvant radiation therapy or adjuvant chemoradiation therapy one month postoperatively. Every three postoperative months, for evaluation of recurrence, the patients were checked for the tumor marker carbohydrate antigen 199 (CA 19-9) and carcinoembryonic antigen (CEA). Additionally, ultrasonography or a dynamic CT scan was performed during these follow-up visits. The median postoperative follow-up time was 21 months (range: 3–128 months). When tumor recurred, adjuvant chemotherapy and/or radiation therapy was given dependent upon patient status. Tumor recurrence was categorized as either local recurrence or distant metastases. Local recurrence was further divided into four subtypes according to location (liver parenchymal resection margin, remnant common bile duct, hepatoduodenal lymph nodes, or anastomosis site of bile duct) on the basis of imaging studies such as dynamic CT or MRI.
Statistical analysis
Continuous variables were compared with Student’s t-test and expressed as a mean value with standard deviation. Categorical variables were compared with the chi-square test or Fisher’s exact test, as appropriate. Survival rates were calculated by the Kaplan-Meier method; differences in survival rate were compared by the log rank test. Statistical analyses were performed with SPSS v 18 software for Microsoft Windows (SPSS Inc, Chicago, IL). P values of <0.05 were considered statistically significant.
Results
Operative type
As shown in Table 1, bile duct resection alone was performed in 26 of 52 patients and concomitant liver resection was performed in 26 of 52 patients. We performed several types of liver resections according to tumor invasion and liver disease status. A left hepatectomy or volume-preserving liver resection was performed in patients who were expected to have a small remnant liver following a right hepatectomy. A left side hepatectomy was performed in 6 patients, a right side hepatectomy was performed in 13 patients, and a volume preserving liver resection was performed in 7 patients. Portal vein embolization was performed in 7 of 26 patients. All patients underwent a right hepatectomy.
Patient characteristics and tumor characteristics
Table 2 shows patient characteristics for the two groups. There were no differences in age, gender, or body mass index between the two groups. The proportion of patients with elevated preoperative serum level of tumor marker was not significantly different between the two groups. Surgery time was longer in group A (P < 0.001), but intraoperative blood loss and transfusion requirement were not significantly different between the two groups. Total number of dissected lymph node and metastatic lymph node were not significantly different between the two groups. Curability was higher in group A (P = 0.010). All patients in group A underwent R0 resection without additional resection of the proximal bile duct. However, 7 patients in group B underwent R1 resection. Except the patients who underwent R1 resection, additional resection of the proximal bile duct was performed in six patients in group B because of tumor invasion found on frozen section. The proportion of patients who received additional resection of the distal bile duct did not differ between the two groups. Among the patients who underwent R1 resection, two patients had microscopic tumor present on the resection margin of the distal bile duct and five patients had microscopic tumor present on the resection margin of the proximal bile duct in group B. Pathologic data and tumor stage are presented in Table 3. The proportion of patients with Bismuth type I was higher in group B. There were no differences in macroscopic type, histologic type, lymphatic permeation, perineural invasion, T stage, and N stage between the two groups.
Morbidity and mortality
Postoperative complications occurred in 16 of 52 patients (Table 4). There were no significant differences in complication rates between the two groups. Intra-abdominal abscess followed by bile leakage was the most common complication (7 patients). This was treated by additional external abdominal drainage in all but two patients who had no clinical symptoms. A pleural effusion occurred in one patient in group A. Reoperation was performed on one patient in each group because of wound dehiscence. The group A patient was treated in the intensive care unit because of a cerebral infarction; this patient was subsequently discharged without sequelae. Pleural effusion, wound dehiscence, and cerebral infarction occurred in patients who underwent right hepatectomy. Postoperative mortality was not observed in this study. All patients were discharged in good condition.
Tumor recurrence
During follow-up, tumor recurred in 18 of 45 patients, excluding 7 patients with a R1 resection (Table 5). Local recurrence occurred in 13 patients and the local recurrence rate was higher in group B (P = 0.006). Anastomosis site (hepaticojejunostomy) was the most common site of local recurrence (6 patients); no patients in group A experienced a recurrence at the anastomosis site. Recurrence at the liver parenchymal resection margin occurred in two patients in group A. These patients underwent a segmentectomy 4 with a caudate lobectomy. Recurrence at the remnant common bile duct occurred in one patient in each group. Distant metastases occurred in 9 patients; 4 patients had both a local recurrence and distant metastases. There was no difference in distant metastases between the two groups (P = 1.0). Multiple liver metastases occurred in 4 patients, carcinomatosis occurred in 3 patients, and lung metastases occurred in 2 patients.
