Abstract
Purpose
This retrospective study aimed to clarify whether the postoperative prognosis differs between right and left hepatectomy for Bismuth type I/II perihilar cholangiocarcinoma.
Methods
Preoperative images of 195 patients with perihilar cholangiocarcinoma were reexamined. Patients with Bismuth type I/II perihilar cholangiocarcinoma without a difference in extraductal tumor invasion between the right and left sides of the hepatic portal region were classified into those undergoing left (L group) or right (R group) hepatectomy.
Results
Twenty-three patients (11.8%) were classified into the L group and 33 (16.9%) into the R group. All eight patients with pTis/1 belonged to the L group. The L group had significantly less liver failure than the R group (p = 0.001). One patient (4.3%) in the L group and four patients (12.1%) in the R group died from postoperative complications. Among 48 patients with pT2, the L group tended to have better overall survival (median, 12.2 vs. 5.6 years; p = 0.072), but not recurrence-free survival (median, 9.1 vs. 3.6 years; p = 0.477), in comparison to the R group.
Conclusions
Postoperative survival after left hepatectomy for Bismuth type I/II perihilar cholangiocarcinoma is expected to be as long as that after right hepatectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perihilar cholangiocarcinoma (PHC) is a highly malignant tumor that involves the main biliary confluence of the right and left hepatic ducts [1]. In 1992, Bismuth et al. [2] divided PHC into four anatomical types based on the ductal spread of the tumor: type I, hepatic duct involvement below the bifurcation; type II, bifurcation involvement; type IIIa, involvement of right-sided second-order ducts; type IIIb, involvement of left-sided second-order ducts; and type IV, bilateral involvement of right- and left-sided second-order ducts. In 2003, Kawasaki et al. [3] proposed right hepatectomy as the optimal surgical approach for Bismuth type I/II PHC based on the following anatomical considerations: (1) the left hepatic duct is longer than the right hepatic duct; (2) the right hepatic artery is more likely to be invaded than the left hepatic artery, because the right hepatic artery passes behind the common hepatic duct; and (3) in patients for whom portal venous resection at the hepatic hilum is necessary, it is easier to perform venous reconstruction with the left portal vein than the right portal vein, because of the long extrahepatic portion of the transverse portion of the left portal vein.
In 2004, we reported on 40 consecutive resections for PHC (Bismuth type I, n = 7; type II, n = 12; type III, n = 13; and IV, n = 6) [4]. The analysis showed that the survival of patients treated with right hepatectomy was significantly better than that of patients who underwent other procedures (left hepatectomy, isolated caudate lobectomy, or hilar resection alone). Based on these findings, we suggested performing right hepatectomy, even for Bismuth type I/II PHC. However, in our previous study [4], among 19 patients with Bismuth type I/II PHC, 14 did not undergo major hepatectomy and only 1 underwent left hepatectomy. In 2007, Ikeyama et al. [5] reported that patients who underwent right hepatectomy for Bismuth I/II type PHC had significantly longer postoperative survival than those who underwent other procedures, and recommended right hepatectomy for Bismuth type I/II PHC. However, only 4 of the 54 patients with Bismuth type I/II PHC underwent left hepatectomy in that study [5]. Despite the limitations of the surgical procedures used in these studies [4, 5], right hepatectomy has been considered the standard treatment for Bismuth type I/II PHC.
One reason for preferring right hepatectomy for PHC is that right hepatectomy with routine portal vein resection, using the so-called “no-touch” technique, is thought to prevent the microscopic dissemination of cancer cells [6,7,8]. Recently, however, the oncological superiority of right hepatectomy over left hepatectomy for PHC has been called into question [9, 10]. In the benchmark study, which analyzed a large cohort of consecutive patients who underwent major liver surgery for PHC at 24 high-volume centers in three continents, a comparison between anatomical right and left hepatectomy revealed a significant difference in overall survival (OS), which favored left hepatectomy. There was no evidence of the oncological superiority of routine portal vein resection [10]. Moreover, in 2015, Hirose et al. [11] measured the length of the resected right hepatic duct in right hepatectomy, and that of the resected left hepatic duct in left hepatectomy, and reported that the lengths of the resected bile ducts were similar. The authors concluded that the assumption that the left hepatic duct is longer than the right hepatic duct was the surgeon’s biased view [11].
As mentioned previously, the oncological superiority of right hepatectomy over left hepatectomy for Bismuth type I/II PHC remains unclear. Hence, we conducted a retrospective study to determine with postoperative prognosis differs between right and left hepatectomy for Bismuth type I/II PHC.
Methods
Patients
The data of patients with PHC who underwent major hepatectomy in the Department of Gastroenterological Surgery II at Hokkaido University Hospital between March 2004 and March 2019 were retrospectively analyzed. Patients who had undergone preoperative chemotherapy, those who had undergone surgical resection for previous biliary tract carcinoma, and those with distant metastases were excluded from the analysis. Patients diagnosed with advanced cystic duct carcinoma were also excluded, because biliary carcinoma centered in the cystic duct has been classified as gallbladder carcinoma by the American Joint Committee on Cancer (AJCC) (eighth edition) [1]. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study design was approved by the Human Research Review Committee of Hokkaido University Hospital (approval number: 020-0208). Informed consent, including that which permitted the investigators to use the data retrospectively, was obtained from each patient at the time of surgery.
Diagnosis of Bismuth type I/II without a difference in extraductal tumor invasion between the right and left sides of the hepatic portal region
Patients who underwent hepatectomy for Bismuth type I/II PHC were reexamined. The Bismuth type was determined based on preoperative imaging findings, including those of contrast-enhanced computed tomography (CT), cholangiography, and magnetic resonance cholangiopancreatography. The highest priority was placed on CT findings of extramural tumor growth. Consequently, this study included only patients with Bismuth type I/II PHC in whom—according to preoperative imaging findings—the same curative effect could be achieved with either left or right hepatectomy. Therefore, patients with Bismuth type I/II PHC who showed a difference in extraductal tumor invasion between the right and left sides of the hepatic portal region were excluded from the present study (Fig. 1). Additionally, patients with Bismuth type I/II PHC who were diagnosed with cholangiocarcinoma at the second-order or higher biliary radical upstream of the intrahepatic bile duct by preoperative biopsy were also excluded. Finally, the selected patients were classified according to the performance of right (R group) or left (L group) hepatectomy.
a Coronal and b axial views of the same patient with Bismuth type I perihilar cholangiocarcinoma (PHC) without a difference in extraductal tumor invasion between the right and left sides of the hepatic portal region (arrows indicate the tumor). c Coronal and d axial views of the same patient with Bismuth type II PHC with extraductal tumor invasion of the right side of the hepatic portal region. c Bismuth type II PHC (arrow). d Tumor progression in the right side of the hepatic hilus involving the right hepatic artery (arrow)
Preoperative management
Preoperative biliary decompression was performed to reduce serum bilirubin concentrations to < 2 mg/dL in all patients with jaundice, and to control segmental cholangitis. Patient liver volumes were semiautomatically measured using contrast-enhanced CT (volume or 5-mm-thick axial imaging data), and the ratio of the future remnant liver volume to the total liver volume (%FLR) was calculated [12]. In our department, the standard surgical procedure for Bismuth type I/II PHC was right hepatectomy with caudate lobectomy and extrahepatic bile duct resection [4, 13, 14]. However, when the indocyanine green retention rate at 15 min was > 15%, the %FLR for right hepatectomy was < 30%, or hepatopancreatoduodenectomy was necessary, left hepatectomy was considered. Moreover, when the primary tumors were of the macroscopic papillary growth type, left hepatectomy was considered, because the survival of patients with papillary growth type tumors is reported to be fairly good, even after extrahepatic bile duct resection without hepatectomy [5]. In all patients, the side of the hepatectomy was determined preoperatively, and was not changed intraoperatively. When right hepatectomy was planned, preoperative portal vein embolization (PVE) on the side of the liver to be resected was considered. If PVE was performed, the %FLR was calculated at approximately 2–4 weeks after PVE to determine the degree of hypertrophy [15].