Carcinomatosis was only observed in group A patients (one with a right side hepatectomy and one with a left side hepatectomy and one with a volume-preserving liver resection). In regard to tumor recurrence after R1 resection, tumor recurrence occurred in 4 patients (Table 6), 3 of whom experienced a recurrence at the anastomosis site; the fourth patient suffered lung metastases. Among three patients with no recurrence, one patient died from an unrelated cause eight months postoperatively. Two patients have survived for more than one year without recurrence.
Survival
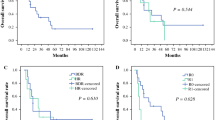
The 1- and 3-year disease-free survival rates of group A were 68.4 and 55.9 %, respectively; the rates for group B were 78.7 and 52.8 %, respectively. The 1- and 3-year overall survival rates of group A were 92.0 and 87.4 %, respectively; those for group B were 95.8 and 76.1 %, respectively. The median survival after resection was 19.0 months (range: 5–128 months) in group A and 23.5 months (range: 2–78 months) in group B. There was no difference in the disease-free survival rate between the two groups (P = 0.334; Fig. 2); however, group A had a better overall survival than group B (P = 0.047; Fig. 3). In addition, there were no differences in disease-free survival and overall survival rates among the liver resection subtypes (data not shown).
Discussion
Nowadays, many studies have reported that a concomitant liver resection of HCCA obtained both a negative resection margin and an increase in the number of patients eligible for resection [16–18]. However, controversy exists regarding the appropriate surgical procedure for patients with Bismuth type I and II HCCA. Some authors are of the opinion that bile duct resection without liver resection is an acceptable procedure for Bismuth type I and II HCCA, especially papillary type and below T2 tumors [20–22]. In the present study, when compared with bile duct resection alone, concomitant liver resection had a similar complication rate, a high curability, and a low local recurrence rate. Thus, our results bear a similarity with other recent reports.
Most surgeons favor a right hepatectomy because the distance from the confluence of the bile duct to the segmental ramification is much greater on the left side and the right hepatic artery runs close to the confluence of the bile duct [21, 25–27]. However, the right liver volume is generally 60–70 % of the total liver volume [28–30], and portal vein embolization is often necessary to avoid postoperative liver failure. In contrast, left hepatectomy has the advantage of liver parenchymal preservation. Shimizu et al. demonstrated the radicality as well as the safety of a left side hepatectomy in patients with HCCA predominantly on the left side [31]. They showed a similar postoperative morbidity and curability between left hepatectomy and right hepatectomy in patients with Bismuth type III HCCA. In the present study, survival and recurrence rate after a left hepatectomy was not different from that of a right hepatectomy. R0 resection was achieved in all patients who underwent a left hepatectomy.
In this study, limited liver resections for preservation of liver parenchyma were performed in 7 patients. Segmentectomy 4 with caudate lobectomy is noted to be a minimal resection procedure to prevent residual tumor in the bile duct [32, 33]. Although this procedure can preserve more liver parenchymal volume than a left hepatectomy, segmentectomy 4 with caudate lobectomy required a longer operative time (hepatectomy with caudate lobectomy: 439.5 ± 136.5 min, segmentectomy 4 with caudate lobectomy: 592.5 ± 92.0 min; P = 0.019) because of the multiple transection planes and duct anastomoses. In terms of outcome, a segmentectomy 4 with caudate lobectomy did not have a significant advantage over left hepatectomy. Recently, in our institution, left hepatectomy with caudate lobectomy has been the preferred procedure in Bismuth type I and II HCCA patients with a high risk for postoperative liver dysfunction.
Hilar cholangiocarcinoma has a tendency to spread intraluminally along the bile ducts into the liver; in addition, it extends into surrounding tissue, with perineural invasion and hepatic parenchymal invasion. Therefore, HCCA often requires extensive hepatic parenchymal resection. Shimada et al. [34], in a pathologic analysis of 29 patients with bile duct cancer, reported that the mean distance from microscopic invasion to the gross margin was 16.8 mm toward the liver. Adequacy of margin clearance for HCCA should be > 5 mm [35]. Shingu et al. [36] reported that additional resection of the bile duct for achievement of a negative margin did not improve survival because additional resection of > 5 mm in the proximal bile duct is difficult. In the present study, R1 resection was only observed in the bile duct resection alone group. Furthermore, additional resection of the proximal bile duct was performed in 6 patients in the bile duct resection alone group. Concomitant liver resection did not require additional resection of the proximal bile duct. These results indicate that bile duct resection alone does not guarantee the absence of microscopic residual tumor; therefore, concomitant liver resection is effective for the achievement of a tumor-free resection.