Surgical procedures
The standard surgical procedure for PHC included major hepatectomy with caudate lobectomy and extrahepatic bile duct resection. Combined pancreatoduodenectomy was performed when an invasive carcinoma had spread to the intrapancreatic bile duct. In patients who did not undergo combined pancreatoduodenectomy, the hepatic artery and portal vein were skeletonized after lymphadenectomy around the pancreatic head and division of the common bile duct. From 2005 to 2012, we adopted a “no-touch” technique for right hepatectomy, when preoperative imaging findings showed involvement of the duct neighboring the portal bifurcation, as we have reported previously [7, 8, 16]. In right hepatectomy, hepatic dissection was performed along the right side of the middle hepatic vein towards the right side of the umbilical portion, and division of the left hepatic duct was performed adjacent to the umbilical portion [13]. In left hepatectomy, hepatic dissection was performed along the left side of the middle hepatic vein towards the right side of the inferior vena cava, and division of the right anterior branch of the bile duct behind the middle hepatic vein was performed. The right posterior branch of the bile duct was then divided along the cranial surface of the right portal vein [14, 17, 18]. If the hepatic artery could not be separated from the surrounding nerve sheath, resection and reconstruction of the hepatic artery were performed after hepatic dissection. In patients who did not undergo pancreatoduodenectomy, bilioenteric Roux-en-Y anastomosis was performed using the ropeway method [19]. A modified Child’s method was used for reconstruction in patients who underwent hepatopancreatoduodenectomy.
Evaluation of postoperative complications
Medical records were used to acquire information on postoperative complications in all patients, which were assessed and graded according to the Clavien–Dindo classification [20]. Post-hepatectomy liver failure [21], intraabdominal hemorrhage [22], and bile leakage [23] that occurred during hospitalization were evaluated according to the definition and grading of the International Study Group of Liver Surgery.
Postoperative follow-up
Patients attended regular follow-up assessments every 3–6 months. The follow-up period ended in March 2021. At each visit, contrast-enhanced CT was performed. When tumor recurrence was difficult to determine based on the CT scan alone, ultrasonography, magnetic resonance imaging, bone scintigraphy, and/or positron emission tomography were used to confirm tumor recurrence. Although adjuvant chemotherapy was not routinely administered, some patients received adjuvant chemotherapy at the discretion of the attending physician, or by participating in clinical trials. Therapies after tumor recurrence were also determined by the attending physician. These included surgical resection, chemotherapy, radiotherapy, and best supportive care. Surgical resection for tumor recurrence was considered in patients who met the following criteria: new sites of recurrence that had not been detected during an observation period of > 3 months after the first recurrence was detected, even when the size of the lesions had increased, and all recurrent lesions were amenable to complete resection [24].
Pathological examination
All resected specimens were fixed in 10% buffered formalin. Serial sections were prepared at 3- to 6-mm intervals for microscopic examination using hematoxylin and eosin staining. The patients’ pathological findings were documented according to the AJCC cancer staging manual (eighth edition) [1].
Survival analyses
OS and recurrence-free survival (RFS) after surgical resection were estimated. RFS was defined as the interval between surgery and the date of the clinical diagnosis of the first recurrence or death from any cause. The impact of clinicopathological factors on OS and RFS was examined using univariate and multivariate analyses.
Statistical analyses
Statistical analyses were performed using the JMP software program (version 14.0; SAS Institute Inc., Cary, NC). p values of < 0.05 were considered to indicate statistical significance. Categorical data were analyzed using chi-squared tests. Survival rates were estimated using the Kaplan–Meier method, and log-rank tests were used to analyze associations between survival rates and various clinicopathological factors. Prognostic factors that were significant in the univariate analysis and the side of the hepatectomy (L or R group) were included in the multivariate analysis. Factors strongly associated with the side of the hepatectomy were excluded from the Cox proportional hazards model, because the most interesting factor in this study was the side of the hepatectomy.
Results
Patients
There were 23 patients in the L group and 33 in the R group (Fig. 2). In the L group, the main reasons for choosing left hepatectomy were insufficient %FLR (n = 7), a high indocyanine green retention rate at 15 min (n = 6), a large liver volume in reserve for hepatopancreatoduodenectomy (n = 5), tumors of macroscopic papillary growth type (n = 4), and PVE for portal hypertension (n = 1).
Clinicopathological features
The clinicopathological features are shown in Table 1. According to the pathological T (pT) classification of the AJCC cancer staging manual (eighth edition), all patients categorized into the R or L groups were classified as having pTis/1/2. All of the patients with pTis/1 (n = 8) belonged to the L group. In contrast, all 33 patients in the R group were diagnosed with pT2. Therefore, patients with pT2 in the L group were subclassified. The clinicopathological factors of patients with pT2 in the L and R groups were compared, and there was a significant difference in %FLR. Only one patient in the L group underwent hepatic artery resection and reconstruction. Histopathological examination of this patient revealed involvement of the surrounding nerve sheath, but not the wall of the hepatic artery. Only one patient in the L group was diagnosed with invasive carcinoma at the exfoliated margin of the transectional plate of the peripheral hepatic hilus. This patient’s tumor had not recurred within 48 months after surgical resection. Two patients in the R group were diagnosed with invasive carcinoma at the exfoliated margin of the extrahepatic bile duct. One patient died from postoperative complications; the other did not have recurrence within 24 months after surgical resection.
Postoperative complications
The frequencies of representative complications are shown in Table 2. There was no significant difference in Clavien–Dindo classification between the L and R groups. One patient in the L group who underwent hepatopancreatoduodenectomy died from bleeding due to rupture of the arterial aneurysm. Seven patients in the R group had severe complications (Clavien–Dindo grade IV/V). The frequency of liver failure in the L group was significantly less than that in the R group.
Postoperative prognosis
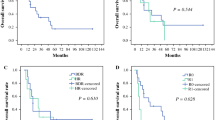
The OS and RFS curves of all 56 patients are shown in Fig. 3a, b. None of the eight patients with pTis/1, all of whom belonged to the L group (as mentioned above), died from recurrence during the follow-up period. Therefore, to avoid bias, survival curves were examined only for patients with pT2. Patients with pT2 in the L group tended to have better OS than those in the R group (median OS: 12.2 vs. 5.6 years, respectively; p = 0.072). There was no significant difference in RFS between the L and R groups of patients with pT2 (median RFS: 9.1 vs. 3.6 years, respectively; p = 0.477).
In the 48 patients with pT2, a low %FLR (< 50%) (p = 0.030) and regional lymph node metastasis (p = 0.012) were significant adverse prognostic factors for OS in univariate analysis (Table 3). Conversely, there was no significant difference in RFS between patients with a low and high %FLR (Table 3). Regional lymph node metastasis and the side of the hepatectomy were included in the multivariate analysis. However, %FLR was excluded, because it was strongly associated with the side of the hepatectomy in the clinicopathological analysis. Therefore, regional lymph node metastasis was selected as the only independent prognostic factor for both OS and RFS (Table 3).
The detailed data of six patients in the L group who had recurrence during the follow-up period are shown in Table 4. In five of these six patients, the site of recurrence was the liver. Moreover, four of these five patients underwent partial liver resection for recurrence. One patient developed local recurrence (peripheral bilioenteric anastomosis) 9.0 years after primary surgery (patient 6 in Table 4). During the observation period, the primary site of recurrence in 5 of the 17 patients with recurrence in the R group was the liver. However, only two patients underwent liver resection for recurrence (Online Resource 1). In the R group, only one patient (without carcinoma at the resected surgical margin) experienced local recurrence (peripheral bilioenteric anastomosis) 9.7 years after surgical resection.
Discussion
The main finding of this study was that there was no significant difference in OS between patients with pT2 in the two groups, although the patients with pT2 in the L group tended to have better OS than did those in the R group. Moreover, RFS was similar between patients with pT2 in both groups.
Unexpectedly, the pT classification (AJCC cancer staging manual [eighth edition]) [1] of the patients in this study was pTis/1/2. The common hepatic duct runs at a right slant. Therefore, when ventral invasion of Bismuth type I/II tumors progresses, it is more likely to invade the right side than the left side of the hepatic hilus. This study was limited to patients with Bismuth type I/II PHC in whom, according to preoperative imaging findings, the same curative effect could be achieved with left hepatectomy as with right hepatectomy. Therefore, patients with Bismuth type I/II PHC who have less vertical infiltration (i.e., pTis/1/2) were selected.
One reason why right hepatectomy is recommended for Bismuth type I/II PHC is local tumor clearance. In this study, however, one patient was diagnosed with local recurrence in each group. Additionally, both patients were negative for invasive carcinoma at the resected surgical margin, and were diagnosed with local recurrence ≥ 9 years after surgical resection. Therefore, these two tumors diagnosed as recurrent tumors may have in fact been de novo tumors. Thus, the risk of ductal recurrence appears to be comparable between left and right hepatectomy for Bismuth type I/II PHC without a difference in extraductal tumor invasion between the right and left sides of the hepatic hilus.