Inflammatory stromal infiltration is an innate histologic characteristic of HCCA [37]. Several studies have reported discontinuous submucosal invasion of the bile duct in patients with HCCA [38, 39]. Therefore, frozen section analysis to determine the status of the bile duct margin can be inaccurate, especially after preoperative biliary drainage. In the present study, recurrences at the anastomosis site were observed in 6 patients with R0 resection. All underwent bile duct resection alone. Three of 6 patients underwent additional resection of the proximal bile duct. However, no patient in the concomitant liver resection group experienced a recurrence at an anastomosis site. These results support the premise that the survival rate of bile duct resection alone is lower than that of concomitant liver resection, even when comparing only R0 resection; in addition, they corroborate that a sufficient negative margin width of the bile duct is related to improved survival after R0 resection [35].
The literature contains many studies that demonstrate improved outcome with concomitant liver resection [8, 14–19]. However, to the best of our knowledge, only three studies have reported that survival rate was significantly better after concomitant liver resection, compared to bile duct resection alone [1, 14, 15]. Those studies analyzed only R0 resection to exclude survival factors related to curability. Our study analyzed survival rate of R0 and R1 resection concurrently because R1 resection and additional resection of the proximal bile duct was only observed in the bile duct resection alone group. The local recurrence rate after bile duct resection alone was higher than that of concomitant liver resection (P = 0.006). These results suggest that bile duct resection alone is impacted in terms of curability or radicality in patients with Bismuth type I and II HCCA. This was an obligate feature of bile duct resection alone.
Several studies have demonstrated that bile duct resection without liver resection has a role in patients with Bismuth type I and II HCCA. Ikeyama et al. [21] reported good survival after bile duct resection with or without limited hepatectomy in patients with Bismuth type I and II papillary tumors. Otani et al. [22] suggested that bile duct resection alone may be indicated for papillary tumors graded below a T2 lesion and negative for lymph node metastases. In the present study, 3 patients in the bile duct resection alone group were alive more than five years without recurrence. All had a papillary tumor and the tumor stage was confirmed to be below T2 stage. This finding demonstrates the role of bile duct resection alone in patients with Bismuth type I and II HCCA. However, in the bile duct resection alone group, cancer recurred in another 3 patients with papillary tumors. One patient underwent a R1 resection and the other two underwent additional resection of the proximal bile duct due to positive margins on frozen section. Bile duct resection alone should be meticulously performed after preoperative evaluation of superficial cancer spread.
There are two limitations to our study. One is that it did not have clear criteria for the application of resection type. This is a limitation of a retrospective study. At our institution, the surgical strategy for Bismuth type I and II HCCA has slowly evolved from bile duct resection alone to concomitant liver resection. However, bile duct resection alone was principally performed in patients with early T stage and/or Bismuth type I HCCA. Another limitation is the small number of cases. This is a result of the low incidence of HCCA and the difficulty of early diagnosis. In this study, overall survival rate in the combined liver resection group was better than that of the local resection group (P = 0.047). However, disease free survival was not significantly different in two groups. Future studies may clarify the survival benefit of concomitant liver resection in patients with Bismuth type I and II HCCA.
In conclusion, concomitant liver resection in patients with Bismuth type I and II HCCA requires a long surgery time. Nonetheless, concomitant liver resection achieved a R0 resection without additional resection of the bile duct in all patients with Bismuth type I and II HCCA; furthermore it had a lower local recurrence rate than bile duct resection alone. The complication rate was not different between patients with concomitant liver resection and those with bile duct resection alone. The overall survival rate was better in patients with concomitant liver resection. Therefore, concomitant liver resection should be considered in patients with Bismuth type I and II HCCA.