Another reason why right hepatectomy is recommended for Bismuth type I/II PHC is that when right hepatic artery involvement is suspected in patients with Bismuth type I/II PHC, the right hepatic artery may easily be resected during right hepatectomy; however, reconstruction of the right hepatic artery is needed in left hepatectomy. Mizuno et al. [25] reported that hepatic artery-related complications had occurred in 19 (13.0%) of 146 patients who underwent hepatic artery reconstruction, 59% of whom underwent left trisectionectomy, in comparison to 9 (1.9%) of 484 patients without hepatic artery-related complications; however, the incidence of Clavien–Dindo grade ≥ III complications did not differ between the two groups to a statistically significant extent. Matsuyama et al. [26] also reported the incidence of complications between patients who underwent surgical resection for PHC with and without hepatic artery resection. Thus, in high-volume centers, hepatic artery resection and reconstruction for PHC have been performed relatively safely. Moreover, Sugiura et al. [27] insist that reconstruction of the right hepatic artery in left hepatectomy is relatively easy, due to the limited involvement and wide caliber of the distal side of the right hepatic artery. Therefore, in patients with Bismuth type I/II PHC without extraductal tumor progression of the right hepatic hilus, left hepatectomy with resection and reconstruction of the right hepatic artery appears not to be significantly inferior to right hepatectomy. A high %FLR after hepatectomy is an important indicator for preventing severe liver failure. The benchmark study [10] demonstrated that the median standard preoperative FLR in patients who underwent right hepatectomy was significantly higher than that in those who underwent left hepatectomy (65% vs. 40%, respectively; p < 0.001), and that the former group had a significantly higher rate of liver failure than the latter group (23.3% vs. 10.9%, respectively; p < 0.001). These trends were similar to those in our study. The benchmark study [10] also showed that patients who underwent right hepatectomy had a significantly higher in-hospital mortality rate than those who underwent left hepatectomy (6.6% vs. 2.9%, respectively; p = 0.02). In the present study, the mortality rates did not differ to a statistically significant extent (Clavien–Dindo grade V); however, this may be due to the limited number of patients. Therefore, with respect to preventing severe postoperative complications, there is no doubt that left hepatectomy is preferable for Bismuth type I/II PHC.
In this study, all patients with pTis/1 belonged to the L group. This may be attributed to the surgical procedure selection policy of our department (i.e., when primary tumors are of the macroscopic papillary growth type, left hepatectomy is considered), as mentioned in the Preoperative management section. Therefore, to avoid selection bias, comparisons of survival between the L and R groups were analyzed only in patients with pT2. Among patients with pT2, the L group tended to have better OS than the R group; however, this was not the case for RFS. These results suggested that despite recurrence in patients in the L group, they were still expected to survive longer than those in the R group. Interestingly, the benchmark study [10] demonstrated that there was a significant difference in OS, but not disease-free survival, between right and left hepatectomy, favoring left hepatectomy. These results were similar to ours. In this study, four patients with tumor recurrence in the liver in the L group underwent partial liver resection, and these patients survived longer. Additional liver resection in the L group may be permitted after a high remnant liver function is observed. Indeed, the survival analysis showed that a higher %FLR was a significantly better prognostic factor for OS, but not RFS, than a lower %FLR. Therefore, patients in the L group had a higher tolerance to therapy for recurrent disease in comparison to patients in the R group, because of the larger remnant liver volume.
Conclusions
Left hepatectomy for patients with Bismuth type I/II PHC without a difference in extraductal tumor invasion between the right and left sides of the hepatic portal region is expected to have a similar prognosis to right hepatectomy. In addition, a larger residual liver volume may not only reduce the risk of liver failure but also improve tolerance to therapy for recurrent disease. The main limitation of this retrospective study is the small sample size. Therefore, future large multicenter studies using consistent imaging modalities are warranted to validate our findings. Currently, however, we recommend left hepatectomy for Bismuth type I/II PHC without extraductal tumor invasion in the right side of the hepatic portal region.
Change history
18 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00595-021-02410-6
References
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2016.
Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–8.
Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92.
Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101.
Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 2007;246:1052–7.
Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012;19:1602–8.
Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K. No-touch resection of hilar malignancies with right hepatectomy and routine portal reconstruction. J Hepatobiliary Pancreat Surg. 2009;16:502–7.