References
Klatskin G (1965) Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatic. An unusual tumor with distinctive clinical and pathological features. Am J Med 38:241–256
Nanashima A, Tobinaga S, Abo T et al (2012) Experience of surgical resection for hilar cholangiocarcinomas at a Japanese single cancer institute. Hepatogastroenterology 59:347–350
Cannon RM, Brock G, Buell JF (2012) Surgical resection for hilar cholangiocarcinoma: experience improves respectability. HPB (Oxf) 14:142–149
Saxena A, Chua TC, Chu FC et al (2011) Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg 202:310–320
Ito F, Cho CS, Rikkers LF et al (2009) Hilar cholangiocarcinoma: current management. Ann Surg 250:210–218
Ruys AT, van Haelst S, Busch OR et al (2012) Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg 36:2179–2186. doi:10.1007/s00268-012-1638-5
Nakeeb A, Pitt HA, Sohn TA et al (1996) Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 224:463–473 discussion 473–475
Miyazaki M, Ito H, Nakagawa K et al (1998) Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery 123:131–136
Young AL, Igami T, Senda Y et al (2011) Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion. HPB (Oxford) 13:483–493
Nagino M, Kamiya J, Nishio H et al (2006) Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 243:364–372
Miyazaki M, Kimura F, Shimizu H et al (2007) Recent advance in the treatment of hilar cholangiocarcinoma: hepatectomy with vascular resection. J Hepatobiliary Pancreat Surg 14:463–468
Igami T, Nishio H, Ebata T et al (2010) Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepato-biliary-pancreatic Sci 17:449–454
Konstadoulakis MM, Roayaie S, Gomatos IP et al (2008) Aggressive surgical resection for hilar cholangiocarcinoma: is it justified? Audit of a single center’s experience. Am J Surg 196:160–169
Kondo S, Hirano S, Ambo Y et al (2004) Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg 240:95–101
Jarnagin WR, Fong Y, DeMatteo RP et al (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234:507–517 discussion 517–509
Dinant S, Gerhards MF, Rauws EA et al (2006) Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol 13:872–880
Ito F, Agni R, Rettammel RJ et al (2008) Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg 248:273–279
DeOliveira ML, Cunningham SC, Cameron JL et al (2007) Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 245:755–762
Kow AW, Wook CD, Song SC et al (2012) Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg 36:1112–1121. doi:10.1007/s00268-012-1497-0
Launois B, Terblanche J, Lakehal M et al (1999) Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg 230:266–275
Ikeyama T, Nagino M, Oda K et al (2007) Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg 246:1052–1057
Otani K, Chijiiwa K, Kai M et al (2012) Role of hilar resection in the treatment of hilar cholangiocarcinoma. Hepatogastroenterology 59:696–700
Bismuth H, Corlette MB (1975) Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 140:170–178
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Kawasaki S, Imamura H, Kobayashi A et al (2003) Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg 238:84–92
Jonas S, Benckert C, Thelen A et al (2008) Radical surgery for hilar cholangiocarcinoma. Eur J Surg Oncol 34:263–271
Neuhaus P, Jonas S, Settmacher U et al (2003) Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg 388:194–200
Farges O, Belghiti J, Kianmanesh R et al (2003) Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 237:208–217
Abdalla EK, Denys A, Chevalier P et al (2004) Total and segmental liver volume variations: implications for liver surgery. Surgery 135:404–410
Tongyoo A, Pomfret EA, Pomposelli JJ (2012) Accurate estimation of living donor right hemi-liver volume from portal vein diameter measurement and standard liver volume calculation. Am J Transplant 12:1229–1239
Shimizu H, Kimura F, Yoshidome H et al (2010) Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg 251:281–286
Miyazaki M, Ito H, Nakagawa K et al (1998) Segments I and IV resection as a new approach for hepatic hilar cholangiocarcinoma. Am J Surg 175:229–231
Kawarada Y, Isaji S, Taoka H et al (1999) S4a + S5 with caudate lobe (S1) resection using the Taj Mahal liver parenchymal resection for carcinoma of the biliary tract. J Gastrointest Surg 3:369–373
Shimada H, Niimoto S, Matsuba A et al (1988) The infiltration of bile duct carcinoma along the bile duct wall. Int Surg 73:87–90
Seyama Y, Kubota K, Sano K et al (2003) Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 238:73–83
Shingu Y, Ebata T, Nishio H et al (2010) Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery 147:49–56
Bosma A (1990) Surgical pathology of cholangiocarcinoma of the liver hilus (Klatskin tumor). Semin Liver Dis 10:85–90
Sakamoto E, Nimura Y, Hayakawa N et al (1998) The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg 227:405–411
Hayashi S, Miyazaki M, Kondo Y et al (1994) Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer 73:2922–2929
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, J.H., Choi, G.H., Choi, S.H. et al. Liver Resection for Bismuth Type I and Type II Hilar Cholangiocarcinoma. World J Surg 37, 829–837 (2013). https://doi.org/10.1007/s00268-013-1909-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-1909-9