Tamoto E, Hirano S, Tsuchikawa T, Tanaka E, Miyamoto M, Matsumoto J, et al. Portal vein resection using the no-touch technique with a hepatectomy for hilar cholangiocarcinoma. HPB (Oxford). 2014;16:56–61.
Nagino M, Ebata T, Mizuno T. Oncological superiority of right-sided hepatectomy over left-sided hepatectomy as surgery for klatskin tumors: truth or biased view? Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004534.
Mueller M, Breuer E, Mizuno T, Bartsch F, Ratti F, Benzing C, et al. Perihilar cholangiocarcinoma-novel benchmark values for surgical and oncological outcomes from 24 expert centers. Ann Surg. 2021. https://doi.org/10.1097/SLA.0000000000005103.
Hirose T, Igami T, Ebata T, Yokoyama Y, Sugawara G, Mizuno T, et al. Surgical and radiological studies on the length of the hepatic ducts. World J Surg. 2015;39:2983–9.
Nakanishi Y, Tsuchikawa T, Okamura K, Nakamura T, Tamoto E, Noji T, et al. Risk factors for a high Comprehensive Complication Index score after major hepatectomy for biliary cancer: a study of 229 patients at a single institution. HPB (Oxford). 2016;18:735–41.
Hirano S, Tanaka E, Shichinohe T, Suzuki O, Hazama K, Kitagami H, et al. Treatment strategy for hilar cholangiocarcinoma, with special reference to the limits of ductal resection in right-sided hepatectomies. J Hepatobiliary Pancreat Surg. 2007;14:429–33.
Noji T, Tanaka K, Matsui A, Nakanishi Y, Asano T, Nakamura T, et al. Transhepatic direct approach to the “limit of the division of the hepatic ducts” leads to a high r0 resection rate in perihilar cholangiocarcinoma. J Gastrointest Surg. 2021;25:2358–67.
Sakuhara Y, Abo D, Hasegawa Y, Shimizu T, Kamiyama T, Hirano S, et al. Preoperative percutaneous transhepatic portal vein embolization with ethanol injection. AJR Am J Roentgenol. 2012;198:914–22.
Nakanishi Y, Tsuchikawa T, Okamura K, Nakamura T, Tamoto E, Murakami S, et al. Prognostic impact of the site of portal vein invasion in patients with surgically resected perihilar cholangiocarcinoma. Surgery. 2016;159:1511–9.
Uesaka K. Left hepatectomy or left trisectionectomy with resection of the caudate lobe and extrahepatic bile duct for hilar cholangiocarcinoma (with video). J Hepatobiliary Pancreat Sci. 2012;19:195–202.
Natsume S, Ebata T, Yokoyama Y, Igami T, Sugawara G, Shimoyama Y, et al. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg. 2012;255:754–62.
Hirano S, Tanaka E, Tsuchikawa T, Matsumoto J, Shichinohe T, Kato K. Techniques of biliary reconstruction following bile duct resection (with video). J Hepatobiliary Pancreat Sci. 2012;19:203–9.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24.
Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 2011;13:528–35.
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–8.
Nakanishi Y, Okamura K, Tsuchikawa T, Nakamura T, Noji T, Asano T, et al. Time to recurrence after surgical resection and survival after recurrence among patients with perihilar and distal cholangiocarcinomas. Ann Surg Oncol. 2020;27:4171–80.
Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, et al. Combined vascular resection for locally advanced perihilar cholangiocarcinoma. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004322.
Matsuyama R, Mori R, Ota Y, Homma Y, Kumamoto T, Takeda K, et al. Significance of vascular resection and reconstruction in surgery for hilar cholangiocarcinoma: with special reference to hepatic arterial resection and reconstruction. Ann Surg Oncol. 2016;23:475–84.
Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, et al. Left hepatectomy with combined resection and reconstruction of right hepatic artery for bismuth type i and ii perihilar cholangiocarcinoma. World J Surg. 2019;43:894–901.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Reference 12 was published incorrectly in the original publication. Reference 12 has been updated in original publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakanishi, Y., Hirano, S., Okamura, K. et al. Clinical and oncological benefits of left hepatectomy for Bismuth type I/II perihilar cholangiocarcinoma. Surg Today 52, 844–852 (2022). https://doi.org/10.1007/s00595-021-02401-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02401-7